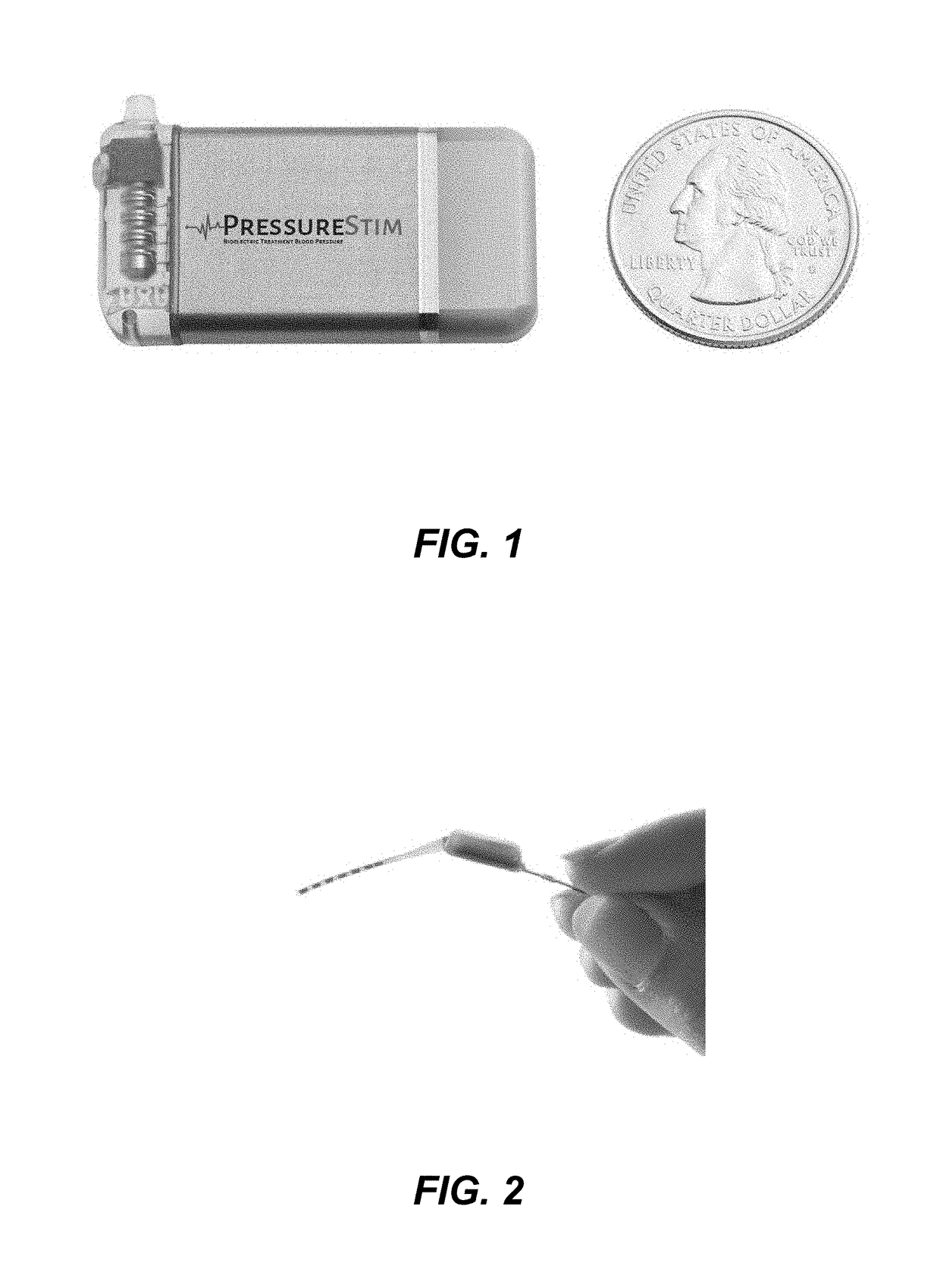
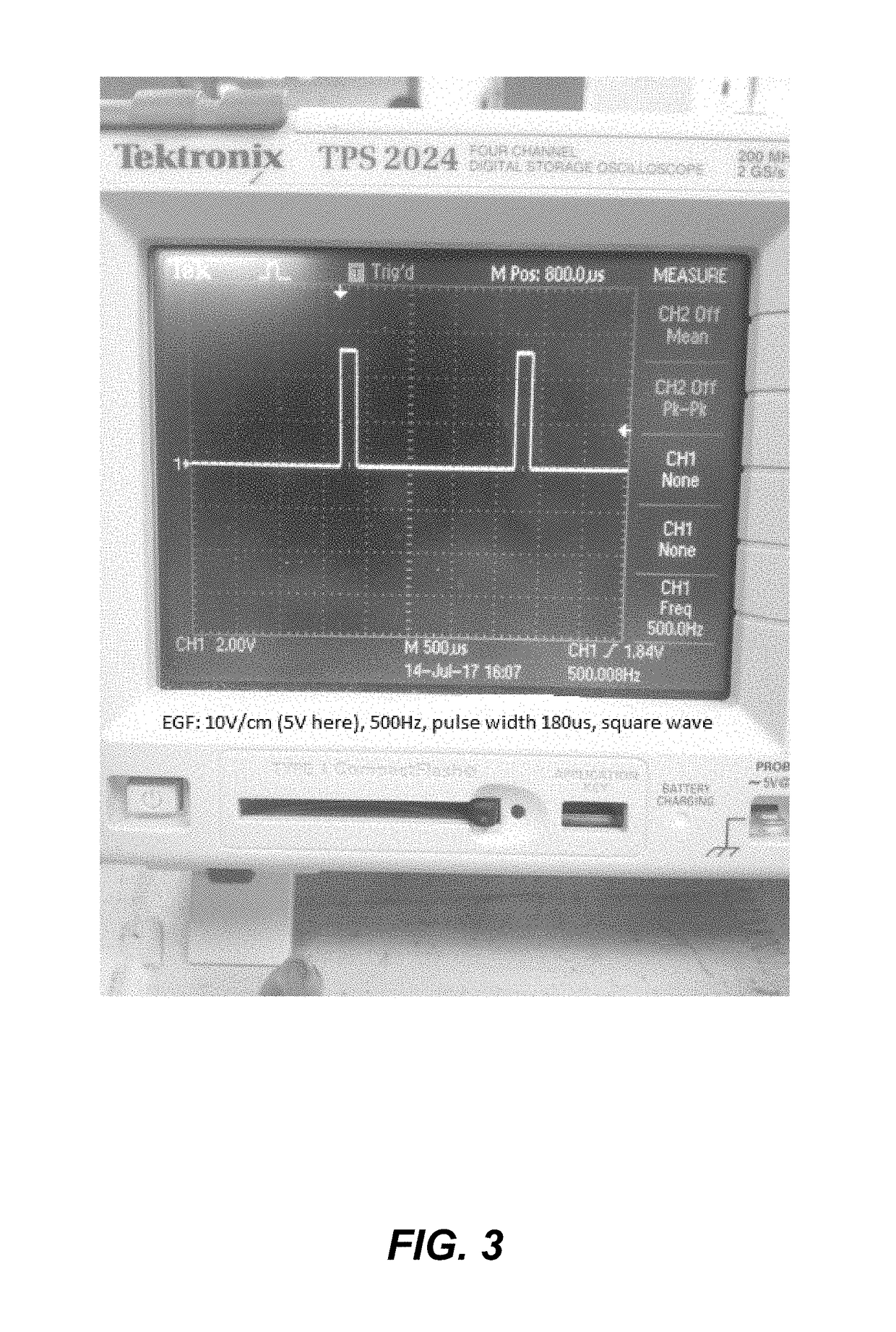
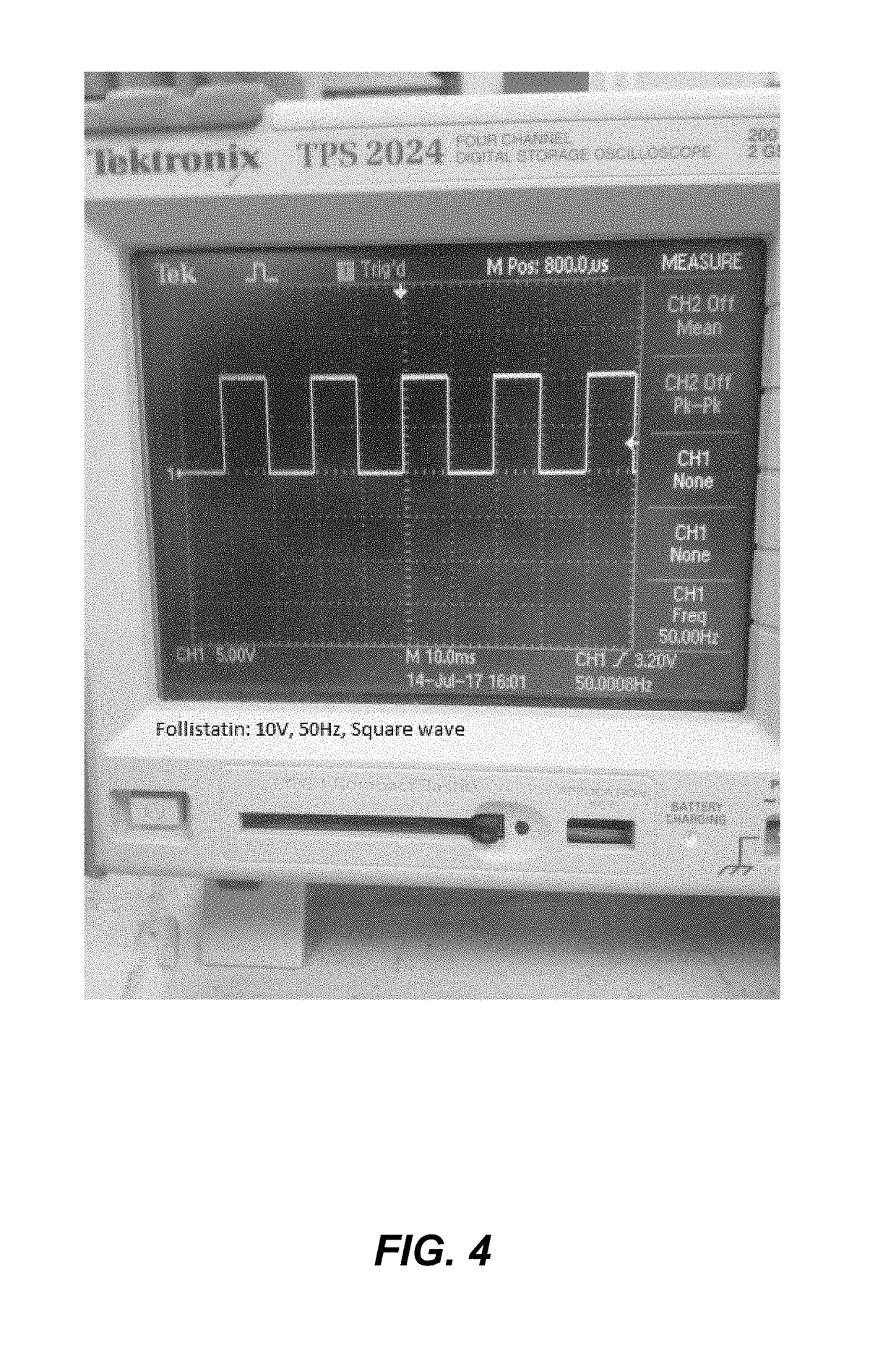
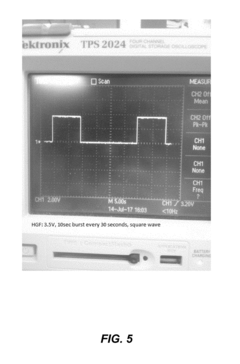
The Impact of Bioresonance on Blood Pressure Regulation
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioresonance and BP Regulation: Background and Objectives
Bioresonance therapy, a controversial alternative medical practice, has gained attention in recent years for its potential impact on various physiological processes, including blood pressure regulation. This emerging field of study aims to explore the intricate relationship between electromagnetic frequencies and the human body's regulatory mechanisms, particularly focusing on cardiovascular health.
The concept of bioresonance originated in the 1970s, based on the premise that all cells and organs in the human body emit unique electromagnetic frequencies. Proponents of this theory suggest that these frequencies can be detected, analyzed, and manipulated to restore balance and promote healing. As research in this area has progressed, scientists and medical professionals have begun to investigate the potential applications of bioresonance in managing chronic conditions, including hypertension.
The primary objective of studying the impact of bioresonance on blood pressure regulation is to determine whether this non-invasive approach can offer a viable alternative or complementary treatment for individuals struggling with hypertension. This research aims to uncover the underlying mechanisms by which electromagnetic frequencies might influence the complex systems involved in blood pressure control, such as the autonomic nervous system, endothelial function, and hormonal regulation.
As the global burden of hypertension continues to rise, with an estimated 1.28 billion adults affected worldwide, the need for innovative and effective management strategies becomes increasingly urgent. Traditional pharmacological interventions, while effective for many patients, often come with side effects and may not be suitable for all individuals. This has led researchers to explore alternative approaches, including bioresonance, in the hope of developing more personalized and holistic treatment options.
The technological evolution in the field of bioresonance has been marked by significant advancements in detection and analysis equipment. Modern devices can now measure and interpret a wide range of electromagnetic frequencies with unprecedented accuracy, allowing for more precise interventions and data collection. This progress has paved the way for more rigorous scientific investigations into the efficacy and mechanisms of bioresonance therapy.
As research in this field continues to evolve, scientists are working to establish standardized protocols for bioresonance therapy in blood pressure management. This includes determining optimal frequency ranges, treatment durations, and application methods. Additionally, efforts are being made to integrate bioresonance data with other physiological measurements to create a more comprehensive understanding of its effects on cardiovascular health.
The concept of bioresonance originated in the 1970s, based on the premise that all cells and organs in the human body emit unique electromagnetic frequencies. Proponents of this theory suggest that these frequencies can be detected, analyzed, and manipulated to restore balance and promote healing. As research in this area has progressed, scientists and medical professionals have begun to investigate the potential applications of bioresonance in managing chronic conditions, including hypertension.
The primary objective of studying the impact of bioresonance on blood pressure regulation is to determine whether this non-invasive approach can offer a viable alternative or complementary treatment for individuals struggling with hypertension. This research aims to uncover the underlying mechanisms by which electromagnetic frequencies might influence the complex systems involved in blood pressure control, such as the autonomic nervous system, endothelial function, and hormonal regulation.
As the global burden of hypertension continues to rise, with an estimated 1.28 billion adults affected worldwide, the need for innovative and effective management strategies becomes increasingly urgent. Traditional pharmacological interventions, while effective for many patients, often come with side effects and may not be suitable for all individuals. This has led researchers to explore alternative approaches, including bioresonance, in the hope of developing more personalized and holistic treatment options.
The technological evolution in the field of bioresonance has been marked by significant advancements in detection and analysis equipment. Modern devices can now measure and interpret a wide range of electromagnetic frequencies with unprecedented accuracy, allowing for more precise interventions and data collection. This progress has paved the way for more rigorous scientific investigations into the efficacy and mechanisms of bioresonance therapy.
As research in this field continues to evolve, scientists are working to establish standardized protocols for bioresonance therapy in blood pressure management. This includes determining optimal frequency ranges, treatment durations, and application methods. Additionally, efforts are being made to integrate bioresonance data with other physiological measurements to create a more comprehensive understanding of its effects on cardiovascular health.
Market Analysis for Bioresonance BP Management
The bioresonance market for blood pressure management is experiencing significant growth, driven by increasing awareness of alternative therapies and a rising prevalence of hypertension worldwide. The global market for bioresonance devices and therapies is projected to expand at a compound annual growth rate (CAGR) of 5.8% from 2021 to 2026, with the blood pressure management segment showing particularly strong potential.
Consumer demand for non-invasive and drug-free approaches to managing hypertension is a key factor fueling market growth. As more individuals seek holistic health solutions, bioresonance therapy is gaining traction as a complementary treatment option. The market is also benefiting from an aging population, as older adults are more likely to experience hypertension and are often more open to alternative therapies.
Geographically, Europe currently leads the bioresonance market, with Germany and Switzerland being major hubs for research and development. However, North America and Asia-Pacific regions are expected to show the fastest growth in the coming years. The United States, in particular, is seeing increased adoption of bioresonance technologies in integrative medicine practices.
The market landscape is characterized by a mix of established medical device manufacturers and specialized bioresonance equipment providers. Key players include Deta Elis Holding, Regumed GmbH, and Sensitiv Imago, among others. These companies are investing heavily in research and development to improve the efficacy and user-friendliness of their devices.
One of the primary challenges facing the market is the lack of standardized protocols and regulations for bioresonance therapy. This has led to skepticism among some healthcare professionals and regulatory bodies. However, ongoing clinical trials and research studies are gradually building a stronger evidence base for the effectiveness of bioresonance in blood pressure management.
The COVID-19 pandemic has had a mixed impact on the market. While it initially disrupted supply chains and reduced access to therapy centers, it has also accelerated the development of home-use bioresonance devices. This shift towards home-based solutions is expected to create new opportunities for market growth in the post-pandemic era.
Looking ahead, the integration of artificial intelligence and machine learning into bioresonance devices is anticipated to be a major trend. These technologies could enhance the precision of frequency detection and personalization of treatment protocols. Additionally, the growing interest in wearable health technologies presents an opportunity for the development of more compact and user-friendly bioresonance devices for continuous blood pressure monitoring and management.
Consumer demand for non-invasive and drug-free approaches to managing hypertension is a key factor fueling market growth. As more individuals seek holistic health solutions, bioresonance therapy is gaining traction as a complementary treatment option. The market is also benefiting from an aging population, as older adults are more likely to experience hypertension and are often more open to alternative therapies.
Geographically, Europe currently leads the bioresonance market, with Germany and Switzerland being major hubs for research and development. However, North America and Asia-Pacific regions are expected to show the fastest growth in the coming years. The United States, in particular, is seeing increased adoption of bioresonance technologies in integrative medicine practices.
The market landscape is characterized by a mix of established medical device manufacturers and specialized bioresonance equipment providers. Key players include Deta Elis Holding, Regumed GmbH, and Sensitiv Imago, among others. These companies are investing heavily in research and development to improve the efficacy and user-friendliness of their devices.
One of the primary challenges facing the market is the lack of standardized protocols and regulations for bioresonance therapy. This has led to skepticism among some healthcare professionals and regulatory bodies. However, ongoing clinical trials and research studies are gradually building a stronger evidence base for the effectiveness of bioresonance in blood pressure management.
The COVID-19 pandemic has had a mixed impact on the market. While it initially disrupted supply chains and reduced access to therapy centers, it has also accelerated the development of home-use bioresonance devices. This shift towards home-based solutions is expected to create new opportunities for market growth in the post-pandemic era.
Looking ahead, the integration of artificial intelligence and machine learning into bioresonance devices is anticipated to be a major trend. These technologies could enhance the precision of frequency detection and personalization of treatment protocols. Additionally, the growing interest in wearable health technologies presents an opportunity for the development of more compact and user-friendly bioresonance devices for continuous blood pressure monitoring and management.
Current Challenges in Bioresonance BP Technology
Bioresonance technology for blood pressure regulation faces several significant challenges that hinder its widespread adoption and clinical efficacy. One of the primary obstacles is the lack of standardization in bioresonance devices and protocols. Different manufacturers employ varying frequencies and treatment methodologies, making it difficult to establish consistent and reproducible results across studies and clinical applications.
The scientific community remains skeptical about the underlying mechanisms of bioresonance therapy, particularly in relation to blood pressure regulation. There is a notable absence of robust, peer-reviewed research that conclusively demonstrates the efficacy of bioresonance in managing hypertension. This lack of evidence-based support creates a barrier to acceptance within mainstream medical practice and limits funding for further research.
Another challenge lies in the complexity of blood pressure regulation itself. The human body's blood pressure control system involves multiple physiological processes, including the renin-angiotensin-aldosterone system, autonomic nervous system, and various hormonal factors. Bioresonance technology must address this complexity to effectively modulate blood pressure, which requires a sophisticated understanding of these interrelated systems.
The placebo effect presents a significant confounding factor in assessing the true impact of bioresonance on blood pressure. Many studies struggle to differentiate between the physiological effects of bioresonance and the psychological impact of undergoing treatment. Designing double-blind, placebo-controlled studies for bioresonance therapy is challenging due to the nature of the treatment, which often involves visible and tactile elements.
Regulatory hurdles pose another substantial challenge. Many health authorities and regulatory bodies have not recognized bioresonance as a validated medical treatment for blood pressure management. This lack of official recognition limits the technology's integration into standard healthcare protocols and restricts its availability to patients through conventional medical channels.
The long-term effects and safety profile of bioresonance therapy for blood pressure regulation remain unclear. While short-term studies have shown promising results, there is a dearth of longitudinal research examining the sustained impact and potential side effects of prolonged bioresonance treatment on cardiovascular health.
Lastly, the cost and accessibility of bioresonance technology present practical challenges. Many bioresonance devices are expensive, and treatments are often not covered by health insurance plans. This financial barrier limits the technology's reach to a broader patient population and hinders large-scale clinical trials necessary for validating its effectiveness in blood pressure management.
The scientific community remains skeptical about the underlying mechanisms of bioresonance therapy, particularly in relation to blood pressure regulation. There is a notable absence of robust, peer-reviewed research that conclusively demonstrates the efficacy of bioresonance in managing hypertension. This lack of evidence-based support creates a barrier to acceptance within mainstream medical practice and limits funding for further research.
Another challenge lies in the complexity of blood pressure regulation itself. The human body's blood pressure control system involves multiple physiological processes, including the renin-angiotensin-aldosterone system, autonomic nervous system, and various hormonal factors. Bioresonance technology must address this complexity to effectively modulate blood pressure, which requires a sophisticated understanding of these interrelated systems.
The placebo effect presents a significant confounding factor in assessing the true impact of bioresonance on blood pressure. Many studies struggle to differentiate between the physiological effects of bioresonance and the psychological impact of undergoing treatment. Designing double-blind, placebo-controlled studies for bioresonance therapy is challenging due to the nature of the treatment, which often involves visible and tactile elements.
Regulatory hurdles pose another substantial challenge. Many health authorities and regulatory bodies have not recognized bioresonance as a validated medical treatment for blood pressure management. This lack of official recognition limits the technology's integration into standard healthcare protocols and restricts its availability to patients through conventional medical channels.
The long-term effects and safety profile of bioresonance therapy for blood pressure regulation remain unclear. While short-term studies have shown promising results, there is a dearth of longitudinal research examining the sustained impact and potential side effects of prolonged bioresonance treatment on cardiovascular health.
Lastly, the cost and accessibility of bioresonance technology present practical challenges. Many bioresonance devices are expensive, and treatments are often not covered by health insurance plans. This financial barrier limits the technology's reach to a broader patient population and hinders large-scale clinical trials necessary for validating its effectiveness in blood pressure management.
Existing Bioresonance Solutions for BP Control
01 Non-invasive blood pressure measurement using bioresonance
Bioresonance technology is utilized for non-invasive blood pressure measurement. This method involves detecting and analyzing electromagnetic signals from the body to determine blood pressure without the need for traditional cuff-based measurements. The technique may offer continuous monitoring capabilities and improved patient comfort.- Non-invasive blood pressure measurement using bioresonance: Bioresonance technology is utilized for non-invasive blood pressure measurement. This method involves detecting and analyzing electromagnetic signals from the body to determine blood pressure without the need for traditional cuff-based measurements. The technique may offer continuous monitoring capabilities and improved patient comfort.
- Wearable devices for bioresonance blood pressure monitoring: Wearable devices are developed to incorporate bioresonance technology for continuous blood pressure monitoring. These devices may be in the form of smartwatches, armbands, or other wearable formats, allowing for real-time tracking of blood pressure throughout daily activities without the need for manual measurements.
- Integration of bioresonance blood pressure monitoring with other health parameters: Systems are designed to combine bioresonance blood pressure monitoring with other health parameters such as heart rate, oxygen saturation, and activity levels. This integrated approach provides a more comprehensive view of cardiovascular health and enables better management of hypertension and related conditions.
- Data analysis and AI algorithms for bioresonance blood pressure interpretation: Advanced data analysis techniques and artificial intelligence algorithms are employed to interpret bioresonance signals related to blood pressure. These methods aim to improve the accuracy of blood pressure estimations and provide insights into blood pressure trends and patterns over time.
- Calibration and validation methods for bioresonance blood pressure devices: Techniques are developed for calibrating and validating bioresonance-based blood pressure measurement devices. These methods ensure the accuracy and reliability of the devices by comparing their results with traditional blood pressure measurement techniques and establishing standardized protocols for device testing and certification.
02 Wearable devices for bioresonance blood pressure monitoring
Wearable devices are developed to incorporate bioresonance technology for continuous blood pressure monitoring. These devices may be in the form of smartwatches, armbands, or other wearable formats, allowing for real-time tracking of blood pressure fluctuations throughout daily activities.Expand Specific Solutions03 Integration of bioresonance blood pressure monitoring with other health parameters
Systems are designed to combine bioresonance blood pressure monitoring with other health parameters such as heart rate, oxygen saturation, and activity levels. This integrated approach provides a more comprehensive view of cardiovascular health and enables better management of hypertension and related conditions.Expand Specific Solutions04 Artificial intelligence and machine learning in bioresonance blood pressure analysis
Advanced algorithms utilizing artificial intelligence and machine learning are developed to enhance the accuracy and reliability of bioresonance blood pressure measurements. These technologies help in processing complex bioresonance signals and adapting to individual physiological variations.Expand Specific Solutions05 Calibration and validation methods for bioresonance blood pressure devices
Techniques are established for calibrating and validating bioresonance blood pressure measurement devices. These methods ensure the accuracy and reliability of the devices by comparing their results with traditional blood pressure measurement techniques and accounting for various physiological factors that may influence readings.Expand Specific Solutions
Key Players in Bioresonance Medical Devices
The bioresonance technology's impact on blood pressure regulation is in an early developmental stage, with a growing market potential due to increasing hypertension prevalence. The technology's maturity varies among key players, with established medical device companies like Medtronic and Edwards Lifesciences leading in related cardiovascular innovations. Emerging firms such as CVRx and Sympara Medical are specifically focusing on bioresonance applications for blood pressure control. Research institutions like Penn State and AIT Austrian Institute of Technology are contributing to the scientific foundation, while companies like OMRON Healthcare and Murata Manufacturing are exploring consumer-oriented applications. The competitive landscape is diverse, spanning from large corporations to specialized startups and academic research centers.
CVRx, Inc.
Technical Solution: CVRx has developed the Barostim neo system, an implantable device that uses bioresonance principles to regulate blood pressure through neuromodulation of the carotid baroreceptors[1]. The system consists of a small pulse generator implanted under the collarbone and a lead attached to the carotid sinus. By delivering electrical pulses to the baroreceptors, it activates the body's natural blood pressure regulation mechanisms[2]. The Barostim neo system utilizes a proprietary algorithm that adapts the stimulation parameters based on the patient's physiological responses, ensuring optimal therapy delivery[3]. Clinical trials have demonstrated significant blood pressure reductions, with patients experiencing an average decrease in systolic blood pressure of 20 mmHg after 6 months of treatment[4]. The system also incorporates remote monitoring capabilities, allowing healthcare providers to adjust therapy settings and track patient progress without the need for frequent in-person visits[5].
Strengths: Targeted approach to blood pressure regulation, potential for long-term blood pressure management, and adaptability to individual patient needs. Weaknesses: Invasive implantation procedure, potential for device-related complications, and limited suitability for patients with certain anatomical variations of the carotid sinus.
Medtronic, Inc.
Technical Solution: Medtronic has developed a bioresonance-based approach to blood pressure regulation using their proprietary Barostim neo system. This technology utilizes electrical stimulation of the carotid sinus to activate the body's natural blood pressure regulation mechanisms[1]. The system consists of a small pulse generator implanted below the collarbone and a lead that attaches to the carotid artery. By delivering carefully calibrated electrical pulses, it modulates baroreceptor activity, which in turn influences the autonomic nervous system to lower blood pressure[2]. Clinical trials have shown significant reductions in systolic blood pressure, with an average decrease of 16.2 mmHg after 6 months of therapy[3].
Strengths: Targeted approach to blood pressure regulation, minimally invasive procedure, and potential for long-term blood pressure management. Weaknesses: Requires surgical implantation, potential for device-related complications, and may not be suitable for all patients with hypertension.
Core Innovations in Bioresonance BP Therapy
Bioelectric blood pressure management
PatentActiveUS20190022396A1
Innovation
- A bioelectric stimulation system that uses low-power signals to target the vagal nerve, promoting the release of proteins like tropoelastin, SDF-1, VEGF, and others to improve arterial elasticity and balance electrical potentials, thereby reducing blood pressure and preventing artery narrowing and spasms.
System and method for mapping baroreceptors
PatentActiveUS20160136432A1
Innovation
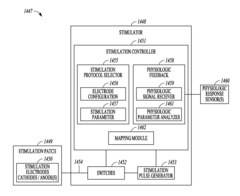
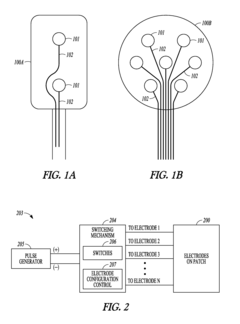
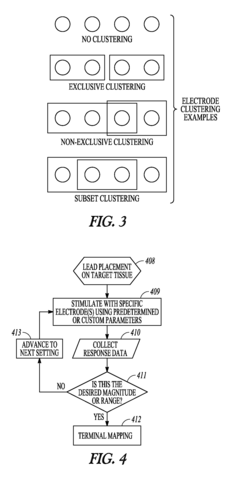
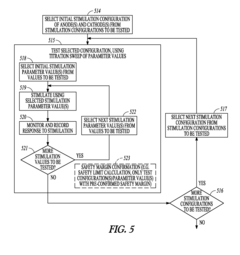
- A system and method using a set of stimulation electrodes with bipolar configurations, including anode and cathode clusters, to systematically map baroreceptor regions by stimulating tissue and monitoring physiological responses, allowing for precise identification of baroreceptor hotspots and reducing unnecessary stimulation of muscle or nerve tissue.
Regulatory Framework for Bioresonance Medical Devices
The regulatory framework for bioresonance medical devices is a complex and evolving landscape, reflecting the growing interest in alternative therapies and their potential impact on health outcomes, such as blood pressure regulation. As bioresonance technology gains traction in the medical field, regulatory bodies worldwide are grappling with the challenge of establishing appropriate guidelines and standards for these devices.
In the United States, the Food and Drug Administration (FDA) classifies bioresonance devices as Class II medical devices, subject to general controls and special controls. This classification requires manufacturers to submit a 510(k) premarket notification, demonstrating substantial equivalence to a legally marketed predicate device. The FDA's approach emphasizes the importance of safety and efficacy data, particularly in relation to claims about blood pressure regulation.
The European Union, through its Medical Device Regulation (MDR), has implemented a more stringent regulatory framework for all medical devices, including bioresonance equipment. Under the MDR, manufacturers must provide clinical evidence supporting the intended use of their devices, which includes any claims related to blood pressure management. The CE marking process now requires a more thorough assessment of clinical data and post-market surveillance plans.
In countries like Germany and Switzerland, where bioresonance therapy has gained significant popularity, regulatory bodies have adopted a more permissive stance. These nations allow the use of bioresonance devices for diagnostic and therapeutic purposes, provided they meet basic safety standards. However, manufacturers are still required to provide evidence supporting any specific health claims, including those related to blood pressure regulation.
The regulatory landscape in Asia varies considerably. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for bioresonance devices, requiring manufacturers to demonstrate both safety and a certain level of efficacy. In contrast, China's National Medical Products Administration (NMPA) has yet to develop a comprehensive regulatory framework for these devices, leading to a more fragmented approach to their approval and use.
International standards organizations, such as the International Organization for Standardization (ISO), are working to develop global standards for bioresonance devices. These efforts aim to harmonize regulatory requirements across different regions and ensure consistent quality and safety standards. The ISO 13485 standard for medical devices quality management systems is particularly relevant for manufacturers of bioresonance equipment.
As research into the impact of bioresonance on blood pressure regulation continues to evolve, regulatory frameworks are likely to adapt. Regulatory bodies are increasingly focusing on real-world evidence and post-market surveillance data to inform their decision-making processes. This approach allows for a more dynamic regulatory environment that can respond to emerging scientific evidence and clinical outcomes.
In the United States, the Food and Drug Administration (FDA) classifies bioresonance devices as Class II medical devices, subject to general controls and special controls. This classification requires manufacturers to submit a 510(k) premarket notification, demonstrating substantial equivalence to a legally marketed predicate device. The FDA's approach emphasizes the importance of safety and efficacy data, particularly in relation to claims about blood pressure regulation.
The European Union, through its Medical Device Regulation (MDR), has implemented a more stringent regulatory framework for all medical devices, including bioresonance equipment. Under the MDR, manufacturers must provide clinical evidence supporting the intended use of their devices, which includes any claims related to blood pressure management. The CE marking process now requires a more thorough assessment of clinical data and post-market surveillance plans.
In countries like Germany and Switzerland, where bioresonance therapy has gained significant popularity, regulatory bodies have adopted a more permissive stance. These nations allow the use of bioresonance devices for diagnostic and therapeutic purposes, provided they meet basic safety standards. However, manufacturers are still required to provide evidence supporting any specific health claims, including those related to blood pressure regulation.
The regulatory landscape in Asia varies considerably. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for bioresonance devices, requiring manufacturers to demonstrate both safety and a certain level of efficacy. In contrast, China's National Medical Products Administration (NMPA) has yet to develop a comprehensive regulatory framework for these devices, leading to a more fragmented approach to their approval and use.
International standards organizations, such as the International Organization for Standardization (ISO), are working to develop global standards for bioresonance devices. These efforts aim to harmonize regulatory requirements across different regions and ensure consistent quality and safety standards. The ISO 13485 standard for medical devices quality management systems is particularly relevant for manufacturers of bioresonance equipment.
As research into the impact of bioresonance on blood pressure regulation continues to evolve, regulatory frameworks are likely to adapt. Regulatory bodies are increasingly focusing on real-world evidence and post-market surveillance data to inform their decision-making processes. This approach allows for a more dynamic regulatory environment that can respond to emerging scientific evidence and clinical outcomes.
Safety and Efficacy Considerations in Bioresonance Therapy
Safety and efficacy are paramount considerations in the application of bioresonance therapy for blood pressure regulation. As this alternative therapeutic approach gains attention, it is crucial to evaluate its potential risks and benefits thoroughly.
The safety profile of bioresonance therapy is generally considered favorable, with minimal reported adverse effects. The non-invasive nature of the treatment, which typically involves placing electrodes on the skin or using handheld devices, contributes to its perceived safety. However, it is essential to note that the long-term effects of repeated exposure to electromagnetic fields used in bioresonance therapy have not been extensively studied.
One potential safety concern is the risk of electromagnetic interference with implanted medical devices such as pacemakers or insulin pumps. Patients with such devices should consult their healthcare providers before undergoing bioresonance therapy. Additionally, pregnant women and individuals with certain medical conditions may need to exercise caution or avoid the treatment altogether.
Regarding efficacy, the scientific evidence supporting bioresonance therapy for blood pressure regulation remains limited and controversial. While some studies have reported positive outcomes, including reductions in blood pressure and improvements in overall cardiovascular health, many of these studies lack rigorous methodological design or have small sample sizes.
Critics argue that the proposed mechanisms of action for bioresonance therapy in blood pressure regulation are not well-established within the framework of conventional medical understanding. The concept of manipulating the body's electromagnetic fields to influence physiological processes is still considered speculative by many in the scientific community.
Despite these challenges, proponents of bioresonance therapy point to anecdotal evidence and patient testimonials as indicators of its potential efficacy. Some practitioners report success in using bioresonance as a complementary approach alongside traditional blood pressure management strategies.
To establish the true efficacy of bioresonance therapy for blood pressure regulation, larger, well-designed clinical trials are necessary. These studies should include appropriate control groups, standardized treatment protocols, and objective outcome measures. Additionally, research into the underlying mechanisms of action could help elucidate how bioresonance might influence cardiovascular function.
In conclusion, while bioresonance therapy appears to have a favorable safety profile, its efficacy in blood pressure regulation remains a subject of debate. As with any alternative therapy, patients considering bioresonance for blood pressure management should approach it with caution and in consultation with their healthcare providers. Integrating bioresonance therapy into a comprehensive treatment plan that includes evidence-based approaches to blood pressure control may be a prudent strategy until more definitive research is available.
The safety profile of bioresonance therapy is generally considered favorable, with minimal reported adverse effects. The non-invasive nature of the treatment, which typically involves placing electrodes on the skin or using handheld devices, contributes to its perceived safety. However, it is essential to note that the long-term effects of repeated exposure to electromagnetic fields used in bioresonance therapy have not been extensively studied.
One potential safety concern is the risk of electromagnetic interference with implanted medical devices such as pacemakers or insulin pumps. Patients with such devices should consult their healthcare providers before undergoing bioresonance therapy. Additionally, pregnant women and individuals with certain medical conditions may need to exercise caution or avoid the treatment altogether.
Regarding efficacy, the scientific evidence supporting bioresonance therapy for blood pressure regulation remains limited and controversial. While some studies have reported positive outcomes, including reductions in blood pressure and improvements in overall cardiovascular health, many of these studies lack rigorous methodological design or have small sample sizes.
Critics argue that the proposed mechanisms of action for bioresonance therapy in blood pressure regulation are not well-established within the framework of conventional medical understanding. The concept of manipulating the body's electromagnetic fields to influence physiological processes is still considered speculative by many in the scientific community.
Despite these challenges, proponents of bioresonance therapy point to anecdotal evidence and patient testimonials as indicators of its potential efficacy. Some practitioners report success in using bioresonance as a complementary approach alongside traditional blood pressure management strategies.
To establish the true efficacy of bioresonance therapy for blood pressure regulation, larger, well-designed clinical trials are necessary. These studies should include appropriate control groups, standardized treatment protocols, and objective outcome measures. Additionally, research into the underlying mechanisms of action could help elucidate how bioresonance might influence cardiovascular function.
In conclusion, while bioresonance therapy appears to have a favorable safety profile, its efficacy in blood pressure regulation remains a subject of debate. As with any alternative therapy, patients considering bioresonance for blood pressure management should approach it with caution and in consultation with their healthcare providers. Integrating bioresonance therapy into a comprehensive treatment plan that includes evidence-based approaches to blood pressure control may be a prudent strategy until more definitive research is available.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!