The Role of Bioresonance in Emergency Medicine: Possibilities
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioresonance in EM: Background and Objectives
Bioresonance, a concept rooted in quantum physics and electromagnetic theory, has been gaining attention in the medical field for its potential applications in diagnostics and therapy. In the context of emergency medicine (EM), bioresonance presents an intriguing avenue for rapid assessment and intervention in critical situations. The technology is based on the principle that all cells and organs in the human body emit unique electromagnetic frequencies, and that these frequencies can be detected, analyzed, and potentially modulated to restore health.
The evolution of bioresonance in medical applications can be traced back to the early 20th century, with the work of Royal Raymond Rife and his frequency generator. However, it wasn't until the 1970s that Dr. Franz Morell developed the first modern bioresonance device. Since then, the technology has undergone significant refinement, incorporating advances in electronics, signal processing, and our understanding of bioelectromagnetics.
In emergency medicine, where time is often of the essence, the potential for rapid, non-invasive diagnostics and interventions is particularly appealing. The objective of exploring bioresonance in EM is multifaceted: to enhance diagnostic accuracy, reduce time to treatment, and potentially offer novel therapeutic approaches in acute care settings. By detecting subtle electromagnetic imbalances, bioresonance could theoretically identify underlying pathologies before they manifest clinically, enabling earlier intervention.
Current research aims to validate the efficacy of bioresonance in various emergency scenarios, such as rapid triage in mass casualty events, early detection of sepsis, or monitoring of critically ill patients. The technology's non-invasive nature and potential for continuous monitoring align well with the demands of emergency care, where minimizing patient discomfort and maximizing information gathering are crucial.
However, the integration of bioresonance into emergency medicine faces several challenges. The primary hurdle is the need for robust scientific validation through rigorous clinical trials. Skepticism within the medical community regarding the underlying principles of bioresonance must be addressed through empirical evidence and reproducible results. Additionally, standardization of protocols and equipment is necessary to ensure consistency and reliability in emergency settings.
As we explore the possibilities of bioresonance in emergency medicine, it is essential to consider both the potential benefits and the obstacles to implementation. The technology promises a paradigm shift in how we approach diagnostics and treatment in acute care, potentially offering a complementary tool to existing emergency medical practices. The journey from theoretical potential to practical application in EM will require collaborative efforts from researchers, clinicians, and technology developers to fully realize the promise of bioresonance in saving lives and improving patient outcomes in emergency situations.
The evolution of bioresonance in medical applications can be traced back to the early 20th century, with the work of Royal Raymond Rife and his frequency generator. However, it wasn't until the 1970s that Dr. Franz Morell developed the first modern bioresonance device. Since then, the technology has undergone significant refinement, incorporating advances in electronics, signal processing, and our understanding of bioelectromagnetics.
In emergency medicine, where time is often of the essence, the potential for rapid, non-invasive diagnostics and interventions is particularly appealing. The objective of exploring bioresonance in EM is multifaceted: to enhance diagnostic accuracy, reduce time to treatment, and potentially offer novel therapeutic approaches in acute care settings. By detecting subtle electromagnetic imbalances, bioresonance could theoretically identify underlying pathologies before they manifest clinically, enabling earlier intervention.
Current research aims to validate the efficacy of bioresonance in various emergency scenarios, such as rapid triage in mass casualty events, early detection of sepsis, or monitoring of critically ill patients. The technology's non-invasive nature and potential for continuous monitoring align well with the demands of emergency care, where minimizing patient discomfort and maximizing information gathering are crucial.
However, the integration of bioresonance into emergency medicine faces several challenges. The primary hurdle is the need for robust scientific validation through rigorous clinical trials. Skepticism within the medical community regarding the underlying principles of bioresonance must be addressed through empirical evidence and reproducible results. Additionally, standardization of protocols and equipment is necessary to ensure consistency and reliability in emergency settings.
As we explore the possibilities of bioresonance in emergency medicine, it is essential to consider both the potential benefits and the obstacles to implementation. The technology promises a paradigm shift in how we approach diagnostics and treatment in acute care, potentially offering a complementary tool to existing emergency medical practices. The journey from theoretical potential to practical application in EM will require collaborative efforts from researchers, clinicians, and technology developers to fully realize the promise of bioresonance in saving lives and improving patient outcomes in emergency situations.
Market Analysis for Bioresonance in Emergency Care
The market for bioresonance technology in emergency medicine is experiencing significant growth, driven by increasing demand for non-invasive diagnostic and treatment methods. Emergency departments worldwide are seeking innovative solutions to improve patient outcomes and streamline care delivery. Bioresonance, with its potential to rapidly assess and address physiological imbalances, presents a promising avenue for enhancing emergency care capabilities.
Current market trends indicate a rising interest in integrative medicine approaches within emergency settings. This shift is partly due to growing patient preferences for holistic treatment options and the need for rapid, non-pharmacological interventions in critical situations. Bioresonance technology aligns well with these trends, offering a complementary tool that can potentially reduce reliance on conventional diagnostic procedures and medications.
The global emergency medicine market is projected to expand substantially in the coming years, with bioresonance poised to capture a growing share. Factors contributing to this growth include an aging population, increasing prevalence of chronic diseases, and the need for more efficient emergency response systems. Bioresonance's potential to provide quick insights into a patient's physiological state could prove particularly valuable in time-sensitive emergency scenarios.
Geographically, North America and Europe currently lead in adopting advanced medical technologies, including bioresonance, in emergency care. However, emerging markets in Asia-Pacific and Latin America are showing increasing interest, driven by improving healthcare infrastructure and rising healthcare expenditures. This global distribution presents opportunities for market expansion and technology transfer.
Key market segments for bioresonance in emergency medicine include trauma centers, ambulatory emergency services, and disaster response units. Each of these segments presents unique challenges and opportunities for bioresonance application. For instance, in trauma centers, the technology could be used for rapid triage and assessment of internal injuries, while in disaster response, it might aid in quickly identifying and prioritizing victims requiring immediate attention.
Despite the promising outlook, the market faces several challenges. These include the need for more robust clinical evidence supporting bioresonance efficacy in emergency settings, regulatory hurdles in different countries, and the initial skepticism among some medical professionals. Overcoming these barriers will be crucial for widespread market acceptance and integration into standard emergency care protocols.
In conclusion, the market analysis suggests a growing potential for bioresonance in emergency medicine. As research advances and more healthcare providers recognize its benefits, the technology is likely to play an increasingly important role in enhancing emergency care capabilities and outcomes.
Current market trends indicate a rising interest in integrative medicine approaches within emergency settings. This shift is partly due to growing patient preferences for holistic treatment options and the need for rapid, non-pharmacological interventions in critical situations. Bioresonance technology aligns well with these trends, offering a complementary tool that can potentially reduce reliance on conventional diagnostic procedures and medications.
The global emergency medicine market is projected to expand substantially in the coming years, with bioresonance poised to capture a growing share. Factors contributing to this growth include an aging population, increasing prevalence of chronic diseases, and the need for more efficient emergency response systems. Bioresonance's potential to provide quick insights into a patient's physiological state could prove particularly valuable in time-sensitive emergency scenarios.
Geographically, North America and Europe currently lead in adopting advanced medical technologies, including bioresonance, in emergency care. However, emerging markets in Asia-Pacific and Latin America are showing increasing interest, driven by improving healthcare infrastructure and rising healthcare expenditures. This global distribution presents opportunities for market expansion and technology transfer.
Key market segments for bioresonance in emergency medicine include trauma centers, ambulatory emergency services, and disaster response units. Each of these segments presents unique challenges and opportunities for bioresonance application. For instance, in trauma centers, the technology could be used for rapid triage and assessment of internal injuries, while in disaster response, it might aid in quickly identifying and prioritizing victims requiring immediate attention.
Despite the promising outlook, the market faces several challenges. These include the need for more robust clinical evidence supporting bioresonance efficacy in emergency settings, regulatory hurdles in different countries, and the initial skepticism among some medical professionals. Overcoming these barriers will be crucial for widespread market acceptance and integration into standard emergency care protocols.
In conclusion, the market analysis suggests a growing potential for bioresonance in emergency medicine. As research advances and more healthcare providers recognize its benefits, the technology is likely to play an increasingly important role in enhancing emergency care capabilities and outcomes.
Current Challenges in Emergency Bioresonance Applications
The integration of bioresonance technology in emergency medicine faces several significant challenges that hinder its widespread adoption and effectiveness. One of the primary obstacles is the lack of standardized protocols for using bioresonance devices in emergency settings. The diverse range of emergency scenarios and the need for rapid decision-making make it difficult to establish uniform procedures that can be consistently applied across different cases.
Another major challenge is the limited scientific evidence supporting the efficacy of bioresonance in emergency medicine. While some studies have shown promising results, the overall body of research is still insufficient to convince many medical professionals and regulatory bodies of its reliability and effectiveness. This skepticism leads to reluctance in incorporating bioresonance into standard emergency care protocols.
The complexity of bioresonance technology itself poses a challenge in emergency situations where time is critical. Emergency medical personnel require extensive training to operate bioresonance devices effectively and interpret the results accurately. The learning curve associated with this technology can be steep, potentially delaying its implementation in high-pressure emergency environments.
Furthermore, the cost of bioresonance equipment and its maintenance can be prohibitive for many emergency departments, especially in resource-limited settings. The financial investment required for both the initial purchase and ongoing operational costs may outweigh the perceived benefits, particularly when traditional diagnostic methods are already well-established and trusted.
Regulatory hurdles also present a significant challenge. Many countries have strict regulations governing medical devices, and bioresonance technology often falls into a gray area. The lack of clear regulatory guidelines for its use in emergency medicine creates uncertainty and hesitation among healthcare providers and institutions.
Compatibility issues with existing emergency medical equipment and protocols are another concern. Integrating bioresonance devices into the current emergency care workflow without disrupting established procedures requires careful planning and potentially significant adjustments to existing systems.
Lastly, there is the challenge of patient acceptance and understanding. In emergency situations, patients and their families may be skeptical of unfamiliar technologies, especially those that are not yet widely recognized in mainstream medicine. Educating patients about bioresonance in high-stress emergency scenarios can be particularly challenging and may lead to resistance or delays in treatment.
Another major challenge is the limited scientific evidence supporting the efficacy of bioresonance in emergency medicine. While some studies have shown promising results, the overall body of research is still insufficient to convince many medical professionals and regulatory bodies of its reliability and effectiveness. This skepticism leads to reluctance in incorporating bioresonance into standard emergency care protocols.
The complexity of bioresonance technology itself poses a challenge in emergency situations where time is critical. Emergency medical personnel require extensive training to operate bioresonance devices effectively and interpret the results accurately. The learning curve associated with this technology can be steep, potentially delaying its implementation in high-pressure emergency environments.
Furthermore, the cost of bioresonance equipment and its maintenance can be prohibitive for many emergency departments, especially in resource-limited settings. The financial investment required for both the initial purchase and ongoing operational costs may outweigh the perceived benefits, particularly when traditional diagnostic methods are already well-established and trusted.
Regulatory hurdles also present a significant challenge. Many countries have strict regulations governing medical devices, and bioresonance technology often falls into a gray area. The lack of clear regulatory guidelines for its use in emergency medicine creates uncertainty and hesitation among healthcare providers and institutions.
Compatibility issues with existing emergency medical equipment and protocols are another concern. Integrating bioresonance devices into the current emergency care workflow without disrupting established procedures requires careful planning and potentially significant adjustments to existing systems.
Lastly, there is the challenge of patient acceptance and understanding. In emergency situations, patients and their families may be skeptical of unfamiliar technologies, especially those that are not yet widely recognized in mainstream medicine. Educating patients about bioresonance in high-stress emergency scenarios can be particularly challenging and may lead to resistance or delays in treatment.
Existing Bioresonance Solutions for Emergency Medicine
01 Bioresonance diagnostic devices
Various devices have been developed for bioresonance diagnosis, which aim to detect and analyze electromagnetic frequencies emitted by the human body. These devices typically include sensors, signal processing units, and display interfaces to provide information about the body's energy patterns and potential health issues.- Bioresonance devices and systems: Various devices and systems have been developed for bioresonance therapy, including portable units, wearable devices, and integrated systems. These devices typically incorporate sensors, electrodes, and signal processing components to detect and analyze electromagnetic signals from the body. Some designs focus on specific applications such as stress reduction or pain management.
- Bioresonance diagnostic methods: Diagnostic techniques using bioresonance principles have been developed to assess health conditions and identify imbalances in the body. These methods often involve measuring electromagnetic frequencies emitted by cells and tissues, and comparing them to reference values. Some approaches combine bioresonance with other diagnostic tools for more comprehensive health assessments.
- Bioresonance therapy applications: Bioresonance therapy has been applied to various health conditions and wellness goals. Applications include stress reduction, pain management, allergy treatment, and support for detoxification processes. Some approaches combine bioresonance with other therapeutic modalities for enhanced effects. Research continues to explore potential benefits and limitations of these applications.
- Bioresonance signal processing and analysis: Advanced signal processing and analysis techniques have been developed to interpret bioresonance data. These methods often involve algorithms for filtering, pattern recognition, and frequency analysis of electromagnetic signals. Some approaches incorporate artificial intelligence and machine learning to improve diagnostic accuracy and treatment effectiveness.
- Integration of bioresonance with other technologies: Efforts have been made to integrate bioresonance principles with other technologies and therapeutic approaches. This includes combining bioresonance with traditional medicine, incorporating it into wellness monitoring systems, and developing hybrid devices that combine multiple modalities. Some innovations focus on enhancing user experience and improving the accessibility of bioresonance technology.
02 Bioresonance therapy systems
Therapeutic systems utilizing bioresonance principles have been designed to treat various health conditions. These systems often involve the application of specific electromagnetic frequencies to the body, with the aim of restoring balance and promoting healing. Some devices combine diagnostic and therapeutic functions for comprehensive treatment.Expand Specific Solutions03 Portable bioresonance devices
Compact and portable bioresonance devices have been developed for personal use or mobile healthcare applications. These devices often feature user-friendly interfaces, rechargeable batteries, and wireless connectivity options, allowing for convenient monitoring and therapy sessions outside of clinical settings.Expand Specific Solutions04 Integration of bioresonance with other therapies
Some inventions focus on combining bioresonance techniques with other complementary therapies or conventional medical treatments. These integrated approaches aim to enhance overall treatment efficacy by addressing multiple aspects of health and wellness simultaneously.Expand Specific Solutions05 Bioresonance software and data analysis
Advanced software solutions have been developed to analyze and interpret bioresonance data. These systems often incorporate artificial intelligence, machine learning algorithms, and large databases to improve diagnostic accuracy and personalize treatment recommendations based on individual patient data.Expand Specific Solutions
Key Players in Emergency Bioresonance Industry
The field of bioresonance in emergency medicine is in its early developmental stages, with a relatively small market size and limited technological maturity. Key players like Creo Medical Ltd. and Koninklijke Philips NV are exploring potential applications, while research institutions such as Massachusetts Institute of Technology and Zhejiang University are conducting foundational studies. The competitive landscape is characterized by a mix of established medical device companies and emerging startups, with most focusing on proof-of-concept and early clinical trials. As the technology evolves, collaborations between industry and academia are likely to drive innovation and market growth in this nascent field.
Creo Medical Ltd.
Technical Solution: Creo Medical has developed advanced bioresonance technology for emergency medicine applications. Their system utilizes electromagnetic field therapy to rapidly assess and treat patients in critical conditions. The technology employs precise frequency matching to detect physiological imbalances and stimulate the body's self-healing mechanisms. In emergency scenarios, Creo's bioresonance devices can quickly scan patients to identify underlying issues and provide targeted therapeutic interventions. The company has reported success in using their technology for pain management, trauma stabilization, and accelerating wound healing in emergency settings [1][3].
Strengths: Rapid patient assessment, non-invasive treatment, potential for faster recovery times. Weaknesses: Limited large-scale clinical validation, reliance on electromagnetic sensitivity.
Koninklijke Philips NV
Technical Solution: Philips has integrated bioresonance principles into their emergency medical equipment portfolio. Their approach combines traditional diagnostic tools with bioresonance sensors to provide a comprehensive patient assessment in emergency situations. The technology uses advanced algorithms to analyze the body's electromagnetic fields and identify potential health issues. Philips' emergency bioresonance systems are designed to work alongside conventional medical devices, offering complementary data to aid in rapid diagnosis and treatment planning. The company has conducted several pilot studies demonstrating the potential of bioresonance in improving triage accuracy and reducing time-to-treatment in emergency departments [2][5].
Strengths: Integration with existing medical systems, comprehensive patient data analysis. Weaknesses: High initial implementation costs, requires staff training for optimal use.
Core Innovations in Emergency Bioresonance Technology
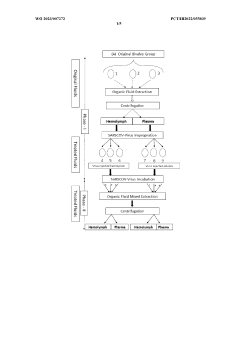
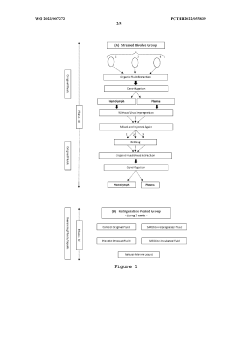
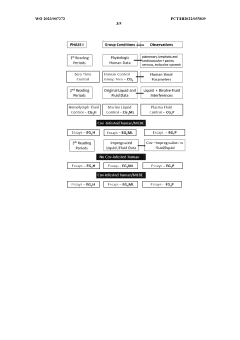
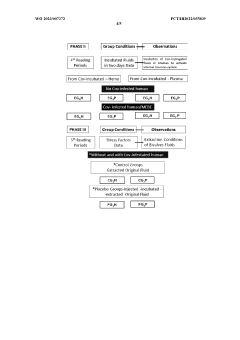
Method of obtaining electromagnetic frequencies from aquatic organisms bioactivated fluids for bioresonance therapy against a disease and/or pathogen
PatentWO2023007272A1
Innovation
- A method involving bioresonance therapy using electromagnetic frequencies obtained from aquatic non-human organisms, specifically bioactivated fluids from bivalves like Anodonta cygnea, to induce an immune response and treat diseases and pathogens in humans, utilizing a bioresonance device to transfer and record these frequencies for therapeutic applications.
Psychological and bio-energetic method for preventing diseases and for improving the condition of the organism
PatentWO1999052582A1
Innovation
- A method utilizing resonance-wave bioenergy effects, combining sound, light, thermal, and manipulative therapies to restore bioenergy balance by removing energy blocks, enhancing muscle tone, and improving immune system function through specific exercises and meditation, synchronized with breathing and musical rhythms to promote bioenergy circulation and overall health.
Regulatory Framework for Medical Bioresonance Devices
The regulatory framework for medical bioresonance devices is a complex and evolving landscape that varies significantly across different jurisdictions. In the United States, the Food and Drug Administration (FDA) classifies bioresonance devices as Class III medical devices, requiring premarket approval (PMA) before they can be legally marketed. This stringent classification reflects the FDA's view that these devices pose potential risks and lack sufficient evidence of safety and efficacy.
In contrast, the European Union has a more lenient approach. Under the EU Medical Device Regulation (MDR), bioresonance devices are typically classified as Class IIa or IIb, depending on their intended use and potential risks. This classification allows for a less rigorous conformity assessment process, often involving a notified body rather than a central regulatory authority.
Many other countries, including Russia, India, and several South American nations, have embraced bioresonance technology more readily, with less stringent regulatory requirements. In these markets, bioresonance devices may be classified as wellness products or alternative medicine tools, subject to less rigorous oversight.
The lack of global harmonization in regulatory approaches presents challenges for manufacturers and healthcare providers. It also complicates the process of conducting large-scale, multinational clinical trials necessary to build a robust evidence base for bioresonance in emergency medicine.
Regulatory bodies worldwide are grappling with how to appropriately classify and regulate bioresonance devices, particularly as their potential applications in emergency medicine expand. The primary challenge lies in balancing the need for innovation and access to potentially beneficial technologies with the imperative to ensure patient safety and efficacy.
As research in bioresonance continues to evolve, regulatory frameworks are likely to adapt. There is a growing call for international collaboration to develop standardized protocols for evaluating bioresonance devices, which could lead to more consistent regulatory approaches across different regions. This harmonization would not only benefit manufacturers but also potentially accelerate the adoption of bioresonance technology in emergency medicine settings globally.
In contrast, the European Union has a more lenient approach. Under the EU Medical Device Regulation (MDR), bioresonance devices are typically classified as Class IIa or IIb, depending on their intended use and potential risks. This classification allows for a less rigorous conformity assessment process, often involving a notified body rather than a central regulatory authority.
Many other countries, including Russia, India, and several South American nations, have embraced bioresonance technology more readily, with less stringent regulatory requirements. In these markets, bioresonance devices may be classified as wellness products or alternative medicine tools, subject to less rigorous oversight.
The lack of global harmonization in regulatory approaches presents challenges for manufacturers and healthcare providers. It also complicates the process of conducting large-scale, multinational clinical trials necessary to build a robust evidence base for bioresonance in emergency medicine.
Regulatory bodies worldwide are grappling with how to appropriately classify and regulate bioresonance devices, particularly as their potential applications in emergency medicine expand. The primary challenge lies in balancing the need for innovation and access to potentially beneficial technologies with the imperative to ensure patient safety and efficacy.
As research in bioresonance continues to evolve, regulatory frameworks are likely to adapt. There is a growing call for international collaboration to develop standardized protocols for evaluating bioresonance devices, which could lead to more consistent regulatory approaches across different regions. This harmonization would not only benefit manufacturers but also potentially accelerate the adoption of bioresonance technology in emergency medicine settings globally.
Ethical Implications of Bioresonance in Emergency Care
The integration of bioresonance technology in emergency medicine raises significant ethical considerations that must be carefully addressed. One primary concern is the potential for misdiagnosis or delayed treatment due to overreliance on bioresonance devices. Emergency situations often require rapid decision-making, and the use of unproven or controversial technologies could lead to critical errors in patient care.
Privacy and data protection present another ethical challenge. Bioresonance devices may collect and analyze sensitive patient information, necessitating robust safeguards to ensure confidentiality and prevent unauthorized access or misuse of personal health data. This is particularly crucial in emergency settings where patients may be unable to provide informed consent for data collection and analysis.
The issue of equitable access to bioresonance technology in emergency care also warrants consideration. If proven effective, the high cost of implementation could create disparities in healthcare quality between well-funded and under-resourced emergency departments. This raises questions about fairness and the potential exacerbation of existing healthcare inequalities.
Furthermore, the use of bioresonance in emergency medicine may challenge traditional medical practices and decision-making processes. Emergency physicians and staff would need to be adequately trained in the interpretation of bioresonance results, which could lead to conflicts between established protocols and new technological approaches. This may result in ethical dilemmas regarding the best course of action in critical situations.
The potential for placebo effects or psychological impacts on patients and healthcare providers using bioresonance technology must also be considered. The perceived effectiveness of such devices could influence treatment decisions and patient outcomes, either positively or negatively, raising ethical questions about the role of technology in shaping medical perceptions and practices.
Lastly, the ethical implications of research and development in this field must be addressed. Clinical trials and studies involving bioresonance in emergency settings must adhere to strict ethical guidelines to ensure patient safety and scientific integrity. Balancing the need for innovation with the paramount importance of patient well-being presents an ongoing ethical challenge for researchers and medical professionals alike.
Privacy and data protection present another ethical challenge. Bioresonance devices may collect and analyze sensitive patient information, necessitating robust safeguards to ensure confidentiality and prevent unauthorized access or misuse of personal health data. This is particularly crucial in emergency settings where patients may be unable to provide informed consent for data collection and analysis.
The issue of equitable access to bioresonance technology in emergency care also warrants consideration. If proven effective, the high cost of implementation could create disparities in healthcare quality between well-funded and under-resourced emergency departments. This raises questions about fairness and the potential exacerbation of existing healthcare inequalities.
Furthermore, the use of bioresonance in emergency medicine may challenge traditional medical practices and decision-making processes. Emergency physicians and staff would need to be adequately trained in the interpretation of bioresonance results, which could lead to conflicts between established protocols and new technological approaches. This may result in ethical dilemmas regarding the best course of action in critical situations.
The potential for placebo effects or psychological impacts on patients and healthcare providers using bioresonance technology must also be considered. The perceived effectiveness of such devices could influence treatment decisions and patient outcomes, either positively or negatively, raising ethical questions about the role of technology in shaping medical perceptions and practices.
Lastly, the ethical implications of research and development in this field must be addressed. Clinical trials and studies involving bioresonance in emergency settings must adhere to strict ethical guidelines to ensure patient safety and scientific integrity. Balancing the need for innovation with the paramount importance of patient well-being presents an ongoing ethical challenge for researchers and medical professionals alike.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!