Exploring the Neurostimulatory Effects of Bioresonance Devices
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioresonance Neurostimulation Background and Objectives
Bioresonance therapy, a concept rooted in the principles of quantum physics and electromagnetic field theory, has been a subject of scientific inquiry and medical exploration for several decades. The fundamental premise of bioresonance is that all living organisms emit and respond to specific electromagnetic frequencies. This theory posits that by manipulating these frequencies, it may be possible to influence cellular function and physiological processes within the body.
The evolution of bioresonance technology has been marked by significant advancements in both theoretical understanding and practical application. Initially developed in the 1970s by German physician Franz Morell, bioresonance devices have undergone numerous iterations and refinements. Early devices focused primarily on diagnostic capabilities, attempting to detect and interpret the body's electromagnetic signals. However, as the field progressed, the emphasis shifted towards therapeutic applications, particularly in the realm of neurostimulation.
The convergence of bioresonance principles with neurostimulation techniques represents a promising frontier in medical technology. This intersection aims to leverage the body's natural electromagnetic properties to modulate neural activity and potentially treat a wide range of neurological and psychiatric conditions. The underlying hypothesis suggests that by delivering specific frequency patterns to targeted areas of the nervous system, it may be possible to influence brain function, alleviate symptoms, and promote healing.
Recent technological advancements have led to the development of more sophisticated bioresonance devices capable of both detecting and emitting precise electromagnetic frequencies. These devices are designed to interact with the body's own electromagnetic field, potentially inducing therapeutic effects at the cellular and systemic levels. The integration of artificial intelligence and machine learning algorithms has further enhanced the capabilities of these devices, allowing for more personalized and adaptive treatment protocols.
The primary objectives of exploring the neurostimulatory effects of bioresonance devices are multifaceted. Researchers aim to elucidate the mechanisms by which electromagnetic frequencies interact with neural tissues and influence brain function. Additionally, there is a strong focus on developing non-invasive, drug-free alternatives for treating neurological disorders, potentially offering new hope for patients who have not responded well to conventional therapies.
Furthermore, the field seeks to establish standardized protocols and evidence-based guidelines for the use of bioresonance neurostimulation. This includes determining optimal frequency ranges, treatment durations, and application methods for various conditions. As the technology continues to evolve, there is also a growing emphasis on miniaturization and portability, with the goal of creating user-friendly devices suitable for home use and long-term therapy.
The evolution of bioresonance technology has been marked by significant advancements in both theoretical understanding and practical application. Initially developed in the 1970s by German physician Franz Morell, bioresonance devices have undergone numerous iterations and refinements. Early devices focused primarily on diagnostic capabilities, attempting to detect and interpret the body's electromagnetic signals. However, as the field progressed, the emphasis shifted towards therapeutic applications, particularly in the realm of neurostimulation.
The convergence of bioresonance principles with neurostimulation techniques represents a promising frontier in medical technology. This intersection aims to leverage the body's natural electromagnetic properties to modulate neural activity and potentially treat a wide range of neurological and psychiatric conditions. The underlying hypothesis suggests that by delivering specific frequency patterns to targeted areas of the nervous system, it may be possible to influence brain function, alleviate symptoms, and promote healing.
Recent technological advancements have led to the development of more sophisticated bioresonance devices capable of both detecting and emitting precise electromagnetic frequencies. These devices are designed to interact with the body's own electromagnetic field, potentially inducing therapeutic effects at the cellular and systemic levels. The integration of artificial intelligence and machine learning algorithms has further enhanced the capabilities of these devices, allowing for more personalized and adaptive treatment protocols.
The primary objectives of exploring the neurostimulatory effects of bioresonance devices are multifaceted. Researchers aim to elucidate the mechanisms by which electromagnetic frequencies interact with neural tissues and influence brain function. Additionally, there is a strong focus on developing non-invasive, drug-free alternatives for treating neurological disorders, potentially offering new hope for patients who have not responded well to conventional therapies.
Furthermore, the field seeks to establish standardized protocols and evidence-based guidelines for the use of bioresonance neurostimulation. This includes determining optimal frequency ranges, treatment durations, and application methods for various conditions. As the technology continues to evolve, there is also a growing emphasis on miniaturization and portability, with the goal of creating user-friendly devices suitable for home use and long-term therapy.
Market Analysis for Bioresonance Devices
The market for bioresonance devices has been experiencing significant growth in recent years, driven by increasing consumer interest in alternative and complementary medicine. This trend is particularly pronounced in Europe, North America, and parts of Asia, where holistic health approaches are gaining traction. The global bioresonance market size was valued at approximately $16.5 billion in 2020 and is projected to reach $28.3 billion by 2027, growing at a CAGR of 8.2% during the forecast period.
Several factors contribute to this market expansion. Firstly, there is a growing preference for non-invasive and drug-free treatment options among patients seeking alternatives to conventional medicine. Bioresonance therapy, which claims to detect and treat imbalances in the body's electromagnetic fields, aligns well with this demand. Additionally, the rising prevalence of chronic diseases and stress-related disorders has led many individuals to explore complementary therapies, further fueling market growth.
The market is segmented based on product type, application, and end-user. Product types include body-worn devices, tabletop devices, and standalone systems. Applications range from allergy detection and treatment to pain management, stress reduction, and overall wellness. Key end-users include hospitals, wellness centers, and home users.
Geographically, Europe holds the largest market share, with Germany being a particularly strong market due to its long-standing tradition of naturopathic medicine. North America follows closely, with the United States showing rapid adoption rates. The Asia-Pacific region is expected to witness the fastest growth, driven by increasing disposable incomes and a cultural affinity for holistic health practices in countries like China and India.
Despite the market's positive outlook, it faces several challenges. The lack of robust scientific evidence supporting the efficacy of bioresonance therapy remains a significant barrier to widespread acceptance in mainstream medical communities. Regulatory hurdles also pose challenges, as many countries have not yet established clear guidelines for bioresonance devices. This regulatory uncertainty can impede market growth and innovation.
Consumer awareness and education represent both a challenge and an opportunity for the market. While interest in alternative therapies is growing, many potential users remain unfamiliar with bioresonance technology. Manufacturers and practitioners are investing in educational initiatives to bridge this knowledge gap and expand their customer base.
Looking ahead, technological advancements are expected to play a crucial role in shaping the market's future. Integration of artificial intelligence and machine learning algorithms into bioresonance devices could enhance their diagnostic and therapeutic capabilities, potentially increasing their appeal to both consumers and healthcare professionals. The development of more portable and user-friendly devices is also anticipated, which could further drive adoption in the home-use segment.
Several factors contribute to this market expansion. Firstly, there is a growing preference for non-invasive and drug-free treatment options among patients seeking alternatives to conventional medicine. Bioresonance therapy, which claims to detect and treat imbalances in the body's electromagnetic fields, aligns well with this demand. Additionally, the rising prevalence of chronic diseases and stress-related disorders has led many individuals to explore complementary therapies, further fueling market growth.
The market is segmented based on product type, application, and end-user. Product types include body-worn devices, tabletop devices, and standalone systems. Applications range from allergy detection and treatment to pain management, stress reduction, and overall wellness. Key end-users include hospitals, wellness centers, and home users.
Geographically, Europe holds the largest market share, with Germany being a particularly strong market due to its long-standing tradition of naturopathic medicine. North America follows closely, with the United States showing rapid adoption rates. The Asia-Pacific region is expected to witness the fastest growth, driven by increasing disposable incomes and a cultural affinity for holistic health practices in countries like China and India.
Despite the market's positive outlook, it faces several challenges. The lack of robust scientific evidence supporting the efficacy of bioresonance therapy remains a significant barrier to widespread acceptance in mainstream medical communities. Regulatory hurdles also pose challenges, as many countries have not yet established clear guidelines for bioresonance devices. This regulatory uncertainty can impede market growth and innovation.
Consumer awareness and education represent both a challenge and an opportunity for the market. While interest in alternative therapies is growing, many potential users remain unfamiliar with bioresonance technology. Manufacturers and practitioners are investing in educational initiatives to bridge this knowledge gap and expand their customer base.
Looking ahead, technological advancements are expected to play a crucial role in shaping the market's future. Integration of artificial intelligence and machine learning algorithms into bioresonance devices could enhance their diagnostic and therapeutic capabilities, potentially increasing their appeal to both consumers and healthcare professionals. The development of more portable and user-friendly devices is also anticipated, which could further drive adoption in the home-use segment.
Current Challenges in Bioresonance Neurostimulation
Despite the growing interest in bioresonance neurostimulation, the field faces several significant challenges that hinder its widespread adoption and clinical application. One of the primary obstacles is the lack of standardized protocols for device calibration and treatment administration. The wide variety of bioresonance devices on the market, each with its proprietary algorithms and frequency ranges, makes it difficult to establish consistent treatment parameters across studies and clinical practices.
Another major challenge lies in the limited understanding of the precise mechanisms by which bioresonance devices interact with the nervous system. While theories exist about frequency-based resonance and electromagnetic field interactions, the exact neurophysiological pathways involved remain unclear. This knowledge gap impedes the development of more targeted and effective neurostimulation techniques.
The issue of reproducibility also plagues the field of bioresonance neurostimulation. Many studies reporting positive outcomes have been criticized for small sample sizes, inadequate control groups, and potential placebo effects. The subjective nature of many neurological symptoms further complicates the assessment of treatment efficacy, making it challenging to draw definitive conclusions about the effectiveness of bioresonance devices.
Regulatory hurdles present another significant challenge. The classification and approval process for bioresonance devices varies widely between countries, with some regulatory bodies viewing them as medical devices and others as wellness products. This inconsistency creates barriers to market entry and limits the ability to conduct large-scale, multi-center clinical trials necessary for establishing the technology's credibility.
The integration of bioresonance neurostimulation with existing medical practices and technologies also poses challenges. Many healthcare professionals are unfamiliar with the principles of bioresonance and may be skeptical of its potential benefits. This lack of acceptance within the mainstream medical community hampers the adoption of bioresonance devices in clinical settings and limits research funding opportunities.
Technical limitations of current bioresonance devices also present obstacles. Many devices struggle with precise frequency targeting, especially in deep brain structures. Additionally, the long-term effects of repeated neurostimulation using bioresonance techniques are not well understood, raising concerns about potential adverse effects with prolonged use.
Lastly, the field faces challenges in developing personalized treatment approaches. Individual variations in neurophysiology and response to bioresonance stimulation necessitate more sophisticated methods for tailoring treatments to each patient's unique characteristics. Overcoming this challenge requires advancements in both device technology and our understanding of neuroplasticity and individual frequency responses.
Another major challenge lies in the limited understanding of the precise mechanisms by which bioresonance devices interact with the nervous system. While theories exist about frequency-based resonance and electromagnetic field interactions, the exact neurophysiological pathways involved remain unclear. This knowledge gap impedes the development of more targeted and effective neurostimulation techniques.
The issue of reproducibility also plagues the field of bioresonance neurostimulation. Many studies reporting positive outcomes have been criticized for small sample sizes, inadequate control groups, and potential placebo effects. The subjective nature of many neurological symptoms further complicates the assessment of treatment efficacy, making it challenging to draw definitive conclusions about the effectiveness of bioresonance devices.
Regulatory hurdles present another significant challenge. The classification and approval process for bioresonance devices varies widely between countries, with some regulatory bodies viewing them as medical devices and others as wellness products. This inconsistency creates barriers to market entry and limits the ability to conduct large-scale, multi-center clinical trials necessary for establishing the technology's credibility.
The integration of bioresonance neurostimulation with existing medical practices and technologies also poses challenges. Many healthcare professionals are unfamiliar with the principles of bioresonance and may be skeptical of its potential benefits. This lack of acceptance within the mainstream medical community hampers the adoption of bioresonance devices in clinical settings and limits research funding opportunities.
Technical limitations of current bioresonance devices also present obstacles. Many devices struggle with precise frequency targeting, especially in deep brain structures. Additionally, the long-term effects of repeated neurostimulation using bioresonance techniques are not well understood, raising concerns about potential adverse effects with prolonged use.
Lastly, the field faces challenges in developing personalized treatment approaches. Individual variations in neurophysiology and response to bioresonance stimulation necessitate more sophisticated methods for tailoring treatments to each patient's unique characteristics. Overcoming this challenge requires advancements in both device technology and our understanding of neuroplasticity and individual frequency responses.
Existing Bioresonance Neurostimulation Techniques
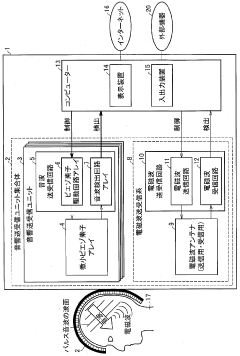
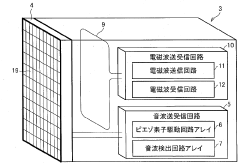
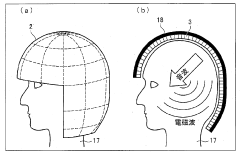
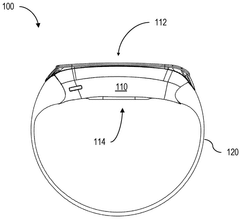
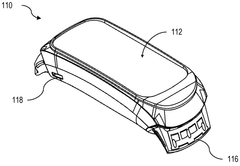
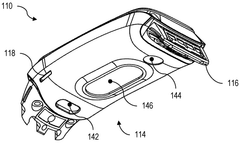
01 Bioresonance devices for neurostimulation
Bioresonance devices are designed to stimulate the nervous system using specific frequencies. These devices can be used for various therapeutic purposes, including pain management, stress reduction, and improving overall well-being. The neurostimulatory effects are achieved by emitting electromagnetic waves that interact with the body's natural frequencies.- Bioresonance devices for neurostimulation: Bioresonance devices are designed to stimulate the nervous system using specific frequencies. These devices can be used for various therapeutic purposes, including pain management, stress reduction, and improving overall well-being. The neurostimulatory effects are achieved by applying electromagnetic fields or electrical impulses to specific areas of the body.
- Non-invasive neurostimulation techniques: Non-invasive neurostimulation techniques utilize external devices to modulate neural activity without the need for surgical intervention. These methods may include transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), or other forms of electromagnetic stimulation. Such techniques can be used to treat various neurological and psychiatric conditions.
- Frequency-specific bioresonance therapy: Frequency-specific bioresonance therapy involves the use of specific electromagnetic frequencies to target particular physiological processes or conditions. This approach is based on the concept that different tissues and organs respond to specific frequencies, allowing for targeted therapeutic interventions. The therapy aims to restore balance and promote healing within the body.
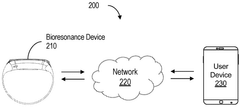
- Portable bioresonance devices for personal use: Portable bioresonance devices are designed for personal use, allowing individuals to receive neurostimulatory treatments at home or on-the-go. These devices are typically compact, user-friendly, and may be connected to smartphones or other digital platforms for personalized therapy programs and monitoring of treatment progress.
- Combination of bioresonance and other therapeutic modalities: Some bioresonance devices incorporate additional therapeutic modalities to enhance their neurostimulatory effects. These may include light therapy, sound therapy, or vibrational therapy. The combination of multiple modalities aims to provide a more comprehensive approach to treatment and potentially improve overall therapeutic outcomes.
02 Non-invasive neurostimulation techniques
Non-invasive neurostimulation methods utilize external devices to modulate neural activity without surgical intervention. These techniques may include transcranial magnetic stimulation, transcranial direct current stimulation, or other forms of electromagnetic stimulation. The devices are designed to target specific brain regions or neural pathways to achieve desired therapeutic outcomes.Expand Specific Solutions03 Frequency-based therapies for neurological disorders
Bioresonance devices can be programmed to emit specific frequencies that may have therapeutic effects on various neurological disorders. These frequencies are believed to interact with the body's electromagnetic field, potentially influencing neural activity and promoting healing. The devices may be used to address conditions such as migraines, anxiety, or sleep disorders.Expand Specific Solutions04 Portable and wearable neurostimulation devices
Advancements in technology have led to the development of portable and wearable bioresonance devices for neurostimulation. These compact devices allow for convenient and continuous treatment, enabling users to receive therapy while going about their daily activities. The wearable nature of these devices may improve patient compliance and treatment efficacy.Expand Specific Solutions05 Combination of bioresonance and other therapeutic modalities
Some bioresonance devices integrate multiple therapeutic modalities to enhance neurostimulatory effects. These may include the combination of electromagnetic stimulation with light therapy, sound therapy, or other complementary approaches. The synergistic effects of these combined therapies aim to provide more comprehensive and effective treatment for various neurological and psychological conditions.Expand Specific Solutions
Key Players in Bioresonance Device Industry
The field of neurostimulatory effects of bioresonance devices is in an early developmental stage, with a growing market driven by increasing interest in non-invasive therapeutic approaches. The global market for neuromodulation devices is expanding, expected to reach significant value in the coming years. Technologically, the field is evolving rapidly, with companies like Boston Scientific Neuromodulation Corp. and Medtronic leading in implantable devices. Meanwhile, emerging players such as Cognito Therapeutics are exploring non-invasive approaches. Academic institutions like Arizona State University and École Polytechnique Fédérale de Lausanne are contributing to fundamental research, pushing the boundaries of our understanding of bioresonance effects on neural systems.
Boston Scientific Neuromodulation Corp.
Technical Solution: Boston Scientific Neuromodulation Corp. has developed advanced bioresonance devices for neurostimulation, focusing on spinal cord stimulation (SCS) and deep brain stimulation (DBS) technologies. Their WaveWriter Alpha™ SCS System utilizes multiple independent current control and neural targeting, allowing for personalized therapy delivery[1]. The system incorporates a proprietary algorithm that automatically adjusts stimulation parameters based on patient feedback and activity levels, optimizing therapeutic outcomes[2]. Additionally, their Vercise™ DBS platform employs directional lead technology, enabling precise stimulation of specific brain regions while minimizing side effects[3]. The company has also invested in research on closed-loop neurostimulation systems that can adapt in real-time to changes in brain activity, potentially improving treatment efficacy for conditions such as Parkinson's disease and essential tremor[4].
Strengths: Advanced targeting capabilities, personalized therapy delivery, and adaptive stimulation technologies. Weaknesses: Limited to invasive neurostimulation methods, which may not be suitable for all patients or conditions.
Koninklijke Philips NV
Technical Solution: Koninklijke Philips NV has developed non-invasive bioresonance devices for neurostimulation, focusing on transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) technologies. Their Neuro 200 TMS system utilizes high-precision coil positioning and neuronavigation for targeted stimulation of specific brain regions[1]. The company has also introduced a novel tDCS device that employs multi-channel stimulation and real-time impedance monitoring to ensure optimal current delivery[2]. Philips has invested in research on combining neurostimulation with neuroimaging techniques, such as fMRI-guided TMS, to enhance treatment precision and efficacy[3]. Additionally, they are exploring the use of artificial intelligence algorithms to personalize stimulation parameters based on individual patient characteristics and treatment responses[4].
Strengths: Non-invasive technologies, integration with neuroimaging, and AI-driven personalization. Weaknesses: Limited efficacy compared to invasive methods for certain conditions, and potential variability in treatment outcomes.
Core Innovations in Bioresonance Neurostimulation
Biological measurement system and biological stimulation system
PatentWO2009122485A1
Innovation
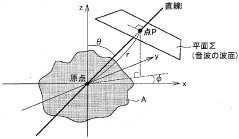
- A bioinstrumentation device that uses a vibration imparting element to generate electromagnetic waves in nerve cells without contact, allowing for non-invasive measurement and stimulation by analyzing the electromagnetic waves generated, utilizing sound waves with frequencies between 1 MHz and 1 GHz to achieve high temporal and spatial resolution.
Device for bioresonance modulation of muscle temperature and blood oxygen levels
PatentWO2025151179A1
Innovation
- A bioresonance device equipped with a processor, temperature and optical sensors, and an electromagnetic frequency generator, capable of emitting frequencies based on real-time physiological data and remote instructions, allowing for dynamic and personalized bioresonance modalities.
Safety and Efficacy Considerations
The safety and efficacy of bioresonance devices for neurostimulation are critical considerations that require thorough examination. These devices, which purport to influence neural activity through electromagnetic resonance, have garnered attention in both medical and consumer markets. However, their mechanisms of action and potential effects on the nervous system remain subjects of ongoing research and debate.
From a safety perspective, the primary concerns revolve around the potential for unintended neural modulation and long-term effects on brain function. The human nervous system is a complex and delicate network, and any external stimulation must be carefully controlled to avoid adverse outcomes. Current studies have shown mixed results, with some reporting minimal side effects and others raising concerns about potential disruptions to normal neural patterns.
Efficacy considerations are equally complex. While some proponents claim significant benefits for various neurological conditions, the scientific evidence supporting these claims is often limited or inconclusive. Clinical trials have shown varying degrees of effectiveness, with results often difficult to replicate consistently across different patient populations and study designs.
One of the key challenges in assessing the efficacy of bioresonance devices is the placebo effect, which can be particularly strong in neurostimulation therapies. Distinguishing between genuine neurophysiological changes and perceived improvements due to expectation bias requires rigorous, double-blind studies with appropriate control groups.
Regulatory bodies, such as the FDA in the United States, have approached bioresonance devices with caution. Many of these devices are classified as wellness products rather than medical devices, which limits the oversight and testing requirements. This classification gap presents challenges for ensuring consistent safety and efficacy standards across the industry.
As research in this field progresses, there is a growing need for standardized protocols to evaluate both the safety and efficacy of bioresonance devices. These protocols should include comprehensive neuroimaging studies to track changes in brain activity, long-term follow-up assessments to monitor for delayed effects, and rigorous clinical trials that adhere to the highest standards of evidence-based medicine.
The potential for bioresonance devices to offer non-invasive neurostimulation options is promising, particularly for conditions that are resistant to conventional treatments. However, the balance between innovation and patient safety must be carefully maintained. Future developments in this field will likely focus on refining the specificity of stimulation, improving our understanding of the underlying mechanisms, and developing more targeted approaches for individual patient needs.
From a safety perspective, the primary concerns revolve around the potential for unintended neural modulation and long-term effects on brain function. The human nervous system is a complex and delicate network, and any external stimulation must be carefully controlled to avoid adverse outcomes. Current studies have shown mixed results, with some reporting minimal side effects and others raising concerns about potential disruptions to normal neural patterns.
Efficacy considerations are equally complex. While some proponents claim significant benefits for various neurological conditions, the scientific evidence supporting these claims is often limited or inconclusive. Clinical trials have shown varying degrees of effectiveness, with results often difficult to replicate consistently across different patient populations and study designs.
One of the key challenges in assessing the efficacy of bioresonance devices is the placebo effect, which can be particularly strong in neurostimulation therapies. Distinguishing between genuine neurophysiological changes and perceived improvements due to expectation bias requires rigorous, double-blind studies with appropriate control groups.
Regulatory bodies, such as the FDA in the United States, have approached bioresonance devices with caution. Many of these devices are classified as wellness products rather than medical devices, which limits the oversight and testing requirements. This classification gap presents challenges for ensuring consistent safety and efficacy standards across the industry.
As research in this field progresses, there is a growing need for standardized protocols to evaluate both the safety and efficacy of bioresonance devices. These protocols should include comprehensive neuroimaging studies to track changes in brain activity, long-term follow-up assessments to monitor for delayed effects, and rigorous clinical trials that adhere to the highest standards of evidence-based medicine.
The potential for bioresonance devices to offer non-invasive neurostimulation options is promising, particularly for conditions that are resistant to conventional treatments. However, the balance between innovation and patient safety must be carefully maintained. Future developments in this field will likely focus on refining the specificity of stimulation, improving our understanding of the underlying mechanisms, and developing more targeted approaches for individual patient needs.
Regulatory Framework for Bioresonance Devices
The regulatory framework for bioresonance devices is a complex and evolving landscape, reflecting the growing interest in and use of these neurostimulatory technologies. In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the safety and efficacy of medical devices, including bioresonance devices. These devices are typically classified as Class II medical devices, requiring premarket notification (510(k)) clearance before they can be legally marketed.
The European Union has implemented the Medical Device Regulation (MDR), which came into full effect in May 2021. Under this framework, bioresonance devices are subject to rigorous safety and performance requirements. Manufacturers must demonstrate compliance with these regulations through clinical evaluations and risk assessments before obtaining CE marking for their devices.
In other regions, such as Asia and Australia, regulatory approaches vary. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for neurostimulation devices, while China's National Medical Products Administration (NMPA) requires extensive clinical data for approval.
Regulatory bodies worldwide are grappling with the challenge of keeping pace with rapid technological advancements in neurostimulation. This has led to the development of adaptive regulatory pathways, designed to balance innovation with patient safety. For instance, the FDA's Digital Health Software Precertification (Pre-Cert) Program aims to streamline the review process for certain digital health products, potentially benefiting some bioresonance device manufacturers.
Safety considerations are paramount in the regulatory framework for bioresonance devices. Regulators require manufacturers to conduct thorough risk assessments, addressing potential adverse effects such as skin irritation, electrical safety, and electromagnetic compatibility. Long-term safety data is increasingly being demanded to assess the cumulative effects of neurostimulation.
Efficacy claims for bioresonance devices are subject to scrutiny, with regulatory bodies requiring substantial clinical evidence to support marketing claims. This has led to an increase in clinical trials and post-market surveillance studies to gather real-world data on device performance and patient outcomes.
As the field of neurostimulation continues to advance, regulatory frameworks are likely to evolve. There is a growing emphasis on international harmonization of regulations, as exemplified by the International Medical Device Regulators Forum (IMDRF). This collaborative effort aims to streamline approval processes and ensure consistent safety standards across different jurisdictions.
The European Union has implemented the Medical Device Regulation (MDR), which came into full effect in May 2021. Under this framework, bioresonance devices are subject to rigorous safety and performance requirements. Manufacturers must demonstrate compliance with these regulations through clinical evaluations and risk assessments before obtaining CE marking for their devices.
In other regions, such as Asia and Australia, regulatory approaches vary. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for neurostimulation devices, while China's National Medical Products Administration (NMPA) requires extensive clinical data for approval.
Regulatory bodies worldwide are grappling with the challenge of keeping pace with rapid technological advancements in neurostimulation. This has led to the development of adaptive regulatory pathways, designed to balance innovation with patient safety. For instance, the FDA's Digital Health Software Precertification (Pre-Cert) Program aims to streamline the review process for certain digital health products, potentially benefiting some bioresonance device manufacturers.
Safety considerations are paramount in the regulatory framework for bioresonance devices. Regulators require manufacturers to conduct thorough risk assessments, addressing potential adverse effects such as skin irritation, electrical safety, and electromagnetic compatibility. Long-term safety data is increasingly being demanded to assess the cumulative effects of neurostimulation.
Efficacy claims for bioresonance devices are subject to scrutiny, with regulatory bodies requiring substantial clinical evidence to support marketing claims. This has led to an increase in clinical trials and post-market surveillance studies to gather real-world data on device performance and patient outcomes.
As the field of neurostimulation continues to advance, regulatory frameworks are likely to evolve. There is a growing emphasis on international harmonization of regulations, as exemplified by the International Medical Device Regulators Forum (IMDRF). This collaborative effort aims to streamline approval processes and ensure consistent safety standards across different jurisdictions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!