Issues in the Safety and Regulation of Bioresonance Devices
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioresonance Safety Background and Objectives
Bioresonance therapy, a controversial alternative medical practice, has gained attention in recent years due to its purported ability to diagnose and treat various health conditions. The technology behind bioresonance devices is based on the concept that all cells and organs in the human body emit unique electromagnetic frequencies. These devices claim to detect and manipulate these frequencies to identify and address health issues.
The evolution of bioresonance technology can be traced back to the 1970s when Dr. Franz Morell and his son-in-law, engineer Erich Rasche, developed the first bioresonance device called MORA. Since then, various iterations and advancements have been made in the field, leading to a diverse range of bioresonance devices available in the market today.
As the popularity of bioresonance therapy has grown, so have concerns regarding its safety and efficacy. The primary objective of this technical research report is to comprehensively examine the safety issues associated with bioresonance devices and explore the current regulatory landscape surrounding their use and distribution.
One of the key challenges in assessing the safety of bioresonance devices lies in the lack of standardized protocols and rigorous scientific evidence supporting their effectiveness. Many proponents of bioresonance therapy claim that the devices are non-invasive and pose minimal risks to users. However, skeptics argue that the absence of proper regulation and quality control measures may lead to potential harm, especially when these devices are used as alternatives to conventional medical treatments.
The regulatory status of bioresonance devices varies significantly across different countries and regions. In some jurisdictions, these devices are classified as medical devices and subject to strict regulatory oversight. In others, they fall into a gray area, with limited or no specific regulations governing their manufacture, sale, and use.
This report aims to provide a comprehensive overview of the current state of bioresonance technology, focusing on safety concerns and regulatory challenges. By examining the historical context, technological principles, and existing safety data, we seek to identify potential risks associated with bioresonance devices and evaluate the adequacy of current regulatory frameworks in addressing these concerns.
Furthermore, this research will explore the ongoing debates within the scientific and medical communities regarding the validity and safety of bioresonance therapy. By analyzing expert opinions, clinical studies, and reported adverse events, we aim to present a balanced perspective on the potential benefits and risks associated with the use of these devices.
The evolution of bioresonance technology can be traced back to the 1970s when Dr. Franz Morell and his son-in-law, engineer Erich Rasche, developed the first bioresonance device called MORA. Since then, various iterations and advancements have been made in the field, leading to a diverse range of bioresonance devices available in the market today.
As the popularity of bioresonance therapy has grown, so have concerns regarding its safety and efficacy. The primary objective of this technical research report is to comprehensively examine the safety issues associated with bioresonance devices and explore the current regulatory landscape surrounding their use and distribution.
One of the key challenges in assessing the safety of bioresonance devices lies in the lack of standardized protocols and rigorous scientific evidence supporting their effectiveness. Many proponents of bioresonance therapy claim that the devices are non-invasive and pose minimal risks to users. However, skeptics argue that the absence of proper regulation and quality control measures may lead to potential harm, especially when these devices are used as alternatives to conventional medical treatments.
The regulatory status of bioresonance devices varies significantly across different countries and regions. In some jurisdictions, these devices are classified as medical devices and subject to strict regulatory oversight. In others, they fall into a gray area, with limited or no specific regulations governing their manufacture, sale, and use.
This report aims to provide a comprehensive overview of the current state of bioresonance technology, focusing on safety concerns and regulatory challenges. By examining the historical context, technological principles, and existing safety data, we seek to identify potential risks associated with bioresonance devices and evaluate the adequacy of current regulatory frameworks in addressing these concerns.
Furthermore, this research will explore the ongoing debates within the scientific and medical communities regarding the validity and safety of bioresonance therapy. By analyzing expert opinions, clinical studies, and reported adverse events, we aim to present a balanced perspective on the potential benefits and risks associated with the use of these devices.
Market Analysis of Bioresonance Devices
The bioresonance devices market has been experiencing steady growth in recent years, driven by increasing consumer interest in alternative and complementary medicine. This market segment is part of the broader medical devices industry, which is projected to reach a global value of $612.7 billion by 2025, according to a report by Grand View Research. While bioresonance devices represent a small fraction of this market, they have gained traction in certain regions and among specific consumer groups.
Europe, particularly Germany and Russia, has been at the forefront of bioresonance device adoption and market growth. These countries have a long-standing tradition of embracing alternative therapies, which has created a favorable environment for bioresonance technology. In contrast, North America has shown slower adoption rates due to stricter regulatory environments and skepticism from mainstream medical communities.
The Asia-Pacific region is emerging as a significant market for bioresonance devices, with countries like China and India showing increased interest. This growth is attributed to rising disposable incomes, growing awareness of alternative therapies, and a cultural predisposition towards holistic health approaches.
Consumer demographics for bioresonance devices typically include middle to upper-income individuals aged 35-65, who are health-conscious and open to non-traditional medical approaches. This demographic often overlaps with consumers of other alternative health products and services, such as acupuncture, homeopathy, and naturopathy.
Market segmentation for bioresonance devices can be categorized into professional and home-use devices. Professional devices, used in clinics and wellness centers, constitute the larger market share due to their higher price points and perceived efficacy. However, the home-use segment is growing rapidly, driven by increasing consumer desire for personal health management tools.
The competitive landscape of the bioresonance device market is fragmented, with numerous small to medium-sized companies operating in various regions. Key players include BICOM, Rayonex Biomedical, and Sensitiv Imago. These companies often differentiate themselves through proprietary technologies, device features, and marketing claims about health benefits.
Market challenges include regulatory hurdles, particularly in countries where bioresonance is not recognized as a valid medical treatment. The lack of large-scale clinical studies demonstrating efficacy also poses a significant barrier to wider acceptance and market growth. Additionally, the market faces competition from other alternative therapies and conventional medical treatments.
Despite these challenges, the bioresonance device market is expected to continue growing, albeit at a moderate pace. Factors driving this growth include increasing consumer interest in preventive healthcare, rising chronic disease prevalence, and growing acceptance of complementary and alternative medicine in some healthcare systems.
Europe, particularly Germany and Russia, has been at the forefront of bioresonance device adoption and market growth. These countries have a long-standing tradition of embracing alternative therapies, which has created a favorable environment for bioresonance technology. In contrast, North America has shown slower adoption rates due to stricter regulatory environments and skepticism from mainstream medical communities.
The Asia-Pacific region is emerging as a significant market for bioresonance devices, with countries like China and India showing increased interest. This growth is attributed to rising disposable incomes, growing awareness of alternative therapies, and a cultural predisposition towards holistic health approaches.
Consumer demographics for bioresonance devices typically include middle to upper-income individuals aged 35-65, who are health-conscious and open to non-traditional medical approaches. This demographic often overlaps with consumers of other alternative health products and services, such as acupuncture, homeopathy, and naturopathy.
Market segmentation for bioresonance devices can be categorized into professional and home-use devices. Professional devices, used in clinics and wellness centers, constitute the larger market share due to their higher price points and perceived efficacy. However, the home-use segment is growing rapidly, driven by increasing consumer desire for personal health management tools.
The competitive landscape of the bioresonance device market is fragmented, with numerous small to medium-sized companies operating in various regions. Key players include BICOM, Rayonex Biomedical, and Sensitiv Imago. These companies often differentiate themselves through proprietary technologies, device features, and marketing claims about health benefits.
Market challenges include regulatory hurdles, particularly in countries where bioresonance is not recognized as a valid medical treatment. The lack of large-scale clinical studies demonstrating efficacy also poses a significant barrier to wider acceptance and market growth. Additionally, the market faces competition from other alternative therapies and conventional medical treatments.
Despite these challenges, the bioresonance device market is expected to continue growing, albeit at a moderate pace. Factors driving this growth include increasing consumer interest in preventive healthcare, rising chronic disease prevalence, and growing acceptance of complementary and alternative medicine in some healthcare systems.
Current Safety Challenges in Bioresonance Technology
Bioresonance technology, while gaining popularity in alternative medicine circles, faces significant safety challenges that demand urgent attention. The primary concern stems from the lack of comprehensive scientific evidence supporting its efficacy and safety. This absence of robust clinical trials and peer-reviewed studies leaves a considerable gap in understanding the potential risks associated with these devices.
One of the most pressing safety issues is the potential for misdiagnosis or delayed diagnosis of serious medical conditions. Bioresonance devices are often marketed as diagnostic tools, capable of detecting a wide range of health issues. However, reliance on these unproven methods may lead patients to forgo conventional medical diagnostics, potentially missing critical early interventions for severe illnesses.
The calibration and standardization of bioresonance devices present another significant challenge. Without established industry standards or regulatory oversight, there is a high risk of inconsistent results between different devices or even between sessions using the same device. This lack of reliability not only undermines the credibility of the technology but also poses potential harm to users who may receive inaccurate health information.
Electromagnetic interference is a further safety concern, particularly for individuals with implanted medical devices such as pacemakers or insulin pumps. The electromagnetic fields generated by bioresonance devices, although generally weak, could potentially interfere with the functioning of these critical medical devices, posing a serious risk to vulnerable patients.
The absence of proper regulation and quality control in the manufacturing of bioresonance devices is another significant safety challenge. Without stringent oversight, there is a risk of substandard devices entering the market, which may malfunction or produce erroneous results. This lack of quality assurance extends to the materials used in the devices, raising concerns about potential allergic reactions or long-term exposure effects to certain components.
User error and misinterpretation of results represent additional safety challenges. Many bioresonance devices are marketed for home use, often without adequate training or guidance. This can lead to improper use of the device, misinterpretation of results, and potentially harmful self-diagnosis or self-treatment based on inaccurate information.
Lastly, the psychological impact of bioresonance technology on users is a growing concern. The promise of easy, non-invasive diagnosis and treatment can create false hope or anxiety, depending on the results provided by these devices. This psychological aspect, often overlooked in discussions of physical safety, can have significant implications for users' overall well-being and their approach to healthcare.
One of the most pressing safety issues is the potential for misdiagnosis or delayed diagnosis of serious medical conditions. Bioresonance devices are often marketed as diagnostic tools, capable of detecting a wide range of health issues. However, reliance on these unproven methods may lead patients to forgo conventional medical diagnostics, potentially missing critical early interventions for severe illnesses.
The calibration and standardization of bioresonance devices present another significant challenge. Without established industry standards or regulatory oversight, there is a high risk of inconsistent results between different devices or even between sessions using the same device. This lack of reliability not only undermines the credibility of the technology but also poses potential harm to users who may receive inaccurate health information.
Electromagnetic interference is a further safety concern, particularly for individuals with implanted medical devices such as pacemakers or insulin pumps. The electromagnetic fields generated by bioresonance devices, although generally weak, could potentially interfere with the functioning of these critical medical devices, posing a serious risk to vulnerable patients.
The absence of proper regulation and quality control in the manufacturing of bioresonance devices is another significant safety challenge. Without stringent oversight, there is a risk of substandard devices entering the market, which may malfunction or produce erroneous results. This lack of quality assurance extends to the materials used in the devices, raising concerns about potential allergic reactions or long-term exposure effects to certain components.
User error and misinterpretation of results represent additional safety challenges. Many bioresonance devices are marketed for home use, often without adequate training or guidance. This can lead to improper use of the device, misinterpretation of results, and potentially harmful self-diagnosis or self-treatment based on inaccurate information.
Lastly, the psychological impact of bioresonance technology on users is a growing concern. The promise of easy, non-invasive diagnosis and treatment can create false hope or anxiety, depending on the results provided by these devices. This psychological aspect, often overlooked in discussions of physical safety, can have significant implications for users' overall well-being and their approach to healthcare.
Existing Safety Measures for Bioresonance Devices
01 Safety standards and certifications
Bioresonance devices are subject to safety standards and certifications to ensure their safe use. These standards may include electromagnetic compatibility testing, electrical safety requirements, and quality management systems. Compliance with these standards helps to minimize potential risks associated with the use of bioresonance devices.- Safety standards and certifications for bioresonance devices: Bioresonance devices are subject to safety standards and certifications to ensure their safe use. These standards may include electromagnetic compatibility testing, electrical safety requirements, and quality management systems. Certification processes help verify that the devices meet established safety criteria before being marketed to consumers.
- Design features for user safety: Bioresonance devices incorporate various design features to enhance user safety. These may include protective casings, insulated components, and fail-safe mechanisms. Some devices also feature automatic shut-off systems and low-voltage operation to minimize potential risks during use.
- Clinical studies and risk assessments: Safety of bioresonance devices is often evaluated through clinical studies and risk assessments. These studies aim to identify potential adverse effects, contraindications, and long-term safety implications. Risk assessments help manufacturers and regulatory bodies determine the overall safety profile of the devices.
- User guidelines and precautions: Manufacturers provide comprehensive user guidelines and precautions for the safe operation of bioresonance devices. These may include instructions on proper device placement, treatment duration, and frequency of use. Precautions often highlight potential risks for specific user groups, such as pregnant women or individuals with implanted medical devices.
- Regulatory compliance and monitoring: Bioresonance devices are subject to regulatory compliance and ongoing monitoring to ensure their continued safety. This includes adherence to medical device regulations, post-market surveillance, and reporting of adverse events. Regulatory bodies may conduct periodic reviews and inspections to verify compliance with safety standards.
02 Non-invasive nature of bioresonance therapy
Bioresonance devices are designed to be non-invasive, which contributes to their safety profile. These devices typically use electrodes or sensors placed on the skin to measure and interact with the body's electromagnetic fields, without penetrating the skin or causing physical harm to the user.Expand Specific Solutions03 Low-intensity electromagnetic fields
Bioresonance devices generally operate using low-intensity electromagnetic fields, which are considered to be safe for human exposure. The strength of these fields is typically well below the thresholds set by international safety guidelines, minimizing the risk of adverse effects from electromagnetic radiation.Expand Specific Solutions04 User guidelines and contraindications
Manufacturers of bioresonance devices provide user guidelines and specify contraindications to ensure safe usage. These guidelines may include recommendations for treatment duration, frequency of use, and proper electrode placement. Contraindications may be listed for certain medical conditions or for use with other medical devices, such as pacemakers.Expand Specific Solutions05 Ongoing research and clinical studies
The safety of bioresonance devices is subject to ongoing research and clinical studies. These studies aim to evaluate the long-term effects of bioresonance therapy and identify any potential risks or side effects. Continuous research helps to improve the safety profile of these devices and provides valuable information for users and healthcare professionals.Expand Specific Solutions
Key Manufacturers and Regulatory Bodies
The safety and regulation of bioresonance devices present a complex competitive landscape in an emerging market. The industry is in its early stages, with a growing market size driven by increasing interest in alternative therapies. However, the technology's maturity remains low, with significant challenges in establishing scientific validity and regulatory compliance. Key players like Koninklijke Philips NV and Siemens Healthineers AG are exploring potential applications, while smaller companies and research institutions such as Massachusetts Institute of Technology and The Johns Hopkins University are conducting studies to validate the technology. The involvement of established medical device manufacturers like Medtronic and Stryker Corporation indicates growing interest, but also highlights the need for rigorous safety and efficacy standards to be developed and implemented across the industry.
Koninklijke Philips NV
Technical Solution: Philips has developed advanced bioresonance devices incorporating safety features and regulatory compliance measures. Their technology utilizes electromagnetic field therapy to analyze and treat various health conditions. The devices employ sophisticated sensors and algorithms to detect subtle energy imbalances in the body[1]. Philips has implemented multiple safety mechanisms, including automatic shut-off features and power output limitations, to ensure patient safety during use[3]. The company has also invested in clinical trials and regulatory submissions to validate the efficacy and safety of their bioresonance devices, working closely with regulatory bodies to address potential concerns[5].
Strengths: Strong R&D capabilities, established regulatory expertise, and global market presence. Weaknesses: High development costs and potential skepticism from traditional medical community.
Medtronic, Inc.
Technical Solution: Medtronic has developed bioresonance devices with a focus on integrating them into existing medical treatment protocols. Their approach combines bioresonance technology with traditional medical devices to enhance diagnostic accuracy and treatment efficacy. The company has implemented rigorous safety protocols, including real-time monitoring systems and fail-safe mechanisms, to address potential risks associated with electromagnetic field exposure[2]. Medtronic has also invested in extensive clinical trials to demonstrate the safety and effectiveness of their bioresonance devices, particularly in pain management and neurological applications[4]. The company actively engages with regulatory bodies to establish clear guidelines for bioresonance device usage in clinical settings.
Strengths: Extensive medical device experience, strong clinical trial infrastructure, and established relationships with healthcare providers. Weaknesses: Potential resistance from conservative medical practitioners and high regulatory hurdles.
Critical Safety Patents and Research
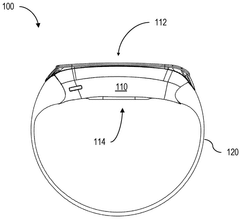
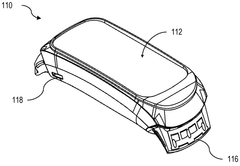
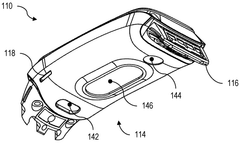
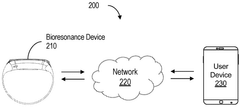
Device for bioresonance modulation of muscle temperature and blood oxygen levels
PatentWO2025151179A1
Innovation
- A bioresonance device equipped with a processor, temperature and optical sensors, and an electromagnetic frequency generator, capable of emitting frequencies based on real-time physiological data and remote instructions, allowing for dynamic and personalized bioresonance modalities.
Electronic bio-resonance device
PatentInactiveEP0885628A2
Innovation
- An electronic bioresonance device that uses a transmission coil as an antenna to emit amplified signals, allowing for non-invasive signal reception and transmission within a limited range of 1-2 meters, enabling signal distribution over a larger body area without direct skin contact, thereby reducing heart load and expanding therapeutic options.
Clinical Efficacy and Evidence Base
The clinical efficacy and evidence base for bioresonance devices remain highly controversial and largely unsubstantiated by rigorous scientific research. While proponents claim these devices can diagnose and treat a wide range of health conditions, the majority of mainstream medical organizations and regulatory bodies consider bioresonance therapy to be pseudoscientific and lacking credible evidence of effectiveness.
Systematic reviews of available clinical studies on bioresonance have consistently found insufficient high-quality evidence to support its use for any medical purpose. A 2015 review published in the European Journal of Integrative Medicine concluded that the evidence base for bioresonance diagnostic and therapeutic applications was "not sufficient to justify further research." The review noted significant methodological flaws in existing studies, including small sample sizes, lack of proper controls, and potential bias.
Some small-scale studies have reported positive outcomes for bioresonance in treating certain conditions like allergies, but these results have not been replicated in larger, well-designed clinical trials. A 2017 randomized controlled trial investigating bioresonance for smoking cessation found no significant difference between the treatment and control groups, contradicting earlier positive claims.
The lack of a plausible scientific mechanism of action further undermines the clinical credibility of bioresonance devices. The fundamental principles of bioresonance therapy, which involve detecting and manipulating purported electromagnetic oscillations of the body, are not supported by established biophysical principles or empirical evidence.
Notably, several professional medical associations have issued position statements explicitly rejecting bioresonance as a valid diagnostic or therapeutic tool. The American Academy of Allergy, Asthma & Immunology, for instance, states that bioresonance "has no proven efficacy" and warns that its use may lead to delayed diagnosis and treatment of genuine medical conditions.
Given the current state of evidence, regulatory bodies in many countries classify bioresonance devices as low-risk medical devices, if at all, and do not recognize them for diagnostic or therapeutic purposes. This regulatory stance reflects the overall lack of robust clinical evidence supporting the efficacy and safety of bioresonance technology in healthcare applications.
Systematic reviews of available clinical studies on bioresonance have consistently found insufficient high-quality evidence to support its use for any medical purpose. A 2015 review published in the European Journal of Integrative Medicine concluded that the evidence base for bioresonance diagnostic and therapeutic applications was "not sufficient to justify further research." The review noted significant methodological flaws in existing studies, including small sample sizes, lack of proper controls, and potential bias.
Some small-scale studies have reported positive outcomes for bioresonance in treating certain conditions like allergies, but these results have not been replicated in larger, well-designed clinical trials. A 2017 randomized controlled trial investigating bioresonance for smoking cessation found no significant difference between the treatment and control groups, contradicting earlier positive claims.
The lack of a plausible scientific mechanism of action further undermines the clinical credibility of bioresonance devices. The fundamental principles of bioresonance therapy, which involve detecting and manipulating purported electromagnetic oscillations of the body, are not supported by established biophysical principles or empirical evidence.
Notably, several professional medical associations have issued position statements explicitly rejecting bioresonance as a valid diagnostic or therapeutic tool. The American Academy of Allergy, Asthma & Immunology, for instance, states that bioresonance "has no proven efficacy" and warns that its use may lead to delayed diagnosis and treatment of genuine medical conditions.
Given the current state of evidence, regulatory bodies in many countries classify bioresonance devices as low-risk medical devices, if at all, and do not recognize them for diagnostic or therapeutic purposes. This regulatory stance reflects the overall lack of robust clinical evidence supporting the efficacy and safety of bioresonance technology in healthcare applications.
Consumer Protection and Education
Consumer protection and education play a crucial role in addressing the safety and regulatory issues surrounding bioresonance devices. As these devices gain popularity in alternative medicine circles, it is essential to ensure that consumers are well-informed about their potential risks and limitations.
One of the primary concerns in consumer protection is the lack of standardized regulations for bioresonance devices. Many of these devices are marketed as diagnostic or therapeutic tools without sufficient scientific evidence to support their claims. This regulatory gap leaves consumers vulnerable to misleading information and potentially harmful treatments. To address this issue, regulatory bodies must work towards establishing clear guidelines for the manufacturing, marketing, and use of bioresonance devices.
Education is equally important in empowering consumers to make informed decisions about bioresonance treatments. Many individuals may be unaware of the scientific controversies surrounding these devices and their unproven effectiveness. Developing comprehensive educational materials that explain the principles of bioresonance, its limitations, and potential risks is essential. These resources should be easily accessible to the public and written in clear, non-technical language.
Healthcare professionals also play a vital role in consumer protection and education. They should be equipped with up-to-date information about bioresonance devices to provide accurate guidance to their patients. This includes understanding the current scientific evidence, potential interactions with conventional treatments, and the importance of not replacing proven medical interventions with unverified alternative therapies.
Another critical aspect of consumer protection is the implementation of strict labeling requirements for bioresonance devices. These labels should clearly state that the devices are not approved for diagnostic or therapeutic purposes and include warnings about potential risks. Additionally, manufacturers should be required to provide comprehensive information about the device's intended use, limitations, and any known adverse effects.
Monitoring and reporting systems for adverse events related to bioresonance devices are also crucial for consumer safety. Establishing a centralized database for collecting and analyzing reports of side effects or complications can help identify potential safety issues and inform future regulatory decisions. This system should be easily accessible to both healthcare professionals and consumers.
In conclusion, effective consumer protection and education strategies are essential to address the safety and regulatory challenges posed by bioresonance devices. By implementing comprehensive regulations, providing clear and accessible information, and fostering collaboration between regulatory bodies, healthcare professionals, and consumers, we can create a safer environment for those considering or using these alternative therapies.
One of the primary concerns in consumer protection is the lack of standardized regulations for bioresonance devices. Many of these devices are marketed as diagnostic or therapeutic tools without sufficient scientific evidence to support their claims. This regulatory gap leaves consumers vulnerable to misleading information and potentially harmful treatments. To address this issue, regulatory bodies must work towards establishing clear guidelines for the manufacturing, marketing, and use of bioresonance devices.
Education is equally important in empowering consumers to make informed decisions about bioresonance treatments. Many individuals may be unaware of the scientific controversies surrounding these devices and their unproven effectiveness. Developing comprehensive educational materials that explain the principles of bioresonance, its limitations, and potential risks is essential. These resources should be easily accessible to the public and written in clear, non-technical language.
Healthcare professionals also play a vital role in consumer protection and education. They should be equipped with up-to-date information about bioresonance devices to provide accurate guidance to their patients. This includes understanding the current scientific evidence, potential interactions with conventional treatments, and the importance of not replacing proven medical interventions with unverified alternative therapies.
Another critical aspect of consumer protection is the implementation of strict labeling requirements for bioresonance devices. These labels should clearly state that the devices are not approved for diagnostic or therapeutic purposes and include warnings about potential risks. Additionally, manufacturers should be required to provide comprehensive information about the device's intended use, limitations, and any known adverse effects.
Monitoring and reporting systems for adverse events related to bioresonance devices are also crucial for consumer safety. Establishing a centralized database for collecting and analyzing reports of side effects or complications can help identify potential safety issues and inform future regulatory decisions. This system should be easily accessible to both healthcare professionals and consumers.
In conclusion, effective consumer protection and education strategies are essential to address the safety and regulatory challenges posed by bioresonance devices. By implementing comprehensive regulations, providing clear and accessible information, and fostering collaboration between regulatory bodies, healthcare professionals, and consumers, we can create a safer environment for those considering or using these alternative therapies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!