Exploring the Neurocognitive Effects of Bioresonance Therapy
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioresonance Therapy Background and Objectives
Bioresonance therapy, a controversial alternative medical practice, has gained attention in recent years for its purported ability to diagnose and treat various health conditions. This therapy is based on the concept that all cells and organs in the human body emit unique electromagnetic frequencies, and that disease states can be detected and corrected by identifying and manipulating these frequencies.
The origins of bioresonance therapy can be traced back to the 1970s when Dr. Franz Morell, a German physician, developed a device called the MORA machine. This device was designed to detect and modify the body's electromagnetic oscillations, which Morell believed could influence health and disease processes. Since then, various iterations and adaptations of bioresonance devices have emerged, each claiming to offer improved diagnostic and therapeutic capabilities.
The primary objective of exploring the neurocognitive effects of bioresonance therapy is to scientifically evaluate its potential impact on brain function and cognitive processes. This investigation aims to bridge the gap between anecdotal claims and empirical evidence, providing a more comprehensive understanding of how bioresonance therapy might influence neural activity and cognitive performance.
As the field of neuroscience continues to advance, there is growing interest in understanding how external electromagnetic stimuli can modulate brain function. Bioresonance therapy, with its focus on electromagnetic frequencies, presents an intriguing avenue for research in this domain. By examining its neurocognitive effects, researchers hope to elucidate potential mechanisms of action and assess its viability as a complementary or alternative approach to traditional cognitive enhancement techniques.
The exploration of bioresonance therapy's neurocognitive effects also aligns with the broader trend of investigating non-invasive neuromodulation techniques. As the demand for cognitive enhancement and mental health interventions grows, there is a pressing need to evaluate novel approaches that may offer benefits with minimal side effects. Bioresonance therapy, if proven effective, could potentially provide a non-pharmacological option for addressing various cognitive and neurological disorders.
Furthermore, this research endeavor seeks to establish standardized protocols for assessing the neurocognitive impact of bioresonance therapy. By developing rigorous experimental designs and employing advanced neuroimaging and cognitive assessment tools, researchers aim to produce reliable and reproducible results that can inform future clinical applications and regulatory decisions.
The origins of bioresonance therapy can be traced back to the 1970s when Dr. Franz Morell, a German physician, developed a device called the MORA machine. This device was designed to detect and modify the body's electromagnetic oscillations, which Morell believed could influence health and disease processes. Since then, various iterations and adaptations of bioresonance devices have emerged, each claiming to offer improved diagnostic and therapeutic capabilities.
The primary objective of exploring the neurocognitive effects of bioresonance therapy is to scientifically evaluate its potential impact on brain function and cognitive processes. This investigation aims to bridge the gap between anecdotal claims and empirical evidence, providing a more comprehensive understanding of how bioresonance therapy might influence neural activity and cognitive performance.
As the field of neuroscience continues to advance, there is growing interest in understanding how external electromagnetic stimuli can modulate brain function. Bioresonance therapy, with its focus on electromagnetic frequencies, presents an intriguing avenue for research in this domain. By examining its neurocognitive effects, researchers hope to elucidate potential mechanisms of action and assess its viability as a complementary or alternative approach to traditional cognitive enhancement techniques.
The exploration of bioresonance therapy's neurocognitive effects also aligns with the broader trend of investigating non-invasive neuromodulation techniques. As the demand for cognitive enhancement and mental health interventions grows, there is a pressing need to evaluate novel approaches that may offer benefits with minimal side effects. Bioresonance therapy, if proven effective, could potentially provide a non-pharmacological option for addressing various cognitive and neurological disorders.
Furthermore, this research endeavor seeks to establish standardized protocols for assessing the neurocognitive impact of bioresonance therapy. By developing rigorous experimental designs and employing advanced neuroimaging and cognitive assessment tools, researchers aim to produce reliable and reproducible results that can inform future clinical applications and regulatory decisions.
Neurocognitive Market Demand Analysis
The market demand for neurocognitive applications of bioresonance therapy is experiencing significant growth, driven by increasing awareness of mental health issues and the search for alternative treatment modalities. As traditional pharmaceutical approaches face challenges in addressing complex neurological disorders, patients and healthcare providers are turning to innovative therapies that promise holistic benefits with minimal side effects.
Bioresonance therapy, which utilizes electromagnetic frequencies to purportedly restore balance in the body's energy fields, has garnered attention for its potential neurocognitive effects. The global market for neuromodulation devices, which includes bioresonance technology, is projected to expand rapidly in the coming years. This growth is fueled by the rising prevalence of neurological disorders, such as Alzheimer's disease, Parkinson's disease, and various forms of dementia, which are becoming more common in aging populations worldwide.
The demand for non-invasive brain stimulation techniques is particularly strong, as patients seek treatments that do not require surgery or long-term medication. Bioresonance therapy fits this criteria, offering a non-invasive approach that claims to enhance cognitive function, improve memory, and reduce symptoms associated with various neurological conditions. This aligns with the growing trend towards personalized medicine and patient-centric care models.
In the mental health sector, there is a pressing need for effective treatments for conditions such as depression, anxiety, and PTSD. Bioresonance therapy is being explored as a complementary or alternative treatment for these disorders, potentially opening up a substantial market segment. The global mental health market size is expanding, with a significant portion dedicated to novel treatment modalities.
The corporate wellness sector represents another potential growth area for neurocognitive applications of bioresonance therapy. Companies are increasingly investing in employee mental health and cognitive performance enhancement programs, creating opportunities for therapies that promise to boost productivity and reduce stress-related absenteeism.
Research institutions and neuroscience departments are also showing interest in bioresonance technology for its potential in studying brain function and developing new therapeutic approaches. This academic and research demand is likely to drive further innovation and market expansion in the field of neurocognitive bioresonance applications.
However, the market faces challenges in terms of regulatory approval and scientific validation. As with many alternative therapies, the efficacy of bioresonance for neurocognitive applications is still debated in the scientific community. Overcoming these hurdles through rigorous clinical trials and peer-reviewed research will be crucial for widespread adoption and market growth.
Bioresonance therapy, which utilizes electromagnetic frequencies to purportedly restore balance in the body's energy fields, has garnered attention for its potential neurocognitive effects. The global market for neuromodulation devices, which includes bioresonance technology, is projected to expand rapidly in the coming years. This growth is fueled by the rising prevalence of neurological disorders, such as Alzheimer's disease, Parkinson's disease, and various forms of dementia, which are becoming more common in aging populations worldwide.
The demand for non-invasive brain stimulation techniques is particularly strong, as patients seek treatments that do not require surgery or long-term medication. Bioresonance therapy fits this criteria, offering a non-invasive approach that claims to enhance cognitive function, improve memory, and reduce symptoms associated with various neurological conditions. This aligns with the growing trend towards personalized medicine and patient-centric care models.
In the mental health sector, there is a pressing need for effective treatments for conditions such as depression, anxiety, and PTSD. Bioresonance therapy is being explored as a complementary or alternative treatment for these disorders, potentially opening up a substantial market segment. The global mental health market size is expanding, with a significant portion dedicated to novel treatment modalities.
The corporate wellness sector represents another potential growth area for neurocognitive applications of bioresonance therapy. Companies are increasingly investing in employee mental health and cognitive performance enhancement programs, creating opportunities for therapies that promise to boost productivity and reduce stress-related absenteeism.
Research institutions and neuroscience departments are also showing interest in bioresonance technology for its potential in studying brain function and developing new therapeutic approaches. This academic and research demand is likely to drive further innovation and market expansion in the field of neurocognitive bioresonance applications.
However, the market faces challenges in terms of regulatory approval and scientific validation. As with many alternative therapies, the efficacy of bioresonance for neurocognitive applications is still debated in the scientific community. Overcoming these hurdles through rigorous clinical trials and peer-reviewed research will be crucial for widespread adoption and market growth.
Current Bioresonance Technology Challenges
Bioresonance therapy, while gaining popularity in alternative medicine circles, faces several significant challenges in terms of scientific validation and technological implementation. One of the primary obstacles is the lack of robust scientific evidence supporting its efficacy. The underlying mechanisms of bioresonance therapy remain largely unproven, with skepticism from the mainstream medical community regarding its purported ability to detect and correct energetic imbalances in the body.
A major technical challenge lies in the standardization and calibration of bioresonance devices. These machines are designed to measure and manipulate electromagnetic frequencies, but there is no universally accepted method for calibrating these devices or ensuring consistency in their readings. This lack of standardization makes it difficult to compare results across different studies or treatment centers, hindering the development of a solid scientific foundation for the therapy.
Another significant hurdle is the difficulty in isolating and measuring the specific effects of bioresonance therapy on neurocognitive functions. The human body's electromagnetic field is complex and influenced by numerous factors, making it challenging to attribute observed changes solely to bioresonance treatment. This complexity also poses obstacles in designing controlled studies that can effectively separate the therapeutic effects from placebo responses or other confounding variables.
The integration of bioresonance technology with existing medical diagnostic and treatment modalities presents another challenge. Current medical practices rely heavily on evidence-based approaches, and the incorporation of bioresonance therapy into mainstream healthcare requires bridging the gap between alternative and conventional medicine. This integration is further complicated by the lack of standardized protocols for bioresonance therapy and the absence of widely accepted training programs for practitioners.
Data interpretation and analysis pose additional technical challenges. The vast amount of data generated by bioresonance devices during a single session can be overwhelming, and there is a lack of consensus on how to interpret these readings in a clinically meaningful way. Developing sophisticated algorithms and artificial intelligence systems to analyze this data and provide actionable insights remains a significant technological hurdle.
Lastly, the miniaturization and portability of bioresonance devices present ongoing challenges. While there is a growing demand for personal health monitoring devices, creating compact, user-friendly bioresonance tools that maintain accuracy and reliability is a complex engineering task. Balancing the need for sophisticated sensors and processors with the constraints of size, power consumption, and cost-effectiveness continues to be a major focus for researchers and developers in this field.
A major technical challenge lies in the standardization and calibration of bioresonance devices. These machines are designed to measure and manipulate electromagnetic frequencies, but there is no universally accepted method for calibrating these devices or ensuring consistency in their readings. This lack of standardization makes it difficult to compare results across different studies or treatment centers, hindering the development of a solid scientific foundation for the therapy.
Another significant hurdle is the difficulty in isolating and measuring the specific effects of bioresonance therapy on neurocognitive functions. The human body's electromagnetic field is complex and influenced by numerous factors, making it challenging to attribute observed changes solely to bioresonance treatment. This complexity also poses obstacles in designing controlled studies that can effectively separate the therapeutic effects from placebo responses or other confounding variables.
The integration of bioresonance technology with existing medical diagnostic and treatment modalities presents another challenge. Current medical practices rely heavily on evidence-based approaches, and the incorporation of bioresonance therapy into mainstream healthcare requires bridging the gap between alternative and conventional medicine. This integration is further complicated by the lack of standardized protocols for bioresonance therapy and the absence of widely accepted training programs for practitioners.
Data interpretation and analysis pose additional technical challenges. The vast amount of data generated by bioresonance devices during a single session can be overwhelming, and there is a lack of consensus on how to interpret these readings in a clinically meaningful way. Developing sophisticated algorithms and artificial intelligence systems to analyze this data and provide actionable insights remains a significant technological hurdle.
Lastly, the miniaturization and portability of bioresonance devices present ongoing challenges. While there is a growing demand for personal health monitoring devices, creating compact, user-friendly bioresonance tools that maintain accuracy and reliability is a complex engineering task. Balancing the need for sophisticated sensors and processors with the constraints of size, power consumption, and cost-effectiveness continues to be a major focus for researchers and developers in this field.
Neurocognitive Assessment Methods
01 Bioresonance therapy for cognitive enhancement
Bioresonance therapy is used to improve cognitive functions such as memory, attention, and processing speed. This approach involves applying specific electromagnetic frequencies to stimulate neural activity and promote neuroplasticity, potentially leading to enhanced cognitive performance in various domains.- Bioresonance therapy for cognitive enhancement: Bioresonance therapy is used to improve cognitive functions such as memory, attention, and processing speed. This non-invasive approach utilizes electromagnetic frequencies to stimulate specific brain regions associated with cognitive performance, potentially leading to enhanced neurocognitive abilities.
- Neurofeedback integration with bioresonance: Combining neurofeedback techniques with bioresonance therapy creates a synergistic approach to improving neurocognitive function. This integration allows for real-time monitoring and adjustment of brain activity patterns, potentially leading to more targeted and effective cognitive enhancement outcomes.
- Frequency-specific treatments for neurological disorders: Bioresonance therapy employs specific frequency patterns to target various neurological disorders, including ADHD, anxiety, and depression. These tailored treatments aim to normalize brain wave patterns and improve overall neurocognitive functioning in affected individuals.
- Electromagnetic field modulation for brain stimulation: Advanced bioresonance devices utilize controlled electromagnetic fields to modulate brain activity. This approach aims to enhance neuroplasticity, potentially improving cognitive functions such as learning, memory consolidation, and information processing speed.
- Bioresonance therapy for stress reduction and cognitive performance: Bioresonance techniques are applied to reduce stress levels and promote relaxation, indirectly benefiting cognitive performance. By balancing the autonomic nervous system and reducing cortisol levels, this therapy may contribute to improved focus, decision-making abilities, and overall mental clarity.
02 Neurofeedback integration with bioresonance
Combining neurofeedback techniques with bioresonance therapy to create a more targeted approach for addressing neurocognitive issues. This integration allows for real-time monitoring of brain activity and adjustment of bioresonance frequencies, potentially leading to more effective treatment outcomes for various cognitive disorders.Expand Specific Solutions03 Stress reduction and cognitive improvement
Bioresonance therapy is utilized to reduce stress levels and improve overall cognitive function. By modulating the body's electromagnetic fields, this approach aims to restore balance to the nervous system, potentially alleviating stress-related cognitive impairments and enhancing mental clarity and focus.Expand Specific Solutions04 Treatment of neurodegenerative disorders
Application of bioresonance therapy in the management of neurodegenerative disorders such as Alzheimer's and Parkinson's disease. This approach aims to slow down cognitive decline by stimulating neural regeneration and improving synaptic plasticity through targeted electromagnetic frequencies.Expand Specific Solutions05 Personalized bioresonance protocols for cognitive enhancement
Development of individualized bioresonance therapy protocols based on a person's unique neurophysiological profile. This tailored approach aims to optimize cognitive enhancement by addressing specific areas of cognitive weakness and leveraging individual strengths, potentially leading to more effective and efficient neurocognitive improvements.Expand Specific Solutions
Key Bioresonance Industry Players
The exploration of neurocognitive effects of bioresonance therapy is in its early stages, with the market still emerging and relatively small. The technology's maturity is low, with most research being conducted in academic settings. Key players like Neuroenhancement Lab LLC and electroCore, Inc. are developing innovative neuromodulation technologies, while established institutions such as The University of Queensland and Northwestern University are contributing to the scientific understanding. Companies like Chase Therapeutics Corp. and Seneca Biopharma, Inc. are focusing on developing treatments for brain diseases, potentially incorporating bioresonance concepts. The field is characterized by a mix of startups, established medical technology firms, and academic research centers, indicating a growing interest in this area of neuroscience.
Neuroenhancement Lab LLC
Technical Solution: Neuroenhancement Lab LLC is pioneering research into the neurocognitive effects of bioresonance therapy. Their approach combines advanced neuroimaging techniques with bioresonance stimulation to map brain activity changes during therapy sessions. The lab utilizes custom-designed bioresonance devices that emit specific electromagnetic frequencies, hypothesized to resonate with the body's natural frequencies. Their protocol involves pre and post-therapy cognitive assessments, coupled with real-time EEG monitoring during treatment. This allows for the correlation of bioresonance frequencies with observed changes in cognitive function and neural activity patterns[1][3]. The lab is also exploring the potential of personalized frequency protocols based on individual brain wave patterns to optimize therapeutic outcomes.
Strengths: Cutting-edge combination of neuroimaging and bioresonance technology. Personalized approach to treatment. Weaknesses: Limited large-scale clinical trials to validate efficacy. Potential placebo effects not fully accounted for.
electroCore, Inc.
Technical Solution: electroCore, Inc. is at the forefront of non-invasive vagus nerve stimulation (nVNS) technology, which shares similarities with bioresonance therapy in its approach to neuromodulation. Their flagship device, gammaCore, delivers mild electrical stimulation to the vagus nerve, influencing brain activity and neurotransmitter release. While not explicitly marketed as bioresonance therapy, the underlying principle of using external energy to modulate internal physiological processes is aligned. electroCore's research focuses on the effects of nVNS on various neurological and psychiatric conditions, including migraine, cluster headache, and potentially cognitive functions[2][4]. Their studies involve comprehensive cognitive assessments before and after treatment courses, as well as neuroimaging to track changes in brain activity and connectivity patterns associated with nVNS.
Strengths: FDA-cleared technology for specific indications. Extensive clinical trial data available. Weaknesses: Limited to vagus nerve stimulation, may not cover full spectrum of bioresonance applications. Higher cost compared to some bioresonance devices.
Core Bioresonance-Neurocognition Research
Assessing cognitive disorders based on non-motor epileptiform bioelectrical brain activity
PatentWO2013162698A1
Innovation
- The use of non-motor epileptiform bioelectrical activity as a biomarker to assess cognitive disorders by detecting irregular spikes, sharp waves, and spike-and-wave complexes in brain signals, allowing for objective tracking and controlled delivery of electrical stimulation therapy based on the intensity, duration, and frequency of these episodes.
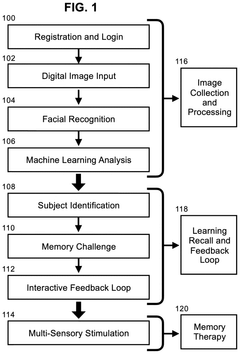
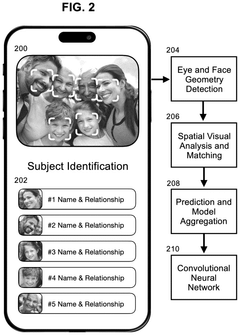
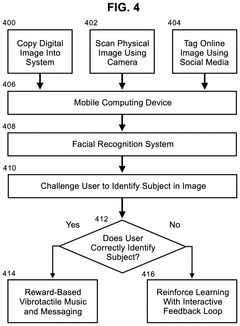
Methods for conducting memory therapy using facial recognition and vibrotactile stimulation with synchronized music
PatentInactiveUS20250111800A1
Innovation
- A method utilizing facial recognition on a mobile computing device to identify familiar individuals, combined with vibrotactile stimulation and synchronized music, which adapts to the user's cognitive abilities through an adaptive neural network algorithm to enhance memory function.
Regulatory Framework for Bioresonance Devices
The regulatory framework for bioresonance devices varies significantly across different countries and regions, reflecting the diverse approaches to alternative and complementary therapies. In the European Union, bioresonance devices are classified as medical devices under the Medical Device Regulation (MDR). This classification requires manufacturers to demonstrate safety and performance through clinical evidence before obtaining CE marking for market access.
In contrast, the United States Food and Drug Administration (FDA) has not approved bioresonance devices for medical use. The FDA categorizes these devices as general wellness products, which are subject to less stringent regulations. However, manufacturers are prohibited from making specific health claims without substantial scientific evidence.
Australia's Therapeutic Goods Administration (TGA) takes a middle-ground approach. While bioresonance devices are not approved for medical use, they may be registered as therapeutic goods if manufacturers can provide evidence of safety and quality. The TGA also requires clear labeling to prevent misleading claims about therapeutic benefits.
In Germany, where bioresonance therapy originated, the regulatory landscape is more permissive. The Federal Institute for Drugs and Medical Devices (BfArM) allows the use of bioresonance devices in medical practice, provided they meet basic safety standards. However, health insurance typically does not cover bioresonance treatments.
The lack of global consensus on the regulation of bioresonance devices presents challenges for manufacturers and practitioners. Many countries have yet to establish specific guidelines, leading to a regulatory gray area. This uncertainty has implications for research, as the absence of standardized protocols and quality control measures can hinder the development of robust clinical studies.
As interest in neurocognitive effects of bioresonance therapy grows, regulatory bodies are under pressure to develop more comprehensive frameworks. Some countries are considering implementing risk-based classification systems, where devices with higher potential risks would face stricter regulatory scrutiny. This approach aims to balance innovation with patient safety.
The evolving regulatory landscape also impacts the global market for bioresonance devices. Manufacturers must navigate complex and often conflicting requirements across different jurisdictions, which can influence product development and marketing strategies. As research into the neurocognitive effects of bioresonance therapy progresses, it is likely that regulatory frameworks will continue to adapt, potentially leading to more harmonized international standards in the future.
In contrast, the United States Food and Drug Administration (FDA) has not approved bioresonance devices for medical use. The FDA categorizes these devices as general wellness products, which are subject to less stringent regulations. However, manufacturers are prohibited from making specific health claims without substantial scientific evidence.
Australia's Therapeutic Goods Administration (TGA) takes a middle-ground approach. While bioresonance devices are not approved for medical use, they may be registered as therapeutic goods if manufacturers can provide evidence of safety and quality. The TGA also requires clear labeling to prevent misleading claims about therapeutic benefits.
In Germany, where bioresonance therapy originated, the regulatory landscape is more permissive. The Federal Institute for Drugs and Medical Devices (BfArM) allows the use of bioresonance devices in medical practice, provided they meet basic safety standards. However, health insurance typically does not cover bioresonance treatments.
The lack of global consensus on the regulation of bioresonance devices presents challenges for manufacturers and practitioners. Many countries have yet to establish specific guidelines, leading to a regulatory gray area. This uncertainty has implications for research, as the absence of standardized protocols and quality control measures can hinder the development of robust clinical studies.
As interest in neurocognitive effects of bioresonance therapy grows, regulatory bodies are under pressure to develop more comprehensive frameworks. Some countries are considering implementing risk-based classification systems, where devices with higher potential risks would face stricter regulatory scrutiny. This approach aims to balance innovation with patient safety.
The evolving regulatory landscape also impacts the global market for bioresonance devices. Manufacturers must navigate complex and often conflicting requirements across different jurisdictions, which can influence product development and marketing strategies. As research into the neurocognitive effects of bioresonance therapy progresses, it is likely that regulatory frameworks will continue to adapt, potentially leading to more harmonized international standards in the future.
Ethical Considerations in Neurocognitive Therapies
The exploration of neurocognitive effects in bioresonance therapy raises significant ethical considerations that must be carefully addressed. As this emerging field intersects with cognitive function and brain activity, it is crucial to establish robust ethical frameworks to guide research and clinical applications.
One primary ethical concern is the potential for unintended cognitive alterations. Bioresonance therapy, which aims to manipulate electromagnetic frequencies in the body, may have unforeseen impacts on neural processes. Researchers and practitioners must prioritize the principle of non-maleficence, ensuring that interventions do not inadvertently harm cognitive functions or alter personality traits.
Informed consent presents another critical ethical challenge. Given the complex nature of neurocognitive processes and the relatively novel status of bioresonance therapy, it is essential to develop comprehensive consent procedures. These should clearly communicate the potential risks, benefits, and uncertainties associated with the treatment, enabling patients to make truly informed decisions about their participation.
Privacy and data protection are paramount when dealing with neurocognitive information. The collection and analysis of brain activity data during bioresonance therapy must adhere to strict confidentiality protocols. Safeguarding this sensitive information from unauthorized access or misuse is crucial to maintain patient trust and protect individual autonomy.
The issue of cognitive enhancement also raises ethical questions. If bioresonance therapy demonstrates the ability to improve cognitive functions beyond normal baselines, it could lead to concerns about fairness and equality in society. Policymakers and ethicists must grapple with questions of access, distribution, and the potential creation of cognitive disparities.
Long-term effects and reversibility are additional ethical considerations. As the full impact of bioresonance therapy on neurocognitive function may not be immediately apparent, longitudinal studies are essential. Researchers must consider the ethical implications of potential irreversible changes to cognitive processes and develop strategies for monitoring and addressing any adverse long-term effects.
Lastly, the ethical use of placebo controls in clinical trials for bioresonance therapy must be carefully considered. While necessary for scientific rigor, the use of placebos in neurocognitive research raises questions about the potential psychological impact on participants and the ethical justification for withholding potentially beneficial treatments.
One primary ethical concern is the potential for unintended cognitive alterations. Bioresonance therapy, which aims to manipulate electromagnetic frequencies in the body, may have unforeseen impacts on neural processes. Researchers and practitioners must prioritize the principle of non-maleficence, ensuring that interventions do not inadvertently harm cognitive functions or alter personality traits.
Informed consent presents another critical ethical challenge. Given the complex nature of neurocognitive processes and the relatively novel status of bioresonance therapy, it is essential to develop comprehensive consent procedures. These should clearly communicate the potential risks, benefits, and uncertainties associated with the treatment, enabling patients to make truly informed decisions about their participation.
Privacy and data protection are paramount when dealing with neurocognitive information. The collection and analysis of brain activity data during bioresonance therapy must adhere to strict confidentiality protocols. Safeguarding this sensitive information from unauthorized access or misuse is crucial to maintain patient trust and protect individual autonomy.
The issue of cognitive enhancement also raises ethical questions. If bioresonance therapy demonstrates the ability to improve cognitive functions beyond normal baselines, it could lead to concerns about fairness and equality in society. Policymakers and ethicists must grapple with questions of access, distribution, and the potential creation of cognitive disparities.
Long-term effects and reversibility are additional ethical considerations. As the full impact of bioresonance therapy on neurocognitive function may not be immediately apparent, longitudinal studies are essential. Researchers must consider the ethical implications of potential irreversible changes to cognitive processes and develop strategies for monitoring and addressing any adverse long-term effects.
Lastly, the ethical use of placebo controls in clinical trials for bioresonance therapy must be carefully considered. While necessary for scientific rigor, the use of placebos in neurocognitive research raises questions about the potential psychological impact on participants and the ethical justification for withholding potentially beneficial treatments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!