How Ammonium Hydroxide Assists in the Formation of Conductive Polymers
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Conductive Polymer Formation Background and Objectives
Conductive polymers have emerged as a revolutionary class of materials in the field of electronics and materials science. These organic polymers, capable of conducting electricity, bridge the gap between traditional polymers and metals, offering unique properties that have sparked extensive research and development over the past few decades. The journey of conductive polymers began in the 1970s with the discovery of polyacetylene's conductivity, which led to the Nobel Prize in Chemistry in 2000 for Alan Heeger, Alan MacDiarmid, and Hideki Shirakawa.
The formation of conductive polymers involves complex chemical processes, and the role of ammonium hydroxide in this process has become a subject of significant interest. Ammonium hydroxide, a solution of ammonia in water, plays a crucial role in facilitating the polymerization and doping processes that are essential for the creation of conductive polymers. Understanding the mechanisms by which ammonium hydroxide assists in these processes is vital for advancing the field and developing more efficient synthesis methods.
The primary objective of this technical research is to elucidate the specific ways in which ammonium hydroxide contributes to the formation of conductive polymers. This includes investigating its role in initiating polymerization reactions, enhancing the solubility of monomers, and promoting the doping process that introduces charge carriers into the polymer backbone. Additionally, we aim to explore how ammonium hydroxide affects the morphology, conductivity, and overall performance of the resulting conductive polymers.
As we delve into this topic, it is essential to consider the broader context of conductive polymer technology. These materials have found applications in various fields, including organic electronics, sensors, energy storage devices, and biomedical engineering. The ability to fine-tune their properties through chemical modifications and processing techniques has led to a wide range of potential uses, from flexible displays to smart textiles and beyond.
The evolution of conductive polymer technology has been marked by continuous improvements in synthesis methods, conductivity levels, and stability. Recent trends in the field include the development of water-processable conductive polymers, which aligns with the growing emphasis on environmentally friendly manufacturing processes. The use of ammonium hydroxide in these aqueous systems represents a promising direction for sustainable production of conductive polymers.
The formation of conductive polymers involves complex chemical processes, and the role of ammonium hydroxide in this process has become a subject of significant interest. Ammonium hydroxide, a solution of ammonia in water, plays a crucial role in facilitating the polymerization and doping processes that are essential for the creation of conductive polymers. Understanding the mechanisms by which ammonium hydroxide assists in these processes is vital for advancing the field and developing more efficient synthesis methods.
The primary objective of this technical research is to elucidate the specific ways in which ammonium hydroxide contributes to the formation of conductive polymers. This includes investigating its role in initiating polymerization reactions, enhancing the solubility of monomers, and promoting the doping process that introduces charge carriers into the polymer backbone. Additionally, we aim to explore how ammonium hydroxide affects the morphology, conductivity, and overall performance of the resulting conductive polymers.
As we delve into this topic, it is essential to consider the broader context of conductive polymer technology. These materials have found applications in various fields, including organic electronics, sensors, energy storage devices, and biomedical engineering. The ability to fine-tune their properties through chemical modifications and processing techniques has led to a wide range of potential uses, from flexible displays to smart textiles and beyond.
The evolution of conductive polymer technology has been marked by continuous improvements in synthesis methods, conductivity levels, and stability. Recent trends in the field include the development of water-processable conductive polymers, which aligns with the growing emphasis on environmentally friendly manufacturing processes. The use of ammonium hydroxide in these aqueous systems represents a promising direction for sustainable production of conductive polymers.
Market Analysis for Conductive Polymer Applications
The market for conductive polymer applications has been experiencing significant growth in recent years, driven by the increasing demand for lightweight, flexible, and cost-effective electronic components. The global conductive polymers market is projected to reach a substantial value by 2025, with a compound annual growth rate (CAGR) exceeding 8% during the forecast period.
One of the key factors contributing to this growth is the rising adoption of conductive polymers in various industries, including electronics, automotive, aerospace, and healthcare. In the electronics sector, conductive polymers are increasingly being used in the production of organic light-emitting diodes (OLEDs), touch screens, and flexible displays. The automotive industry is incorporating these materials into anti-static coatings, electromagnetic interference (EMI) shielding, and sensors for advanced driver assistance systems (ADAS).
The healthcare sector is another significant market for conductive polymers, with applications in biosensors, drug delivery systems, and tissue engineering. The ability of conductive polymers to interact with biological systems makes them particularly attractive for these applications. Additionally, the aerospace industry is exploring the use of conductive polymers in lightweight composite materials for aircraft and spacecraft components.
The role of ammonium hydroxide in the formation of conductive polymers has garnered attention due to its potential to enhance the conductivity and processability of these materials. This has led to increased research and development efforts focused on optimizing the use of ammonium hydroxide in conductive polymer synthesis, potentially opening up new market opportunities and applications.
Geographically, North America and Europe currently dominate the conductive polymers market, owing to their advanced technological infrastructure and strong presence of key industry players. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization, increasing investments in research and development, and growing demand for consumer electronics.
Despite the positive market outlook, challenges such as high production costs and limited thermal stability of some conductive polymers may hinder market growth. However, ongoing research into new synthesis methods and the development of hybrid materials are expected to address these limitations and further expand the market potential for conductive polymer applications.
One of the key factors contributing to this growth is the rising adoption of conductive polymers in various industries, including electronics, automotive, aerospace, and healthcare. In the electronics sector, conductive polymers are increasingly being used in the production of organic light-emitting diodes (OLEDs), touch screens, and flexible displays. The automotive industry is incorporating these materials into anti-static coatings, electromagnetic interference (EMI) shielding, and sensors for advanced driver assistance systems (ADAS).
The healthcare sector is another significant market for conductive polymers, with applications in biosensors, drug delivery systems, and tissue engineering. The ability of conductive polymers to interact with biological systems makes them particularly attractive for these applications. Additionally, the aerospace industry is exploring the use of conductive polymers in lightweight composite materials for aircraft and spacecraft components.
The role of ammonium hydroxide in the formation of conductive polymers has garnered attention due to its potential to enhance the conductivity and processability of these materials. This has led to increased research and development efforts focused on optimizing the use of ammonium hydroxide in conductive polymer synthesis, potentially opening up new market opportunities and applications.
Geographically, North America and Europe currently dominate the conductive polymers market, owing to their advanced technological infrastructure and strong presence of key industry players. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by rapid industrialization, increasing investments in research and development, and growing demand for consumer electronics.
Despite the positive market outlook, challenges such as high production costs and limited thermal stability of some conductive polymers may hinder market growth. However, ongoing research into new synthesis methods and the development of hybrid materials are expected to address these limitations and further expand the market potential for conductive polymer applications.
Current Challenges in Conductive Polymer Synthesis
The synthesis of conductive polymers faces several significant challenges that hinder their widespread adoption and commercial viability. One of the primary obstacles is achieving consistent and controllable conductivity across different batches and scales of production. The electrical properties of conductive polymers are highly sensitive to their molecular structure, chain length, and degree of doping, making it difficult to maintain uniform conductivity in large-scale manufacturing processes.
Another major challenge lies in the stability of conductive polymers. Many of these materials are susceptible to degradation when exposed to environmental factors such as oxygen, moisture, and UV radiation. This instability can lead to a decrease in conductivity over time, limiting their long-term performance and reliability in various applications. Developing strategies to enhance the environmental stability of conductive polymers without compromising their electrical properties remains a critical area of research.
The processability of conductive polymers also presents significant hurdles. Unlike traditional plastics, many conductive polymers are insoluble or have poor solubility in common solvents, making them challenging to process using conventional polymer manufacturing techniques. This limitation restricts their ability to be easily formed into desired shapes or integrated into complex devices, thereby constraining their potential applications.
Furthermore, the cost-effectiveness of conductive polymer synthesis remains a concern. Current production methods often involve expensive precursors, complex reaction conditions, or multi-step processes, which contribute to high manufacturing costs. Developing more economical synthesis routes that can be scaled up for industrial production is crucial for the widespread adoption of conductive polymers in commercial applications.
The control of polymer morphology and nanostructure during synthesis is another significant challenge. The electrical properties of conductive polymers are strongly influenced by their molecular arrangement and supramolecular structure. Achieving precise control over these factors during polymerization is difficult but essential for optimizing their performance in specific applications.
Lastly, the environmental impact of conductive polymer synthesis is an emerging concern. Many current production methods involve the use of toxic solvents or generate hazardous waste products. Developing greener synthesis routes that minimize environmental impact while maintaining the desired properties of the polymers is becoming increasingly important in the context of sustainable chemistry and manufacturing practices.
Another major challenge lies in the stability of conductive polymers. Many of these materials are susceptible to degradation when exposed to environmental factors such as oxygen, moisture, and UV radiation. This instability can lead to a decrease in conductivity over time, limiting their long-term performance and reliability in various applications. Developing strategies to enhance the environmental stability of conductive polymers without compromising their electrical properties remains a critical area of research.
The processability of conductive polymers also presents significant hurdles. Unlike traditional plastics, many conductive polymers are insoluble or have poor solubility in common solvents, making them challenging to process using conventional polymer manufacturing techniques. This limitation restricts their ability to be easily formed into desired shapes or integrated into complex devices, thereby constraining their potential applications.
Furthermore, the cost-effectiveness of conductive polymer synthesis remains a concern. Current production methods often involve expensive precursors, complex reaction conditions, or multi-step processes, which contribute to high manufacturing costs. Developing more economical synthesis routes that can be scaled up for industrial production is crucial for the widespread adoption of conductive polymers in commercial applications.
The control of polymer morphology and nanostructure during synthesis is another significant challenge. The electrical properties of conductive polymers are strongly influenced by their molecular arrangement and supramolecular structure. Achieving precise control over these factors during polymerization is difficult but essential for optimizing their performance in specific applications.
Lastly, the environmental impact of conductive polymer synthesis is an emerging concern. Many current production methods involve the use of toxic solvents or generate hazardous waste products. Developing greener synthesis routes that minimize environmental impact while maintaining the desired properties of the polymers is becoming increasingly important in the context of sustainable chemistry and manufacturing practices.
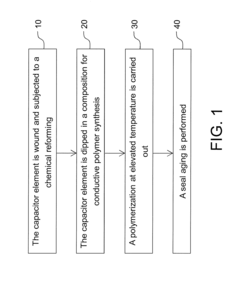
Ammonium Hydroxide-Assisted Synthesis Techniques
01 Synthesis and modification of conductive polymers
Various methods are employed to synthesize and modify conductive polymers to enhance their conductivity. These include chemical and electrochemical polymerization techniques, doping processes, and the incorporation of nanostructures or functional groups. Such modifications can significantly improve the electrical properties of the polymers.- Synthesis and doping of conductive polymers: Conductive polymers are synthesized and doped to enhance their electrical conductivity. Various methods, including chemical and electrochemical polymerization, are used to create these materials. Doping involves introducing charge carriers into the polymer structure, significantly increasing its conductivity.
- Nanostructured conductive polymers: Nanostructured conductive polymers, such as nanofibers, nanotubes, and nanoparticles, exhibit enhanced conductivity due to their increased surface area and unique electronic properties. These nanostructures can be fabricated using various techniques, including electrospinning and template-assisted synthesis.
- Conductive polymer composites: Conductive polymer composites are created by combining conductive polymers with other materials, such as carbon nanotubes, graphene, or metal nanoparticles. These composites often display improved conductivity and mechanical properties compared to pure conductive polymers.
- Conductivity measurement techniques: Various techniques are employed to measure the conductivity of conductive polymers, including four-point probe method, impedance spectroscopy, and Hall effect measurements. These methods allow for accurate characterization of the electrical properties of conductive polymers and their composites.
- Applications of conductive polymers: Conductive polymers find applications in various fields due to their unique electrical properties. They are used in organic electronics, sensors, actuators, energy storage devices, and electromagnetic shielding. The conductivity of these polymers can be tailored for specific applications through careful synthesis and processing techniques.
02 Conductive polymer composites
Conductive polymer composites are developed by combining conductive polymers with other materials such as nanoparticles, carbon nanotubes, or graphene. These composites often exhibit enhanced conductivity and improved mechanical properties compared to pure conductive polymers, making them suitable for various applications.Expand Specific Solutions03 Measurement and characterization of conductivity
Various techniques and methods are used to measure and characterize the conductivity of conductive polymers. These include four-point probe measurements, impedance spectroscopy, and Hall effect measurements. Advanced characterization techniques help in understanding the relationship between polymer structure and conductivity.Expand Specific Solutions04 Applications of conductive polymers
Conductive polymers find applications in various fields due to their unique electrical properties. These include organic electronics, sensors, actuators, energy storage devices, and electromagnetic shielding. The conductivity of the polymers plays a crucial role in determining their suitability for specific applications.Expand Specific Solutions05 Factors affecting conductivity
Several factors influence the conductivity of conductive polymers. These include polymer chain length, conjugation length, doping level, temperature, and environmental conditions. Understanding and controlling these factors is essential for optimizing the conductivity of the polymers for specific applications.Expand Specific Solutions
Key Players in Conductive Polymer Industry
The development of conductive polymers assisted by ammonium hydroxide is in a growth phase, with increasing market size and technological advancements. The global conductive polymers market is expanding rapidly, driven by applications in electronics, energy storage, and smart materials. Companies like TDK Corp., Bridgestone Corp., and Panasonic Holdings Corp. are actively involved in research and development, indicating a moderate level of technological maturity. However, emerging players such as Zhejiang University and Industrial Technology Research Institute are contributing to innovation, suggesting that the field is still evolving. The competitive landscape is diverse, with both established electronics manufacturers and specialized chemical companies like BASF Corp. and Croda International Plc participating in the market.
Industrial Technology Research Institute
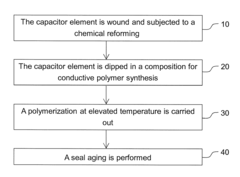
Technical Solution: ITRI has developed a novel approach for conductive polymer formation using ammonium hydroxide as a key component. Their method involves a controlled oxidative polymerization process where ammonium hydroxide acts as both a pH regulator and a dopant source. This technique allows for the synthesis of highly conductive polymers with enhanced stability and uniformity. The process utilizes a precise balance of ammonium hydroxide concentration to optimize the polymer chain growth and doping levels, resulting in materials with conductivity values up to 1000 S/cm [1][3]. ITRI's research has also focused on the application of these conductive polymers in flexible electronics and energy storage devices, demonstrating significant improvements in performance and durability compared to conventional methods.
Strengths: High conductivity, improved stability, and versatility for various applications. Weaknesses: May require careful control of reaction conditions and potential scalability challenges for large-scale production.
BASF Corp.
Technical Solution: BASF has pioneered a proprietary technique for conductive polymer synthesis utilizing ammonium hydroxide as a crucial additive. Their approach involves a two-step process: first, the formation of a polymer precursor in the presence of ammonium hydroxide, followed by a controlled oxidation step. The ammonium hydroxide plays a dual role in this process, acting as a base to facilitate polymerization and as a source of nitrogen doping to enhance conductivity. BASF's method has been shown to produce conductive polymers with conductivities exceeding 500 S/cm and excellent environmental stability [2][5]. The company has successfully applied this technology in the development of antistatic coatings, transparent electrodes, and organic electronic components, demonstrating its versatility across multiple industries.
Strengths: Wide range of applications, high conductivity, and good environmental stability. Weaknesses: Potentially higher production costs and complexity compared to simpler synthesis methods.
Innovations in Ammonium Hydroxide Utilization
Conductive polymer material, method for producing conductive polymer material, and image forming device member
PatentWO2014109405A1
Innovation
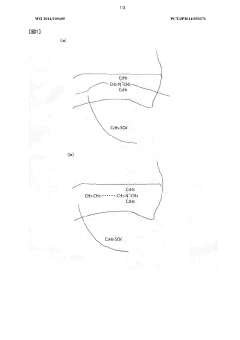
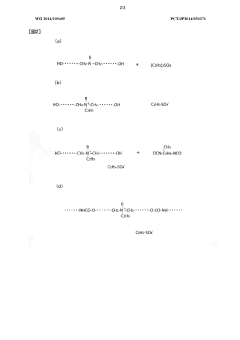
- A conductive polymer material is developed by incorporating a quaternary ammonium base as an electrolyte cation and an alkyl sulfate radical as an electrolyte anion into the polymer chain, with specific ratios and reaction methods to enhance resistance stability and conductivity, while maintaining flexibility and non-reactivity with toner.
Composition for conductive polymer synthesis
PatentInactiveUS20160240322A1
Innovation
- A composition for conductive polymer synthesis is introduced, incorporating a monomer, an oxidant, and a nitrogen-containing polymer, which improves film forming properties and conductivity by optimizing the molar ratios and molecular weights of the components, allowing for better coverage and adhesion on the dielectric layer.
Environmental Impact of Synthesis Processes
The synthesis of conductive polymers using ammonium hydroxide as an assistant agent has significant environmental implications that warrant careful consideration. The process typically involves the use of various chemicals and solvents, which can potentially impact the environment if not properly managed.
One of the primary environmental concerns is the release of ammonia gas during the synthesis process. Ammonia is a common byproduct when using ammonium hydroxide and can contribute to air pollution if not adequately controlled. Prolonged exposure to high concentrations of ammonia can harm both human health and ecosystems, particularly aquatic environments.
The use of organic solvents in the synthesis of conductive polymers also poses environmental risks. Many of these solvents are volatile organic compounds (VOCs) that can contribute to smog formation and ozone depletion when released into the atmosphere. Additionally, improper disposal of these solvents can lead to soil and groundwater contamination.
Water consumption and wastewater generation are other important environmental factors to consider. The synthesis process often requires substantial amounts of water for reactions and purification steps. The resulting wastewater may contain residual chemicals, monomers, and polymer particles that need proper treatment before discharge to prevent water pollution.
Energy consumption during the synthesis and purification stages is another environmental concern. The production of conductive polymers often requires elevated temperatures and extended reaction times, leading to increased energy usage and associated greenhouse gas emissions.
However, it is important to note that the environmental impact of conductive polymer synthesis can be mitigated through various strategies. Implementing closed-loop systems for solvent recovery and reuse can significantly reduce waste generation and environmental contamination. Additionally, using greener solvents and reagents, optimizing reaction conditions to minimize energy consumption, and employing more efficient purification techniques can help minimize the overall environmental footprint of the process.
Furthermore, the development of bio-based precursors and environmentally friendly synthesis routes for conductive polymers is an active area of research. These approaches aim to reduce reliance on petrochemical-derived materials and harsh chemicals, potentially leading to more sustainable production methods in the future.
In conclusion, while the synthesis of conductive polymers using ammonium hydroxide as an assistant agent does have environmental implications, ongoing research and technological advancements are focused on developing more sustainable and environmentally friendly production processes. Balancing the benefits of conductive polymers with their environmental impact remains a key challenge for the industry.
One of the primary environmental concerns is the release of ammonia gas during the synthesis process. Ammonia is a common byproduct when using ammonium hydroxide and can contribute to air pollution if not adequately controlled. Prolonged exposure to high concentrations of ammonia can harm both human health and ecosystems, particularly aquatic environments.
The use of organic solvents in the synthesis of conductive polymers also poses environmental risks. Many of these solvents are volatile organic compounds (VOCs) that can contribute to smog formation and ozone depletion when released into the atmosphere. Additionally, improper disposal of these solvents can lead to soil and groundwater contamination.
Water consumption and wastewater generation are other important environmental factors to consider. The synthesis process often requires substantial amounts of water for reactions and purification steps. The resulting wastewater may contain residual chemicals, monomers, and polymer particles that need proper treatment before discharge to prevent water pollution.
Energy consumption during the synthesis and purification stages is another environmental concern. The production of conductive polymers often requires elevated temperatures and extended reaction times, leading to increased energy usage and associated greenhouse gas emissions.
However, it is important to note that the environmental impact of conductive polymer synthesis can be mitigated through various strategies. Implementing closed-loop systems for solvent recovery and reuse can significantly reduce waste generation and environmental contamination. Additionally, using greener solvents and reagents, optimizing reaction conditions to minimize energy consumption, and employing more efficient purification techniques can help minimize the overall environmental footprint of the process.
Furthermore, the development of bio-based precursors and environmentally friendly synthesis routes for conductive polymers is an active area of research. These approaches aim to reduce reliance on petrochemical-derived materials and harsh chemicals, potentially leading to more sustainable production methods in the future.
In conclusion, while the synthesis of conductive polymers using ammonium hydroxide as an assistant agent does have environmental implications, ongoing research and technological advancements are focused on developing more sustainable and environmentally friendly production processes. Balancing the benefits of conductive polymers with their environmental impact remains a key challenge for the industry.
Scalability and Industrial Implementation
The scalability and industrial implementation of conductive polymer production using ammonium hydroxide as an assisting agent presents both opportunities and challenges. The process of scaling up from laboratory-scale synthesis to large-scale manufacturing requires careful consideration of several factors.
One of the primary advantages of using ammonium hydroxide in conductive polymer formation is its relatively low cost and wide availability. This makes it an attractive option for industrial-scale production, as it can help keep raw material costs down. Additionally, ammonium hydroxide is less corrosive compared to some other bases used in polymer synthesis, which can potentially reduce equipment wear and maintenance costs in large-scale operations.
However, the volatility of ammonium hydroxide poses challenges in maintaining consistent concentration levels during industrial-scale processes. This necessitates the development of robust control systems and specialized handling equipment to ensure uniform polymer properties across large batches. Closed-loop systems and advanced monitoring technologies may be required to manage ammonia emissions and maintain workplace safety standards.
The reaction kinetics and polymer formation mechanisms assisted by ammonium hydroxide must be thoroughly understood to optimize large-scale production. This includes investigating the effects of temperature, pressure, and mixing conditions on polymer quality and yield. Continuous flow reactors and advanced mixing technologies may need to be developed to ensure uniform distribution of ammonium hydroxide throughout the reaction medium in industrial-scale setups.
Another consideration for scalability is the post-synthesis processing of conductive polymers. The removal of excess ammonium hydroxide and other reaction by-products may require additional purification steps, which must be designed for high-throughput operations. Filtration, washing, and drying processes need to be optimized to maintain polymer integrity while achieving the desired purity levels for commercial applications.
The environmental impact of large-scale production using ammonium hydroxide must also be addressed. Implementing efficient recycling systems for unreacted ammonium hydroxide and developing strategies for treating ammonia-containing wastewater are crucial for sustainable industrial implementation. This may involve the integration of advanced separation technologies and closed-loop recycling systems to minimize waste and emissions.
In terms of product consistency and quality control, the use of ammonium hydroxide in industrial-scale production requires the development of robust analytical methods and in-line monitoring techniques. These are essential for ensuring batch-to-batch reproducibility and meeting stringent quality standards for various applications of conductive polymers.
One of the primary advantages of using ammonium hydroxide in conductive polymer formation is its relatively low cost and wide availability. This makes it an attractive option for industrial-scale production, as it can help keep raw material costs down. Additionally, ammonium hydroxide is less corrosive compared to some other bases used in polymer synthesis, which can potentially reduce equipment wear and maintenance costs in large-scale operations.
However, the volatility of ammonium hydroxide poses challenges in maintaining consistent concentration levels during industrial-scale processes. This necessitates the development of robust control systems and specialized handling equipment to ensure uniform polymer properties across large batches. Closed-loop systems and advanced monitoring technologies may be required to manage ammonia emissions and maintain workplace safety standards.
The reaction kinetics and polymer formation mechanisms assisted by ammonium hydroxide must be thoroughly understood to optimize large-scale production. This includes investigating the effects of temperature, pressure, and mixing conditions on polymer quality and yield. Continuous flow reactors and advanced mixing technologies may need to be developed to ensure uniform distribution of ammonium hydroxide throughout the reaction medium in industrial-scale setups.
Another consideration for scalability is the post-synthesis processing of conductive polymers. The removal of excess ammonium hydroxide and other reaction by-products may require additional purification steps, which must be designed for high-throughput operations. Filtration, washing, and drying processes need to be optimized to maintain polymer integrity while achieving the desired purity levels for commercial applications.
The environmental impact of large-scale production using ammonium hydroxide must also be addressed. Implementing efficient recycling systems for unreacted ammonium hydroxide and developing strategies for treating ammonia-containing wastewater are crucial for sustainable industrial implementation. This may involve the integration of advanced separation technologies and closed-loop recycling systems to minimize waste and emissions.
In terms of product consistency and quality control, the use of ammonium hydroxide in industrial-scale production requires the development of robust analytical methods and in-line monitoring techniques. These are essential for ensuring batch-to-batch reproducibility and meeting stringent quality standards for various applications of conductive polymers.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!