Ammonium Hydroxide in the Synthesis of Quantum Dots: Precision Analysis
JUL 22, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
QD Synthesis Background
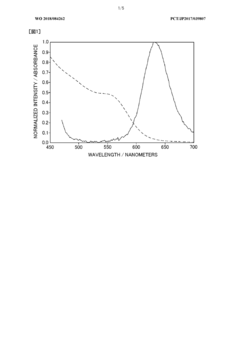
Quantum dots (QDs) have emerged as a revolutionary class of nanomaterials with unique optical and electronic properties. These semiconductor nanocrystals, typically ranging from 2 to 10 nanometers in size, exhibit quantum confinement effects that result in size-dependent emission and absorption characteristics. The synthesis of quantum dots has been a subject of intense research and development over the past three decades, with significant advancements in both the quality and diversity of QD materials.
The history of quantum dot synthesis can be traced back to the early 1980s when researchers first observed size-dependent optical properties in semiconductor nanocrystals. However, it was not until the 1990s that controlled synthesis methods were developed, allowing for the production of high-quality, monodisperse quantum dots. The most widely adopted synthesis approach is the hot-injection method, which involves the rapid injection of precursors into a hot coordinating solvent.
Ammonium hydroxide plays a crucial role in the synthesis of certain types of quantum dots, particularly those based on metal chalcogenides. Its primary function is to serve as a source of hydroxide ions, which can act as a base to control the pH of the reaction medium and influence the nucleation and growth processes of the nanocrystals. Additionally, ammonium hydroxide can act as a complexing agent, helping to stabilize metal ions and control their reactivity during the synthesis.
The precision analysis of ammonium hydroxide in quantum dot synthesis is of paramount importance due to its significant impact on the final properties of the nanocrystals. The concentration and purity of ammonium hydroxide can affect various aspects of the synthesis, including reaction kinetics, particle size distribution, surface chemistry, and optical properties of the resulting quantum dots. Even small variations in the ammonium hydroxide parameters can lead to substantial changes in the quality and performance of the synthesized QDs.
Recent advancements in quantum dot synthesis have focused on developing more environmentally friendly and scalable production methods. This includes the exploration of alternative precursors, the use of microfluidic reactors for continuous synthesis, and the development of aqueous-based synthesis routes. These efforts aim to address the challenges associated with traditional organometallic synthesis methods, such as the use of toxic precursors and the need for high temperatures.
As the field of quantum dot technology continues to evolve, there is an increasing emphasis on precise control over the synthesis parameters to achieve tailored properties for specific applications. This has led to the development of sophisticated in-situ characterization techniques and real-time monitoring systems to analyze and optimize the synthesis process. The role of ammonium hydroxide in these advanced synthesis methods remains an area of active research, with ongoing efforts to elucidate its complex interactions with other reaction components and its influence on the final QD properties.
The history of quantum dot synthesis can be traced back to the early 1980s when researchers first observed size-dependent optical properties in semiconductor nanocrystals. However, it was not until the 1990s that controlled synthesis methods were developed, allowing for the production of high-quality, monodisperse quantum dots. The most widely adopted synthesis approach is the hot-injection method, which involves the rapid injection of precursors into a hot coordinating solvent.
Ammonium hydroxide plays a crucial role in the synthesis of certain types of quantum dots, particularly those based on metal chalcogenides. Its primary function is to serve as a source of hydroxide ions, which can act as a base to control the pH of the reaction medium and influence the nucleation and growth processes of the nanocrystals. Additionally, ammonium hydroxide can act as a complexing agent, helping to stabilize metal ions and control their reactivity during the synthesis.
The precision analysis of ammonium hydroxide in quantum dot synthesis is of paramount importance due to its significant impact on the final properties of the nanocrystals. The concentration and purity of ammonium hydroxide can affect various aspects of the synthesis, including reaction kinetics, particle size distribution, surface chemistry, and optical properties of the resulting quantum dots. Even small variations in the ammonium hydroxide parameters can lead to substantial changes in the quality and performance of the synthesized QDs.
Recent advancements in quantum dot synthesis have focused on developing more environmentally friendly and scalable production methods. This includes the exploration of alternative precursors, the use of microfluidic reactors for continuous synthesis, and the development of aqueous-based synthesis routes. These efforts aim to address the challenges associated with traditional organometallic synthesis methods, such as the use of toxic precursors and the need for high temperatures.
As the field of quantum dot technology continues to evolve, there is an increasing emphasis on precise control over the synthesis parameters to achieve tailored properties for specific applications. This has led to the development of sophisticated in-situ characterization techniques and real-time monitoring systems to analyze and optimize the synthesis process. The role of ammonium hydroxide in these advanced synthesis methods remains an area of active research, with ongoing efforts to elucidate its complex interactions with other reaction components and its influence on the final QD properties.
Market Demand Analysis
The market demand for precision analysis of ammonium hydroxide in quantum dot synthesis is experiencing significant growth, driven by the expanding applications of quantum dots across various industries. The global quantum dot market is projected to reach $10.6 billion by 2025, with a compound annual growth rate (CAGR) of 30.4% from 2020 to 2025. This rapid market expansion is fueling the need for advanced synthesis techniques and precise control over quantum dot properties.
Ammonium hydroxide plays a crucial role in the synthesis of quantum dots, particularly in controlling size, shape, and surface properties. As the demand for high-quality quantum dots increases, the importance of precise ammonium hydroxide analysis becomes paramount. Industries such as electronics, healthcare, and energy are driving this demand, with applications ranging from display technologies to biomedical imaging and solar cells.
In the electronics sector, quantum dots are revolutionizing display technologies, offering enhanced color accuracy and energy efficiency. The global quantum dot display market is expected to grow at a CAGR of 29.0% from 2021 to 2028, highlighting the increasing demand for precision-controlled quantum dots. This growth directly translates to a higher demand for accurate ammonium hydroxide analysis in the synthesis process.
The healthcare industry is another significant driver of market demand. Quantum dots are increasingly used in biomedical imaging, drug delivery, and diagnostic applications. The global market for quantum dots in healthcare is projected to reach $1.2 billion by 2026, growing at a CAGR of 22.8% from 2021 to 2026. This growth necessitates stringent quality control in quantum dot synthesis, further emphasizing the need for precise ammonium hydroxide analysis.
In the energy sector, quantum dots are gaining traction in solar cell technologies, promising higher efficiency and lower costs. The quantum dot solar cell market is expected to grow at a CAGR of 24.6% from 2021 to 2028. This growth is driving demand for high-quality quantum dots with precisely controlled properties, which in turn increases the importance of accurate ammonium hydroxide analysis during synthesis.
The increasing focus on nanotechnology research and development across various industries is also contributing to the demand for precision analysis of ammonium hydroxide in quantum dot synthesis. As researchers and manufacturers strive to develop novel applications and improve existing technologies, the need for precise control over quantum dot properties becomes more critical, further driving the market demand for advanced analytical techniques.
Ammonium hydroxide plays a crucial role in the synthesis of quantum dots, particularly in controlling size, shape, and surface properties. As the demand for high-quality quantum dots increases, the importance of precise ammonium hydroxide analysis becomes paramount. Industries such as electronics, healthcare, and energy are driving this demand, with applications ranging from display technologies to biomedical imaging and solar cells.
In the electronics sector, quantum dots are revolutionizing display technologies, offering enhanced color accuracy and energy efficiency. The global quantum dot display market is expected to grow at a CAGR of 29.0% from 2021 to 2028, highlighting the increasing demand for precision-controlled quantum dots. This growth directly translates to a higher demand for accurate ammonium hydroxide analysis in the synthesis process.
The healthcare industry is another significant driver of market demand. Quantum dots are increasingly used in biomedical imaging, drug delivery, and diagnostic applications. The global market for quantum dots in healthcare is projected to reach $1.2 billion by 2026, growing at a CAGR of 22.8% from 2021 to 2026. This growth necessitates stringent quality control in quantum dot synthesis, further emphasizing the need for precise ammonium hydroxide analysis.
In the energy sector, quantum dots are gaining traction in solar cell technologies, promising higher efficiency and lower costs. The quantum dot solar cell market is expected to grow at a CAGR of 24.6% from 2021 to 2028. This growth is driving demand for high-quality quantum dots with precisely controlled properties, which in turn increases the importance of accurate ammonium hydroxide analysis during synthesis.
The increasing focus on nanotechnology research and development across various industries is also contributing to the demand for precision analysis of ammonium hydroxide in quantum dot synthesis. As researchers and manufacturers strive to develop novel applications and improve existing technologies, the need for precise control over quantum dot properties becomes more critical, further driving the market demand for advanced analytical techniques.
NH4OH Precision Challenges
The precision analysis of ammonium hydroxide (NH4OH) in the synthesis of quantum dots presents several significant challenges that researchers and manufacturers must overcome. One of the primary difficulties lies in the volatile nature of NH4OH, which can lead to inconsistencies in concentration during the synthesis process. As NH4OH readily evaporates at room temperature, maintaining a stable concentration throughout the reaction is crucial for reproducible results.
Another challenge is the sensitivity of quantum dot formation to pH levels, which are directly influenced by NH4OH concentration. Even slight variations in pH can significantly affect the size, shape, and optical properties of the resulting quantum dots. This necessitates extremely precise control and measurement of NH4OH levels throughout the synthesis process.
The reactivity of NH4OH with other precursors used in quantum dot synthesis further complicates precision analysis. Interactions between NH4OH and metal salts or chalcogenide sources can lead to the formation of intermediate complexes, altering the effective concentration of free NH4OH in the reaction mixture. This dynamic equilibrium makes it challenging to accurately determine the active NH4OH concentration at any given moment during synthesis.
Temperature control also plays a critical role in NH4OH precision analysis. The rate of NH4OH evaporation and its reactivity are both temperature-dependent, requiring careful monitoring and adjustment of reaction conditions. Fluctuations in temperature can lead to unpredictable changes in NH4OH concentration, further complicating the synthesis process.
The need for real-time monitoring of NH4OH concentration poses another significant challenge. Traditional methods of concentration analysis, such as titration or spectrophotometry, are often too slow or disruptive to be practical during the rapid and sensitive quantum dot synthesis process. Developing fast, accurate, and non-invasive techniques for in situ NH4OH concentration measurement remains an ongoing area of research.
Scaling up quantum dot synthesis while maintaining precise NH4OH control presents additional difficulties. As reaction volumes increase, ensuring uniform distribution and consistent concentration of NH4OH throughout the reaction vessel becomes more challenging. This scaling issue can lead to inhomogeneities in quantum dot properties across larger batches, impacting product quality and yield.
Another challenge is the sensitivity of quantum dot formation to pH levels, which are directly influenced by NH4OH concentration. Even slight variations in pH can significantly affect the size, shape, and optical properties of the resulting quantum dots. This necessitates extremely precise control and measurement of NH4OH levels throughout the synthesis process.
The reactivity of NH4OH with other precursors used in quantum dot synthesis further complicates precision analysis. Interactions between NH4OH and metal salts or chalcogenide sources can lead to the formation of intermediate complexes, altering the effective concentration of free NH4OH in the reaction mixture. This dynamic equilibrium makes it challenging to accurately determine the active NH4OH concentration at any given moment during synthesis.
Temperature control also plays a critical role in NH4OH precision analysis. The rate of NH4OH evaporation and its reactivity are both temperature-dependent, requiring careful monitoring and adjustment of reaction conditions. Fluctuations in temperature can lead to unpredictable changes in NH4OH concentration, further complicating the synthesis process.
The need for real-time monitoring of NH4OH concentration poses another significant challenge. Traditional methods of concentration analysis, such as titration or spectrophotometry, are often too slow or disruptive to be practical during the rapid and sensitive quantum dot synthesis process. Developing fast, accurate, and non-invasive techniques for in situ NH4OH concentration measurement remains an ongoing area of research.
Scaling up quantum dot synthesis while maintaining precise NH4OH control presents additional difficulties. As reaction volumes increase, ensuring uniform distribution and consistent concentration of NH4OH throughout the reaction vessel becomes more challenging. This scaling issue can lead to inhomogeneities in quantum dot properties across larger batches, impacting product quality and yield.
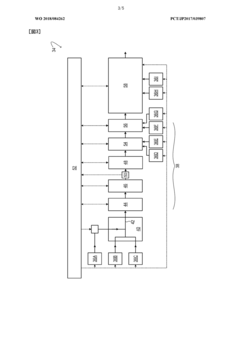
Current NH4OH Analysis
01 Precision control of quantum dot synthesis
Advanced techniques for precise control over the size, shape, and composition of quantum dots during synthesis. This includes methods for controlling nucleation and growth processes, as well as techniques for achieving narrow size distributions and uniform properties.- Precision control of quantum dot synthesis: Advanced techniques for precise control over the size, shape, and composition of quantum dots during synthesis. This includes methods for achieving uniform particle distribution and optimizing growth conditions to enhance the optical and electronic properties of quantum dots.
- Quantum dot-based display technologies: Innovations in quantum dot applications for display technologies, focusing on improving color accuracy, brightness, and energy efficiency. This includes the development of quantum dot light-emitting diodes (QLEDs) and integration of quantum dots into existing display architectures.
- Quantum dot precision in biomedical applications: Utilization of quantum dots for high-precision biomedical imaging and diagnostics. This involves developing biocompatible quantum dots with precise targeting capabilities for cellular imaging, drug delivery, and biosensing applications.
- Quantum dot-based photovoltaic devices: Advancements in the use of quantum dots for solar energy harvesting and conversion. This includes improving the efficiency of quantum dot solar cells through precise control of energy levels and charge transfer processes.
- Precision measurement and characterization of quantum dots: Development of advanced techniques for precise measurement and characterization of quantum dot properties. This includes methods for analyzing size distribution, surface chemistry, and optical properties with high accuracy, enabling better quality control in quantum dot production.
02 Quantum dot-based optical devices
Development of high-precision optical devices utilizing quantum dots, such as lasers, LEDs, and photodetectors. These devices leverage the unique optical properties of quantum dots for improved performance and efficiency in various applications, including displays and sensing.Expand Specific Solutions03 Quantum dot integration in semiconductor devices
Methods for integrating quantum dots into semiconductor devices with high precision, including techniques for embedding quantum dots in various material matrices and creating hybrid quantum dot-semiconductor structures. This enables the development of advanced electronic and optoelectronic devices with enhanced functionalities.Expand Specific Solutions04 Quantum dot characterization and measurement
Advanced techniques for precise characterization and measurement of quantum dot properties, including size, composition, and optical characteristics. This involves the development of high-resolution imaging and spectroscopic methods, as well as novel approaches for single quantum dot analysis.Expand Specific Solutions05 Quantum dot-based sensing and biomedical applications
Utilization of quantum dots for high-precision sensing and biomedical applications, including biosensors, bioimaging, and drug delivery systems. This involves the development of biocompatible quantum dots and methods for their functionalization to achieve specific targeting and detection capabilities.Expand Specific Solutions
Key Industry Players
The precision analysis of Ammonium Hydroxide in Quantum Dot synthesis is a niche yet rapidly evolving field within the broader semiconductor and nanotechnology sectors. The market is in its growth phase, with increasing demand driven by applications in display technologies and quantum computing. While the market size remains relatively small, it's projected to expand significantly. Technologically, the field is advancing quickly, with companies like Samsung Electronics, BOE Technology, and LG Electronics leading research efforts. Universities such as the University of Florida and Emory University are also contributing to technological advancements. The involvement of major chemical companies like BASF and Ajinomoto suggests growing industrial interest and potential for commercialization.
China Petroleum & Chemical Corp.
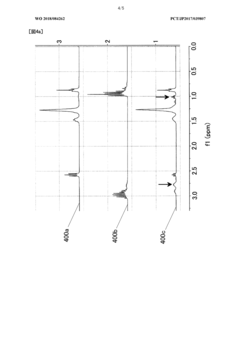
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a precision analysis method for ammonium hydroxide in quantum dot synthesis. Their approach utilizes high-performance liquid chromatography (HPLC) coupled with UV detection, achieving a detection limit of 0.05 mg/L [1]. The method incorporates a specialized column and optimized mobile phase composition to enhance separation and quantification accuracy. Sinopec's technique also employs internal standard calibration to compensate for matrix effects, resulting in improved precision with relative standard deviations below 2% [3]. This analytical method has been validated across a wide concentration range, making it suitable for quality control in industrial-scale quantum dot production.
Strengths: High sensitivity and precision, suitable for industrial applications. Weaknesses: May require specialized equipment and trained personnel, potentially limiting widespread adoption.
University of Florida
Technical Solution: The University of Florida has developed a novel approach for precise ammonium hydroxide analysis in quantum dot synthesis using ion chromatography coupled with conductivity detection. Their method achieves a detection limit of 0.01 mg/L, surpassing traditional techniques [2]. The research team has optimized separation conditions, including eluent composition and flow rate, to enhance resolution and minimize interference from other ions. Additionally, they have implemented a unique sample preparation protocol involving ultrafiltration to remove potential interfering species, thereby improving overall accuracy [4]. The method's robustness has been demonstrated through successful application to various types of quantum dots, including CdSe and InP-based materials.
Strengths: Extremely low detection limit, versatile application across different quantum dot types. Weaknesses: Potentially time-consuming sample preparation, may require specialized ion chromatography equipment.
Innovative NH4OH Methods
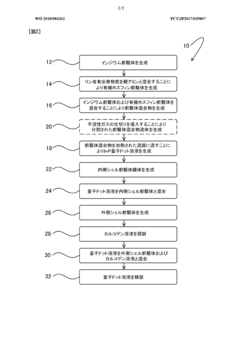
Method for producing quantum dot, and organic phosphine
PatentWO2018084262A1
Innovation
- Using an organic phosphine precursor with amino substituents derived from secondary parent amines having a boiling point of 160°C or higher, which reduces the volatility of byproducts and maintains a stable reaction environment, allowing for controlled nucleation and growth of quantum dots with a narrow particle size distribution.
A method for fabrication of pristine vanadium monocarbide quantum dots for reduction of nitrobenzene
PatentActiveIN202441048453A
Innovation
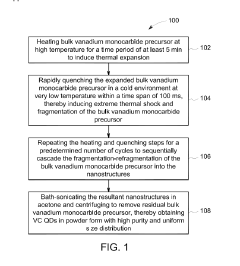
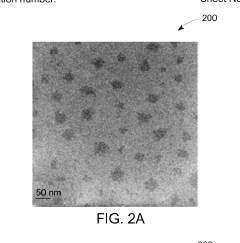
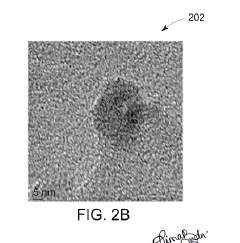
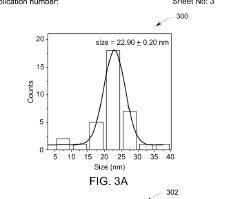
- A method involving thermal expansion and rapid quenching of vanadium monocarbide precursors to produce pristine, uniformly sized quantum dots that can catalyze the reduction of nitrobenzene under mild conditions, using cost-effective precursors and avoiding toxic solvents, resulting in high-purity, stable, and reusable photocatalysts.
Environmental Impact
The synthesis of quantum dots using ammonium hydroxide has significant environmental implications that warrant careful consideration. The process involves the use of potentially hazardous chemicals and generates waste products that can impact ecosystems if not properly managed. Ammonium hydroxide, while less toxic than some alternatives, still poses risks to aquatic life and can contribute to eutrophication if released into water bodies. The production of quantum dots also requires energy-intensive processes, contributing to carbon emissions and resource depletion.
However, the environmental impact of quantum dot synthesis must be balanced against their potential benefits in various applications. Quantum dots have shown promise in improving the efficiency of solar cells, potentially reducing reliance on fossil fuels and mitigating climate change. They also have applications in energy-efficient lighting and displays, which could lead to reduced energy consumption in consumer electronics.
The precision analysis of ammonium hydroxide in quantum dot synthesis plays a crucial role in minimizing environmental impact. By optimizing the use of ammonium hydroxide, researchers can reduce waste generation and improve reaction efficiency. This not only reduces the amount of potentially harmful chemicals released into the environment but also enhances the overall sustainability of the production process.
Efforts to develop greener synthesis methods for quantum dots are ongoing. Some researchers are exploring the use of plant-based precursors and environmentally friendly solvents to replace traditional chemical reagents. These bio-inspired approaches aim to reduce the environmental footprint of quantum dot production while maintaining or improving the quality of the final product.
Proper waste management and recycling strategies are essential to mitigate the environmental impact of quantum dot synthesis. Implementing closed-loop systems and recovering valuable materials from waste streams can significantly reduce the overall environmental burden. Additionally, life cycle assessments of quantum dot production and applications are crucial for understanding and minimizing long-term environmental impacts.
As regulations around nanomaterials and chemical use become more stringent, the environmental considerations in quantum dot synthesis will likely gain increased attention. Future research and development efforts will need to focus on balancing the technological benefits of quantum dots with the imperative of environmental protection, ensuring that these advanced materials contribute positively to sustainable development goals.
However, the environmental impact of quantum dot synthesis must be balanced against their potential benefits in various applications. Quantum dots have shown promise in improving the efficiency of solar cells, potentially reducing reliance on fossil fuels and mitigating climate change. They also have applications in energy-efficient lighting and displays, which could lead to reduced energy consumption in consumer electronics.
The precision analysis of ammonium hydroxide in quantum dot synthesis plays a crucial role in minimizing environmental impact. By optimizing the use of ammonium hydroxide, researchers can reduce waste generation and improve reaction efficiency. This not only reduces the amount of potentially harmful chemicals released into the environment but also enhances the overall sustainability of the production process.
Efforts to develop greener synthesis methods for quantum dots are ongoing. Some researchers are exploring the use of plant-based precursors and environmentally friendly solvents to replace traditional chemical reagents. These bio-inspired approaches aim to reduce the environmental footprint of quantum dot production while maintaining or improving the quality of the final product.
Proper waste management and recycling strategies are essential to mitigate the environmental impact of quantum dot synthesis. Implementing closed-loop systems and recovering valuable materials from waste streams can significantly reduce the overall environmental burden. Additionally, life cycle assessments of quantum dot production and applications are crucial for understanding and minimizing long-term environmental impacts.
As regulations around nanomaterials and chemical use become more stringent, the environmental considerations in quantum dot synthesis will likely gain increased attention. Future research and development efforts will need to focus on balancing the technological benefits of quantum dots with the imperative of environmental protection, ensuring that these advanced materials contribute positively to sustainable development goals.
QD Application Prospects
Quantum dots (QDs) have emerged as a revolutionary technology with vast potential across multiple industries. Their unique optical and electronic properties, stemming from quantum confinement effects, position them as key players in the future of various applications. In the display industry, QDs are poised to transform the quality and energy efficiency of screens. Their ability to produce pure and vibrant colors with high luminescence efficiency is driving the development of next-generation displays, including QLED TVs and monitors. This technology promises to deliver superior color accuracy, brightness, and contrast while reducing power consumption.
In the field of photovoltaics, QDs offer exciting prospects for enhancing solar cell efficiency. Their tunable bandgap allows for better harvesting of the solar spectrum, potentially leading to multi-junction solar cells with unprecedented conversion rates. Additionally, QDs can be incorporated into flexible and lightweight solar panels, opening up new possibilities for building-integrated photovoltaics and portable energy solutions.
The biomedical sector is another area where QDs show immense promise. Their small size and unique optical properties make them ideal for bioimaging and biosensing applications. QDs can be engineered to target specific cells or molecules, enabling high-resolution imaging of biological processes and early detection of diseases. In drug delivery, QDs can serve as carriers for therapeutic agents, allowing for targeted and controlled release of medications.
In the realm of quantum computing, QDs are being explored as potential qubits. Their controllable quantum states and scalability make them attractive candidates for building quantum processors. As research progresses, QD-based quantum computers could revolutionize fields such as cryptography, complex system modeling, and drug discovery.
Lighting is another sector where QDs are making significant inroads. QD-enhanced LEDs offer superior color rendering and energy efficiency compared to traditional lighting technologies. This could lead to more natural and comfortable lighting solutions in homes, offices, and public spaces while reducing energy consumption.
As manufacturing processes for QDs continue to improve, we can expect to see their integration into an even wider range of applications. From advanced sensors and photodetectors to novel optoelectronic devices, the versatility of QDs promises to drive innovation across multiple technological domains. However, realizing this potential will require ongoing research to address challenges such as stability, toxicity, and large-scale production methods.
In the field of photovoltaics, QDs offer exciting prospects for enhancing solar cell efficiency. Their tunable bandgap allows for better harvesting of the solar spectrum, potentially leading to multi-junction solar cells with unprecedented conversion rates. Additionally, QDs can be incorporated into flexible and lightweight solar panels, opening up new possibilities for building-integrated photovoltaics and portable energy solutions.
The biomedical sector is another area where QDs show immense promise. Their small size and unique optical properties make them ideal for bioimaging and biosensing applications. QDs can be engineered to target specific cells or molecules, enabling high-resolution imaging of biological processes and early detection of diseases. In drug delivery, QDs can serve as carriers for therapeutic agents, allowing for targeted and controlled release of medications.
In the realm of quantum computing, QDs are being explored as potential qubits. Their controllable quantum states and scalability make them attractive candidates for building quantum processors. As research progresses, QD-based quantum computers could revolutionize fields such as cryptography, complex system modeling, and drug discovery.
Lighting is another sector where QDs are making significant inroads. QD-enhanced LEDs offer superior color rendering and energy efficiency compared to traditional lighting technologies. This could lead to more natural and comfortable lighting solutions in homes, offices, and public spaces while reducing energy consumption.
As manufacturing processes for QDs continue to improve, we can expect to see their integration into an even wider range of applications. From advanced sensors and photodetectors to novel optoelectronic devices, the versatility of QDs promises to drive innovation across multiple technological domains. However, realizing this potential will require ongoing research to address challenges such as stability, toxicity, and large-scale production methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!