Role of Ammonium Hydroxide in Industrial Scale Biopolymer Synthesis
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biopolymer Synthesis Background and Objectives
Biopolymer synthesis has emerged as a critical field in the quest for sustainable and environmentally friendly materials. The evolution of this technology can be traced back to the mid-20th century when scientists began exploring alternatives to petroleum-based polymers. Over the decades, biopolymer synthesis has progressed from laboratory-scale experiments to industrial-scale production, driven by the growing demand for biodegradable and renewable materials.
The primary objective of biopolymer synthesis is to create polymeric materials from biological sources, such as plants, animals, or microorganisms. These biopolymers offer numerous advantages over traditional synthetic polymers, including biodegradability, biocompatibility, and reduced carbon footprint. As environmental concerns and sustainability initiatives gain prominence, the importance of biopolymer synthesis in various industries has increased significantly.
Ammonium hydroxide plays a crucial role in industrial-scale biopolymer synthesis, particularly in the production of certain types of biopolymers. Its alkaline properties make it an essential component in various synthesis processes, acting as a pH regulator, catalyst, or reactant. The use of ammonium hydroxide in biopolymer synthesis has evolved alongside advancements in biotechnology and chemical engineering, enabling more efficient and cost-effective production methods.
The technological progression in biopolymer synthesis has been marked by several key milestones. These include the development of fermentation processes for producing biopolymers, the discovery of new bacterial strains capable of synthesizing specific biopolymers, and the optimization of extraction and purification techniques. Each of these advancements has contributed to the scalability and economic viability of biopolymer production.
Current research in biopolymer synthesis focuses on improving yield, reducing production costs, and enhancing the properties of the final products. Scientists and engineers are exploring novel feedstocks, developing more efficient catalysts, and investigating innovative processing techniques. The role of ammonium hydroxide in these emerging technologies is being continuously evaluated and optimized to maximize its benefits while minimizing any potential environmental impacts.
As the field of biopolymer synthesis continues to evolve, the integration of sustainable practices and green chemistry principles has become a central theme. Researchers are increasingly focusing on developing processes that minimize waste, reduce energy consumption, and utilize renewable resources. In this context, the use of ammonium hydroxide and other chemicals is being scrutinized to ensure alignment with sustainability goals.
The future of biopolymer synthesis, including the role of ammonium hydroxide, is likely to be shaped by advancements in biotechnology, materials science, and process engineering. As we move forward, the objectives of this field will continue to expand, encompassing not only the creation of sustainable materials but also the development of biopolymers with enhanced functionalities and applications across diverse industries.
The primary objective of biopolymer synthesis is to create polymeric materials from biological sources, such as plants, animals, or microorganisms. These biopolymers offer numerous advantages over traditional synthetic polymers, including biodegradability, biocompatibility, and reduced carbon footprint. As environmental concerns and sustainability initiatives gain prominence, the importance of biopolymer synthesis in various industries has increased significantly.
Ammonium hydroxide plays a crucial role in industrial-scale biopolymer synthesis, particularly in the production of certain types of biopolymers. Its alkaline properties make it an essential component in various synthesis processes, acting as a pH regulator, catalyst, or reactant. The use of ammonium hydroxide in biopolymer synthesis has evolved alongside advancements in biotechnology and chemical engineering, enabling more efficient and cost-effective production methods.
The technological progression in biopolymer synthesis has been marked by several key milestones. These include the development of fermentation processes for producing biopolymers, the discovery of new bacterial strains capable of synthesizing specific biopolymers, and the optimization of extraction and purification techniques. Each of these advancements has contributed to the scalability and economic viability of biopolymer production.
Current research in biopolymer synthesis focuses on improving yield, reducing production costs, and enhancing the properties of the final products. Scientists and engineers are exploring novel feedstocks, developing more efficient catalysts, and investigating innovative processing techniques. The role of ammonium hydroxide in these emerging technologies is being continuously evaluated and optimized to maximize its benefits while minimizing any potential environmental impacts.
As the field of biopolymer synthesis continues to evolve, the integration of sustainable practices and green chemistry principles has become a central theme. Researchers are increasingly focusing on developing processes that minimize waste, reduce energy consumption, and utilize renewable resources. In this context, the use of ammonium hydroxide and other chemicals is being scrutinized to ensure alignment with sustainability goals.
The future of biopolymer synthesis, including the role of ammonium hydroxide, is likely to be shaped by advancements in biotechnology, materials science, and process engineering. As we move forward, the objectives of this field will continue to expand, encompassing not only the creation of sustainable materials but also the development of biopolymers with enhanced functionalities and applications across diverse industries.
Market Analysis for Industrial Biopolymers
The industrial biopolymer market has experienced significant growth in recent years, driven by increasing environmental concerns and the shift towards sustainable materials. The global market for industrial biopolymers was valued at approximately $6.5 billion in 2020 and is projected to reach $18.7 billion by 2027, growing at a CAGR of 16.2% during the forecast period.
The demand for biopolymers is particularly strong in packaging, automotive, and consumer goods industries. In the packaging sector, which accounts for the largest market share, biopolymers are increasingly used as alternatives to conventional plastics due to their biodegradability and reduced carbon footprint. The automotive industry is adopting biopolymers for interior components and lightweight parts to improve fuel efficiency and meet stringent environmental regulations.
Regionally, Europe leads the industrial biopolymer market, followed by North America and Asia-Pacific. Europe's dominance is attributed to strict regulations on single-use plastics and a strong focus on circular economy initiatives. The Asia-Pacific region is expected to witness the highest growth rate, driven by rapid industrialization, increasing environmental awareness, and supportive government policies in countries like China and India.
Key players in the industrial biopolymer market include NatureWorks LLC, Novamont S.p.A., BASF SE, and Total Corbion PLA. These companies are investing heavily in research and development to improve the performance and cost-effectiveness of biopolymers. Collaborations between biopolymer producers and end-use industries are becoming increasingly common to develop tailored solutions for specific applications.
The role of ammonium hydroxide in industrial-scale biopolymer synthesis is gaining attention as manufacturers seek to optimize production processes. Ammonium hydroxide serves as a pH regulator and catalyst in various biopolymer synthesis reactions, particularly in the production of chitosan and certain types of polyhydroxyalkanoates (PHAs). Its use can enhance reaction efficiency, improve product quality, and potentially reduce production costs.
However, challenges remain in the widespread adoption of industrial biopolymers. These include higher production costs compared to conventional polymers, limitations in performance characteristics for certain applications, and the need for specialized waste management infrastructure. Despite these challenges, the market outlook remains positive, driven by increasing consumer demand for eco-friendly products and stringent environmental regulations worldwide.
The demand for biopolymers is particularly strong in packaging, automotive, and consumer goods industries. In the packaging sector, which accounts for the largest market share, biopolymers are increasingly used as alternatives to conventional plastics due to their biodegradability and reduced carbon footprint. The automotive industry is adopting biopolymers for interior components and lightweight parts to improve fuel efficiency and meet stringent environmental regulations.
Regionally, Europe leads the industrial biopolymer market, followed by North America and Asia-Pacific. Europe's dominance is attributed to strict regulations on single-use plastics and a strong focus on circular economy initiatives. The Asia-Pacific region is expected to witness the highest growth rate, driven by rapid industrialization, increasing environmental awareness, and supportive government policies in countries like China and India.
Key players in the industrial biopolymer market include NatureWorks LLC, Novamont S.p.A., BASF SE, and Total Corbion PLA. These companies are investing heavily in research and development to improve the performance and cost-effectiveness of biopolymers. Collaborations between biopolymer producers and end-use industries are becoming increasingly common to develop tailored solutions for specific applications.
The role of ammonium hydroxide in industrial-scale biopolymer synthesis is gaining attention as manufacturers seek to optimize production processes. Ammonium hydroxide serves as a pH regulator and catalyst in various biopolymer synthesis reactions, particularly in the production of chitosan and certain types of polyhydroxyalkanoates (PHAs). Its use can enhance reaction efficiency, improve product quality, and potentially reduce production costs.
However, challenges remain in the widespread adoption of industrial biopolymers. These include higher production costs compared to conventional polymers, limitations in performance characteristics for certain applications, and the need for specialized waste management infrastructure. Despite these challenges, the market outlook remains positive, driven by increasing consumer demand for eco-friendly products and stringent environmental regulations worldwide.
Current Challenges in Ammonium Hydroxide Usage
The use of ammonium hydroxide in industrial-scale biopolymer synthesis faces several significant challenges that hinder its widespread adoption and efficiency. One of the primary issues is the volatility of ammonium hydroxide, which can lead to inconsistent concentrations during the synthesis process. This volatility not only affects the reaction kinetics but also poses safety concerns in large-scale production environments.
Another challenge is the pH sensitivity of many biopolymer synthesis reactions. Ammonium hydroxide's strong basic nature can cause rapid pH fluctuations, potentially leading to undesired side reactions or degradation of the biopolymer products. Maintaining precise pH control throughout the synthesis process is crucial for product quality and consistency, but this becomes increasingly difficult with ammonium hydroxide at industrial scales.
The corrosive nature of ammonium hydroxide presents additional complications in terms of equipment selection and maintenance. Industrial-scale reactors and processing equipment must be constructed from materials resistant to ammonia corrosion, which can significantly increase capital costs and limit design options. Regular maintenance and replacement of corroded components also add to operational expenses.
Environmental concerns associated with ammonia emissions pose another significant challenge. Industrial-scale use of ammonium hydroxide can result in substantial ammonia releases, which contribute to air pollution and may require extensive emission control systems to comply with environmental regulations. This not only adds to the complexity of the production process but also increases overall operational costs.
The handling and storage of large quantities of ammonium hydroxide present safety risks that must be carefully managed. Proper ventilation, personal protective equipment, and emergency response protocols are essential to prevent accidents and protect workers from exposure to ammonia vapors. These safety measures add layers of complexity to industrial operations and require ongoing training and vigilance.
Furthermore, the quality control of ammonium hydroxide in large-scale processes can be challenging. Ensuring consistent purity and concentration across batches is critical for reproducible biopolymer synthesis. Variations in ammonium hydroxide quality can lead to inconsistencies in the final product, potentially affecting its properties and performance.
Lastly, the scalability of processes involving ammonium hydroxide from laboratory to industrial levels presents its own set of challenges. Reactions that work well at small scales may encounter unforeseen issues when scaled up, such as heat transfer limitations, mixing inefficiencies, or unexpected side reactions. Overcoming these scaling challenges often requires significant process engineering and optimization efforts.
Another challenge is the pH sensitivity of many biopolymer synthesis reactions. Ammonium hydroxide's strong basic nature can cause rapid pH fluctuations, potentially leading to undesired side reactions or degradation of the biopolymer products. Maintaining precise pH control throughout the synthesis process is crucial for product quality and consistency, but this becomes increasingly difficult with ammonium hydroxide at industrial scales.
The corrosive nature of ammonium hydroxide presents additional complications in terms of equipment selection and maintenance. Industrial-scale reactors and processing equipment must be constructed from materials resistant to ammonia corrosion, which can significantly increase capital costs and limit design options. Regular maintenance and replacement of corroded components also add to operational expenses.
Environmental concerns associated with ammonia emissions pose another significant challenge. Industrial-scale use of ammonium hydroxide can result in substantial ammonia releases, which contribute to air pollution and may require extensive emission control systems to comply with environmental regulations. This not only adds to the complexity of the production process but also increases overall operational costs.
The handling and storage of large quantities of ammonium hydroxide present safety risks that must be carefully managed. Proper ventilation, personal protective equipment, and emergency response protocols are essential to prevent accidents and protect workers from exposure to ammonia vapors. These safety measures add layers of complexity to industrial operations and require ongoing training and vigilance.
Furthermore, the quality control of ammonium hydroxide in large-scale processes can be challenging. Ensuring consistent purity and concentration across batches is critical for reproducible biopolymer synthesis. Variations in ammonium hydroxide quality can lead to inconsistencies in the final product, potentially affecting its properties and performance.
Lastly, the scalability of processes involving ammonium hydroxide from laboratory to industrial levels presents its own set of challenges. Reactions that work well at small scales may encounter unforeseen issues when scaled up, such as heat transfer limitations, mixing inefficiencies, or unexpected side reactions. Overcoming these scaling challenges often requires significant process engineering and optimization efforts.
Existing Ammonium Hydroxide-based Solutions
01 Use in chemical processes
Ammonium hydroxide is widely used in various chemical processes, including as a reactant, catalyst, or pH regulator. It plays a crucial role in the production of different compounds and materials, such as fertilizers, cleaning agents, and pharmaceuticals.- Use in chemical processes: Ammonium hydroxide is widely used in various chemical processes, including as a reactant, catalyst, or pH adjuster. It plays a crucial role in the synthesis of organic compounds, production of fertilizers, and treatment of industrial waste streams.
- Application in cleaning and surface treatment: Ammonium hydroxide is utilized in cleaning formulations and surface treatment processes. It is effective in removing grease, oils, and other contaminants from various surfaces. Additionally, it can be used in etching and polishing applications for metals and semiconductors.
- Role in textile and leather processing: In the textile and leather industries, ammonium hydroxide is employed for various purposes, including dyeing, tanning, and fiber treatment. It helps in adjusting pH levels, improving dye penetration, and enhancing the overall quality of the finished products.
- Use in environmental applications: Ammonium hydroxide finds applications in environmental processes, such as flue gas treatment, air pollution control, and wastewater treatment. It can be used to neutralize acidic compounds and remove harmful emissions from industrial processes.
- Application in pharmaceutical and personal care products: Ammonium hydroxide is used in the production of certain pharmaceutical compounds and personal care products. It can serve as a pH adjuster, stabilizer, or reactant in the formulation of various medications, cosmetics, and hygiene products.
02 Application in wastewater treatment
Ammonium hydroxide is utilized in wastewater treatment processes for pH adjustment, ammonia removal, and nitrogen recovery. It can help in the precipitation of heavy metals and the neutralization of acidic effluents.Expand Specific Solutions03 Role in cleaning and surface treatment
Ammonium hydroxide is an effective cleaning agent and is used in various surface treatment applications. It can remove grease, oils, and other contaminants from surfaces, and is also used in etching and polishing processes for metals and semiconductors.Expand Specific Solutions04 Use in textile and dyeing industries
Ammonium hydroxide finds applications in textile processing and dyeing. It is used as a pH regulator in dyebaths, helps in the fixation of dyes, and can improve the color fastness of fabrics.Expand Specific Solutions05 Application in agriculture and fertilizers
Ammonium hydroxide is used in the production of nitrogen-based fertilizers and as a direct application fertilizer. It provides a source of readily available nitrogen for plants and can help in adjusting soil pH.Expand Specific Solutions
Key Players in Industrial Biopolymer Production
The industrial scale biopolymer synthesis market, focusing on the role of ammonium hydroxide, is in a growth phase with increasing demand for sustainable materials. The market size is expanding, driven by applications in various sectors such as packaging, agriculture, and healthcare. Technologically, the field is advancing rapidly, with companies like BASF Corp., Biomason, Inc., and Ductor Oy leading innovation. These firms are developing novel processes to enhance efficiency and reduce environmental impact. While established players like The Chemours Co. and China Petroleum & Chemical Corp. maintain significant market presence, emerging companies are introducing disruptive technologies, indicating a dynamic and competitive landscape with potential for further growth and technological advancements.
BASF Corp.
Technical Solution: BASF has developed an innovative approach to biopolymer synthesis using ammonium hydroxide as a key component. Their process involves a controlled pH environment maintained by ammonium hydroxide, which facilitates the polymerization of bio-based monomers. This method allows for the production of high-molecular-weight biopolymers with improved mechanical properties[1]. The company has also implemented a closed-loop system that recycles the ammonium hydroxide, reducing waste and improving process efficiency[2]. BASF's technology enables the production of biodegradable plastics with performance comparable to traditional petroleum-based polymers, addressing the growing demand for sustainable materials in various industries[3].
Strengths: Efficient use of ammonium hydroxide, closed-loop recycling system, production of high-performance biopolymers. Weaknesses: Potential ammonia emissions, energy-intensive process, dependency on specific bio-based feedstocks.
Biomason, Inc.
Technical Solution: Biomason has pioneered a groundbreaking approach to biopolymer synthesis using ammonium hydroxide in conjunction with microbial-induced calcite precipitation (MICP). Their process utilizes specialized bacteria that produce calcium carbonate in the presence of urea and calcium chloride, with ammonium hydroxide playing a crucial role in maintaining optimal pH conditions for bacterial activity[4]. This biocement technology allows for the production of structural materials with properties similar to traditional Portland cement but with a significantly lower carbon footprint[5]. Biomason's method involves precise control of ammonium hydroxide levels to ensure consistent material properties and accelerate the curing process, resulting in biopolymer-based construction materials that can be produced at ambient temperatures[6].
Strengths: Low carbon footprint, ambient temperature production, versatile applications in construction. Weaknesses: Scalability challenges, potential variability in material properties, reliance on specific bacterial strains.
Innovations in Ammonium Hydroxide Application
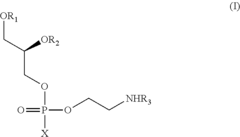
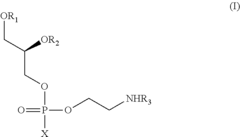
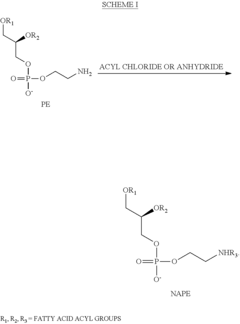
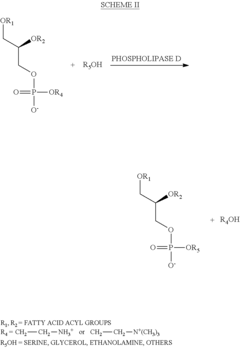
Process for the production of N-acyl-phosphatidyl-ethanolamine
PatentActiveUS8377662B2
Innovation
- A process involving the reaction of lecithin with a limited molar excess of N-Acyl-Ethanolamine in the presence of phospholipase D, using a mixture of water and hydrocarbon solvents, with controlled ratios and conditions, to produce NAPE of high purity and scalability.
Apparatus and method for producing ammonia
PatentWO2023205841A1
Innovation
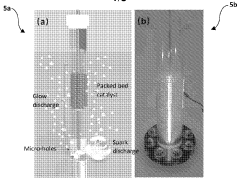
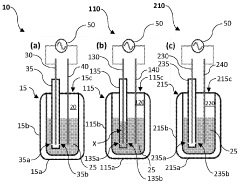
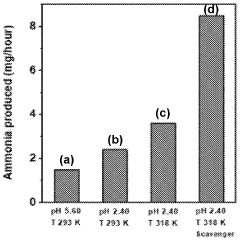
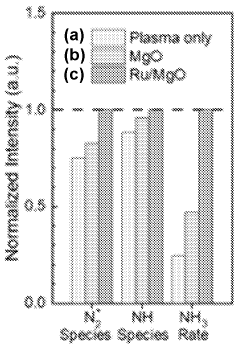
- A plasma-bubble reactor that generates activated nitrogen gas through an electric discharge in a liquid medium, where the activated nitrogen reacts with water to produce ammonia, using a catalyst and oxygen scavenger to enhance efficiency and reduce energy consumption.
Environmental Impact Assessment
The use of ammonium hydroxide in industrial-scale biopolymer synthesis raises significant environmental concerns that require careful assessment and mitigation strategies. One of the primary environmental impacts is the potential for ammonia emissions during the production process. Ammonia is a volatile compound that can contribute to air pollution, particularly in the form of particulate matter and smog formation. These emissions can have adverse effects on local air quality, potentially impacting both human health and ecosystems in the surrounding areas.
Water pollution is another critical environmental consideration. Ammonium hydroxide, if not properly managed, can contaminate water sources through runoff or improper disposal. This can lead to eutrophication in aquatic ecosystems, causing algal blooms and disrupting the balance of aquatic life. Additionally, the high pH of ammonium hydroxide solutions can alter the pH of water bodies, affecting the survival and reproduction of various aquatic species.
The production and transportation of ammonium hydroxide also contribute to the overall carbon footprint of biopolymer synthesis. The energy-intensive processes involved in manufacturing ammonium hydroxide, as well as the fuel consumption during transportation, result in greenhouse gas emissions that contribute to climate change. This aspect of the environmental impact extends beyond the immediate production site and must be considered in the broader context of global environmental sustainability.
Soil contamination is another potential environmental risk associated with the use of ammonium hydroxide in biopolymer synthesis. Accidental spills or improper handling can lead to soil alkalinization, which can negatively affect soil microbial communities and plant growth. This can have long-lasting impacts on local ecosystems and potentially affect agricultural productivity in affected areas.
To mitigate these environmental impacts, industries employing ammonium hydroxide in biopolymer synthesis must implement robust environmental management systems. This includes the use of advanced emission control technologies to minimize air pollution, proper wastewater treatment facilities to prevent water contamination, and stringent protocols for handling and storage to avoid soil contamination. Additionally, exploring alternative, more environmentally friendly catalysts or process modifications could help reduce the reliance on ammonium hydroxide and its associated environmental risks.
Continuous monitoring and regular environmental impact assessments are crucial to ensure compliance with environmental regulations and to identify areas for improvement. This may involve conducting life cycle assessments of the biopolymer production process to comprehensively evaluate its environmental footprint from raw material extraction to final product disposal. By adopting a proactive approach to environmental stewardship, industries can work towards minimizing the ecological impact of ammonium hydroxide use in biopolymer synthesis while maintaining production efficiency and product quality.
Water pollution is another critical environmental consideration. Ammonium hydroxide, if not properly managed, can contaminate water sources through runoff or improper disposal. This can lead to eutrophication in aquatic ecosystems, causing algal blooms and disrupting the balance of aquatic life. Additionally, the high pH of ammonium hydroxide solutions can alter the pH of water bodies, affecting the survival and reproduction of various aquatic species.
The production and transportation of ammonium hydroxide also contribute to the overall carbon footprint of biopolymer synthesis. The energy-intensive processes involved in manufacturing ammonium hydroxide, as well as the fuel consumption during transportation, result in greenhouse gas emissions that contribute to climate change. This aspect of the environmental impact extends beyond the immediate production site and must be considered in the broader context of global environmental sustainability.
Soil contamination is another potential environmental risk associated with the use of ammonium hydroxide in biopolymer synthesis. Accidental spills or improper handling can lead to soil alkalinization, which can negatively affect soil microbial communities and plant growth. This can have long-lasting impacts on local ecosystems and potentially affect agricultural productivity in affected areas.
To mitigate these environmental impacts, industries employing ammonium hydroxide in biopolymer synthesis must implement robust environmental management systems. This includes the use of advanced emission control technologies to minimize air pollution, proper wastewater treatment facilities to prevent water contamination, and stringent protocols for handling and storage to avoid soil contamination. Additionally, exploring alternative, more environmentally friendly catalysts or process modifications could help reduce the reliance on ammonium hydroxide and its associated environmental risks.
Continuous monitoring and regular environmental impact assessments are crucial to ensure compliance with environmental regulations and to identify areas for improvement. This may involve conducting life cycle assessments of the biopolymer production process to comprehensively evaluate its environmental footprint from raw material extraction to final product disposal. By adopting a proactive approach to environmental stewardship, industries can work towards minimizing the ecological impact of ammonium hydroxide use in biopolymer synthesis while maintaining production efficiency and product quality.
Regulatory Framework for Biopolymer Production
The regulatory framework for biopolymer production, particularly in the context of using ammonium hydroxide in industrial-scale synthesis, is a complex and evolving landscape. Governments and regulatory bodies worldwide have established guidelines and standards to ensure the safety, quality, and environmental sustainability of biopolymer production processes.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating biopolymers intended for food contact or medical applications. The FDA's regulations cover the use of chemicals like ammonium hydroxide in the production process, focusing on their potential impact on human health and safety. The Environmental Protection Agency (EPA) also oversees aspects of biopolymer production, particularly concerning environmental impacts and waste management.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to the use of ammonium hydroxide and other chemicals in biopolymer synthesis. This comprehensive framework requires manufacturers to register chemicals, assess their risks, and implement appropriate risk management measures.
In Asia, countries like Japan and South Korea have established their own regulatory frameworks for biopolymers. The Japanese Ministry of Health, Labour and Welfare regulates food-contact biopolymers, while the Korean Ministry of Food and Drug Safety oversees similar applications. These regulations often align with international standards but may have specific national requirements.
International organizations, such as the International Organization for Standardization (ISO), have developed standards for biopolymers, including those related to biodegradability and compostability. These standards provide a common reference point for manufacturers and regulators worldwide.
Specific to ammonium hydroxide use in biopolymer synthesis, regulations often focus on worker safety, environmental release, and potential residues in the final product. Occupational safety and health regulations, such as those enforced by OSHA in the US, mandate proper handling, storage, and use of ammonium hydroxide in industrial settings.
As the biopolymer industry continues to grow, regulatory frameworks are adapting to address new challenges and innovations. There is an increasing emphasis on lifecycle assessment and circular economy principles in biopolymer regulations. This shift is driving manufacturers to consider the entire lifecycle of their products, from raw material sourcing to end-of-life disposal or recycling.
Compliance with these diverse regulatory requirements presents both challenges and opportunities for biopolymer manufacturers. While navigating the complex regulatory landscape can be resource-intensive, it also encourages innovation in safer, more sustainable production methods. As the industry evolves, collaboration between regulators, manufacturers, and researchers will be crucial in developing regulations that balance innovation, safety, and environmental protection in biopolymer production.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating biopolymers intended for food contact or medical applications. The FDA's regulations cover the use of chemicals like ammonium hydroxide in the production process, focusing on their potential impact on human health and safety. The Environmental Protection Agency (EPA) also oversees aspects of biopolymer production, particularly concerning environmental impacts and waste management.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to the use of ammonium hydroxide and other chemicals in biopolymer synthesis. This comprehensive framework requires manufacturers to register chemicals, assess their risks, and implement appropriate risk management measures.
In Asia, countries like Japan and South Korea have established their own regulatory frameworks for biopolymers. The Japanese Ministry of Health, Labour and Welfare regulates food-contact biopolymers, while the Korean Ministry of Food and Drug Safety oversees similar applications. These regulations often align with international standards but may have specific national requirements.
International organizations, such as the International Organization for Standardization (ISO), have developed standards for biopolymers, including those related to biodegradability and compostability. These standards provide a common reference point for manufacturers and regulators worldwide.
Specific to ammonium hydroxide use in biopolymer synthesis, regulations often focus on worker safety, environmental release, and potential residues in the final product. Occupational safety and health regulations, such as those enforced by OSHA in the US, mandate proper handling, storage, and use of ammonium hydroxide in industrial settings.
As the biopolymer industry continues to grow, regulatory frameworks are adapting to address new challenges and innovations. There is an increasing emphasis on lifecycle assessment and circular economy principles in biopolymer regulations. This shift is driving manufacturers to consider the entire lifecycle of their products, from raw material sourcing to end-of-life disposal or recycling.
Compliance with these diverse regulatory requirements presents both challenges and opportunities for biopolymer manufacturers. While navigating the complex regulatory landscape can be resource-intensive, it also encourages innovation in safer, more sustainable production methods. As the industry evolves, collaboration between regulators, manufacturers, and researchers will be crucial in developing regulations that balance innovation, safety, and environmental protection in biopolymer production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!