How Battery Acid Interacts with Different Electrode Materials
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Battery Acid-Electrode Interaction Background
The interaction between battery acid and electrode materials is a fundamental aspect of battery technology, dating back to the invention of the lead-acid battery by Gaston Planté in 1859. This pioneering work laid the foundation for understanding how electrolytes interact with electrodes, a concept that remains crucial in modern battery design and development.
Battery acid, typically a solution of sulfuric acid in water, serves as the electrolyte in many battery systems. Its primary function is to facilitate the movement of ions between the electrodes, enabling the electrochemical reactions that store and release energy. The concentration and composition of the acid significantly influence battery performance, affecting factors such as internal resistance, charge capacity, and cycle life.
Over the years, researchers have explored various electrode materials to optimize battery performance. Traditional lead-acid batteries use lead and lead dioxide electrodes, which react with sulfuric acid to produce electrical energy. However, the quest for higher energy density, longer lifespan, and improved safety has led to the development of diverse electrode materials, each interacting uniquely with battery acids.
The interaction between battery acid and electrodes is a complex process involving multiple phenomena. These include ion exchange, redox reactions, surface adsorption, and in some cases, material degradation. The nature of these interactions depends on factors such as the chemical composition of the electrode, its surface structure, and the specific properties of the electrolyte.
Understanding these interactions is crucial for several reasons. Firstly, it allows for the optimization of battery performance by selecting appropriate electrode-electrolyte combinations. Secondly, it helps in predicting and mitigating degradation mechanisms that can lead to battery failure. Lastly, it guides the development of new battery technologies with enhanced characteristics.
Recent advancements in analytical techniques have significantly improved our ability to study these interactions at the molecular level. Tools such as in-situ X-ray diffraction, atomic force microscopy, and electrochemical impedance spectroscopy now provide unprecedented insights into the dynamic processes occurring at the electrode-electrolyte interface.
The evolution of battery technology has seen a shift from simple acid-based systems to more complex electrolytes and electrode materials. This progression has been driven by the need for batteries with higher energy density, faster charging capabilities, and improved safety profiles. As a result, the study of acid-electrode interactions has expanded to encompass a wide range of materials and electrolyte compositions, each presenting unique challenges and opportunities for optimization.
Battery acid, typically a solution of sulfuric acid in water, serves as the electrolyte in many battery systems. Its primary function is to facilitate the movement of ions between the electrodes, enabling the electrochemical reactions that store and release energy. The concentration and composition of the acid significantly influence battery performance, affecting factors such as internal resistance, charge capacity, and cycle life.
Over the years, researchers have explored various electrode materials to optimize battery performance. Traditional lead-acid batteries use lead and lead dioxide electrodes, which react with sulfuric acid to produce electrical energy. However, the quest for higher energy density, longer lifespan, and improved safety has led to the development of diverse electrode materials, each interacting uniquely with battery acids.
The interaction between battery acid and electrodes is a complex process involving multiple phenomena. These include ion exchange, redox reactions, surface adsorption, and in some cases, material degradation. The nature of these interactions depends on factors such as the chemical composition of the electrode, its surface structure, and the specific properties of the electrolyte.
Understanding these interactions is crucial for several reasons. Firstly, it allows for the optimization of battery performance by selecting appropriate electrode-electrolyte combinations. Secondly, it helps in predicting and mitigating degradation mechanisms that can lead to battery failure. Lastly, it guides the development of new battery technologies with enhanced characteristics.
Recent advancements in analytical techniques have significantly improved our ability to study these interactions at the molecular level. Tools such as in-situ X-ray diffraction, atomic force microscopy, and electrochemical impedance spectroscopy now provide unprecedented insights into the dynamic processes occurring at the electrode-electrolyte interface.
The evolution of battery technology has seen a shift from simple acid-based systems to more complex electrolytes and electrode materials. This progression has been driven by the need for batteries with higher energy density, faster charging capabilities, and improved safety profiles. As a result, the study of acid-electrode interactions has expanded to encompass a wide range of materials and electrolyte compositions, each presenting unique challenges and opportunities for optimization.
Market Analysis for Advanced Battery Technologies
The advanced battery technology market is experiencing significant growth, driven by the increasing demand for electric vehicles, renewable energy storage, and portable electronic devices. As of 2023, the global advanced battery market was valued at approximately $95 billion, with projections indicating a compound annual growth rate (CAGR) of 15% over the next five years. This robust growth is primarily attributed to the rapid adoption of electric vehicles, which accounted for nearly 40% of the market share in 2023.
The interaction between battery acid and different electrode materials plays a crucial role in determining the performance, longevity, and safety of advanced batteries. As such, innovations in this area are driving market trends and shaping consumer preferences. Lithium-ion batteries continue to dominate the market, representing over 70% of the advanced battery market share. However, emerging technologies such as solid-state batteries and lithium-sulfur batteries are gaining traction due to their potential for higher energy density and improved safety profiles.
The automotive sector remains the largest consumer of advanced batteries, with electric vehicle sales surpassing 10 million units globally in 2023. This trend is expected to continue, with major automakers committing to electrify their fleets in the coming years. The energy storage sector is also showing remarkable growth, driven by the increasing integration of renewable energy sources into power grids. The stationary energy storage market is projected to grow at a CAGR of 20% through 2028.
Geographically, Asia-Pacific leads the advanced battery market, accounting for over 50% of global production and consumption. China, in particular, dominates the market due to its strong manufacturing capabilities and government support for electric vehicles and renewable energy. North America and Europe are also significant markets, with both regions investing heavily in research and development of next-generation battery technologies.
The market is characterized by intense competition among established players and new entrants. Key market players are focusing on developing innovative electrode materials and electrolyte formulations to enhance battery performance and reduce costs. Collaborations between battery manufacturers, automotive companies, and research institutions are becoming increasingly common, driving technological advancements and market growth.
Consumer demand for faster charging times, longer battery life, and improved safety is shaping product development strategies. The push for more sustainable and environmentally friendly battery technologies is also influencing market dynamics, with a growing emphasis on recyclable and less toxic battery materials. As the market continues to evolve, the interaction between battery acid and electrode materials remains a critical area of focus for researchers and manufacturers alike, driving innovation and shaping the future of the advanced battery technology market.
The interaction between battery acid and different electrode materials plays a crucial role in determining the performance, longevity, and safety of advanced batteries. As such, innovations in this area are driving market trends and shaping consumer preferences. Lithium-ion batteries continue to dominate the market, representing over 70% of the advanced battery market share. However, emerging technologies such as solid-state batteries and lithium-sulfur batteries are gaining traction due to their potential for higher energy density and improved safety profiles.
The automotive sector remains the largest consumer of advanced batteries, with electric vehicle sales surpassing 10 million units globally in 2023. This trend is expected to continue, with major automakers committing to electrify their fleets in the coming years. The energy storage sector is also showing remarkable growth, driven by the increasing integration of renewable energy sources into power grids. The stationary energy storage market is projected to grow at a CAGR of 20% through 2028.
Geographically, Asia-Pacific leads the advanced battery market, accounting for over 50% of global production and consumption. China, in particular, dominates the market due to its strong manufacturing capabilities and government support for electric vehicles and renewable energy. North America and Europe are also significant markets, with both regions investing heavily in research and development of next-generation battery technologies.
The market is characterized by intense competition among established players and new entrants. Key market players are focusing on developing innovative electrode materials and electrolyte formulations to enhance battery performance and reduce costs. Collaborations between battery manufacturers, automotive companies, and research institutions are becoming increasingly common, driving technological advancements and market growth.
Consumer demand for faster charging times, longer battery life, and improved safety is shaping product development strategies. The push for more sustainable and environmentally friendly battery technologies is also influencing market dynamics, with a growing emphasis on recyclable and less toxic battery materials. As the market continues to evolve, the interaction between battery acid and electrode materials remains a critical area of focus for researchers and manufacturers alike, driving innovation and shaping the future of the advanced battery technology market.
Current Challenges in Electrode-Electrolyte Interface
The electrode-electrolyte interface in battery systems presents several significant challenges that hinder the advancement of battery technology. One of the primary issues is the formation and stability of the Solid Electrolyte Interphase (SEI) layer. This layer, which forms on the electrode surface due to the decomposition of electrolyte components, plays a crucial role in battery performance and longevity. However, controlling its formation and maintaining its stability over numerous charge-discharge cycles remains a complex task.
Another major challenge is the degradation of electrode materials due to continuous interaction with the electrolyte. This interaction can lead to structural changes in the electrode, resulting in capacity loss and reduced cycle life. For instance, in lithium-ion batteries, the repeated insertion and extraction of lithium ions can cause volume changes in the electrode materials, leading to mechanical stress and eventual failure.
Electrolyte decomposition at high voltages is another significant concern, particularly for high-energy-density batteries. As researchers push for higher operating voltages to increase energy density, the stability of conventional electrolytes becomes a limiting factor. This decomposition not only reduces the amount of available electrolyte but also generates gaseous products that can lead to safety issues and decreased battery performance.
The challenge of lithium dendrite formation in lithium metal batteries is also a critical issue at the electrode-electrolyte interface. These dendrites can grow from the anode surface, potentially piercing the separator and causing short circuits. This not only poses safety risks but also limits the practical implementation of high-energy-density lithium metal batteries.
Furthermore, the complex nature of the electrode-electrolyte interface makes it difficult to study and characterize accurately. Advanced in-situ and operando characterization techniques are needed to fully understand the dynamic processes occurring at this interface during battery operation. This lack of comprehensive understanding hampers the development of effective strategies to mitigate interface-related issues.
Lastly, the challenge of designing electrode materials and electrolytes that are mutually compatible and stable over a wide range of operating conditions persists. This includes finding materials that can withstand extreme temperatures, high charge/discharge rates, and long-term cycling without significant degradation at the interface. The intricate balance between high performance and long-term stability at the electrode-electrolyte interface remains a key focus area for battery researchers and developers.
Another major challenge is the degradation of electrode materials due to continuous interaction with the electrolyte. This interaction can lead to structural changes in the electrode, resulting in capacity loss and reduced cycle life. For instance, in lithium-ion batteries, the repeated insertion and extraction of lithium ions can cause volume changes in the electrode materials, leading to mechanical stress and eventual failure.
Electrolyte decomposition at high voltages is another significant concern, particularly for high-energy-density batteries. As researchers push for higher operating voltages to increase energy density, the stability of conventional electrolytes becomes a limiting factor. This decomposition not only reduces the amount of available electrolyte but also generates gaseous products that can lead to safety issues and decreased battery performance.
The challenge of lithium dendrite formation in lithium metal batteries is also a critical issue at the electrode-electrolyte interface. These dendrites can grow from the anode surface, potentially piercing the separator and causing short circuits. This not only poses safety risks but also limits the practical implementation of high-energy-density lithium metal batteries.
Furthermore, the complex nature of the electrode-electrolyte interface makes it difficult to study and characterize accurately. Advanced in-situ and operando characterization techniques are needed to fully understand the dynamic processes occurring at this interface during battery operation. This lack of comprehensive understanding hampers the development of effective strategies to mitigate interface-related issues.
Lastly, the challenge of designing electrode materials and electrolytes that are mutually compatible and stable over a wide range of operating conditions persists. This includes finding materials that can withstand extreme temperatures, high charge/discharge rates, and long-term cycling without significant degradation at the interface. The intricate balance between high performance and long-term stability at the electrode-electrolyte interface remains a key focus area for battery researchers and developers.
Existing Solutions for Electrode Protection
01 Battery acid composition and interaction
The composition of battery acid and its interaction with other materials is a crucial aspect of battery technology. This includes studying the chemical reactions between the acid and electrode materials, as well as the impact on battery performance and longevity. Research in this area focuses on optimizing acid formulations and understanding how different additives can affect the acid's behavior within the battery system.- Battery acid composition and interaction: The composition of battery acid and its interaction with other materials is crucial for battery performance and safety. This includes studying the chemical reactions between the acid and electrode materials, as well as the impact on battery life and efficiency.
- Acid-resistant materials for battery components: Development of acid-resistant materials for battery components, such as separators, casings, and electrodes, to withstand the corrosive nature of battery acid. These materials help improve battery durability and prevent acid leakage.
- Battery acid management systems: Implementation of acid management systems to monitor and control acid levels, concentration, and distribution within the battery. These systems help optimize battery performance, extend lifespan, and enhance safety by preventing acid-related issues.
- Acid circulation and electrolyte mixing: Techniques for improving acid circulation and electrolyte mixing within batteries to enhance performance and efficiency. This includes methods for maintaining uniform acid concentration and preventing stratification of the electrolyte.
- Battery acid recycling and disposal: Methods for safely recycling and disposing of battery acid to minimize environmental impact and recover valuable materials. This includes neutralization techniques, acid treatment processes, and strategies for handling spent battery acid.
02 Acid-resistant materials for battery components
Developing acid-resistant materials for battery components is essential to prevent corrosion and extend battery life. This involves researching and testing various materials that can withstand prolonged exposure to battery acid, such as specialized plastics, coatings, and alloys. These materials are used in the construction of battery casings, separators, and other internal components to improve overall battery durability and performance.Expand Specific Solutions03 Battery acid management and circulation systems
Innovative systems for managing and circulating battery acid are crucial for maintaining optimal battery performance. These systems may include pumps, sensors, and control mechanisms to regulate acid flow, temperature, and concentration within the battery. Advanced acid management techniques can help prevent stratification, reduce maintenance requirements, and extend the overall lifespan of batteries, particularly in large-scale applications.Expand Specific Solutions04 Acid level monitoring and maintenance techniques
Developing effective methods for monitoring and maintaining proper acid levels in batteries is essential for optimal performance and safety. This includes the design of sensors and indicators to accurately measure acid levels, as well as automated systems for adding or removing acid as needed. Advanced monitoring techniques may also incorporate data analysis to predict maintenance requirements and prevent acid-related issues before they occur.Expand Specific Solutions05 Safety measures for handling battery acid
Implementing safety measures for handling battery acid is crucial to protect both personnel and equipment. This involves developing specialized containment systems, personal protective equipment, and emergency response protocols. Research in this area also focuses on creating safer acid formulations and exploring alternative electrolytes that may reduce the risks associated with traditional battery acids while maintaining or improving battery performance.Expand Specific Solutions
Key Players in Battery Industry
The battery acid-electrode interaction field is in a mature development stage, with a substantial market size driven by the growing demand for energy storage solutions. The technology's maturity is evident from the involvement of established players like Duracell, LG Chem, and Toyota, alongside specialized research institutions such as the Commonwealth Scientific & Industrial Research Organisation and the Dalian Institute of Chemical Physics. The competitive landscape is diverse, featuring both traditional battery manufacturers and innovative startups like Ionic Materials, indicating ongoing efforts to improve battery performance and sustainability. The presence of automotive giants and electronics conglomerates suggests a strong focus on applications in electric vehicles and consumer electronics, driving further research and development in this area.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: The Dalian Institute has conducted extensive research on the molecular-level interactions between battery acid and various electrode materials. They have developed advanced in-situ characterization techniques to observe these interactions in real-time, providing crucial insights for electrode design[1]. Their research includes the development of novel electrolyte additives that form protective films on electrode surfaces, mitigating acid-induced degradation[2]. The institute has also pioneered the use of graphene-based materials as protective coatings for electrodes, enhancing their stability in acidic environments[3]. Additionally, they have made significant progress in understanding and optimizing the solid-electrolyte interphase (SEI) formation process, which is critical for battery performance and longevity[4].
Strengths: Cutting-edge research techniques and fundamental understanding of electrode-electrolyte interactions. Weaknesses: Potential gap between laboratory research and practical, large-scale application in commercial batteries.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced electrode materials that interact efficiently with battery acid. Their research focuses on high-nickel cathodes, which offer improved energy density and longer cycle life[1]. They have also introduced silicon-based anodes that enhance the battery's capacity and charging speed[2]. LG Chem's approach involves surface modification of electrode materials to improve their stability in acidic environments, reducing unwanted side reactions and extending battery life[3]. The company has implemented nano-coating techniques to protect electrode particles from acid corrosion, resulting in batteries with higher voltage tolerance and improved safety characteristics[4].
Strengths: High energy density, improved cycle life, and enhanced safety. Weaknesses: Potential higher production costs and complexity in manufacturing processes.
Innovations in Electrode Material Science
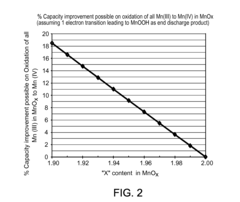
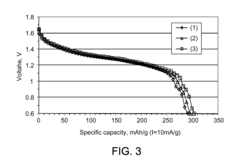
Acid-treated manganese dioxide and methods of making thereof
PatentActiveUS8303840B2
Innovation
- Acid treatment of manganese dioxide at low temperatures to reduce Mn(III) content, increase Mn(IV) content, and remove impurities, resulting in improved cathode active materials with enhanced specific capacity and discharge voltage.
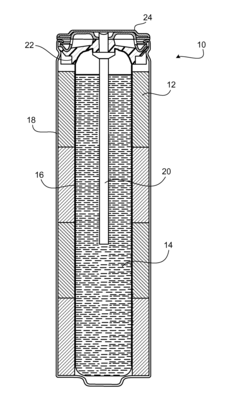
Electrode for lead-acid battery and lead-acid battery including the same
PatentWO2022155234A1
Innovation
- The electrode grid pattern is designed to provide uniform current density, featuring a symmetrical primary current path member extending from the collector lug to the central axis, ensuring consistent current flow and material usage, thereby reducing resistance and promoting deeper current penetration.
Environmental Impact of Battery Production
The production of batteries, particularly those used in electric vehicles and large-scale energy storage systems, has significant environmental implications. The manufacturing process involves the extraction and processing of raw materials, which can lead to habitat destruction, water pollution, and greenhouse gas emissions. Mining activities for lithium, cobalt, and nickel, key components in many battery types, often result in soil degradation and water contamination in surrounding areas.
The energy-intensive nature of battery production contributes to its environmental footprint. The fabrication of electrode materials and the assembly of battery cells require substantial amounts of electricity, often sourced from fossil fuels in many regions. This reliance on non-renewable energy sources during production partially offsets the environmental benefits of battery-powered technologies in their use phase.
Chemical processes involved in battery production generate hazardous waste and emissions. The synthesis of electrode materials and electrolytes involves the use of toxic solvents and reagents, which can pose risks to worker health and local ecosystems if not properly managed. Proper disposal and treatment of these chemical byproducts are crucial to mitigate environmental harm.
Water usage is another critical environmental concern in battery production. The manufacturing process requires large volumes of water for cooling, cleaning, and chemical processes. In water-stressed regions, this high demand can exacerbate local water scarcity issues and impact surrounding communities and ecosystems.
The environmental impact extends to the end-of-life stage of batteries. Improper disposal of spent batteries can lead to soil and water contamination due to the leaching of heavy metals and toxic compounds. Developing efficient recycling technologies and implementing robust recycling programs are essential to reduce the overall environmental footprint of battery production and use.
Efforts to mitigate these environmental impacts are ongoing. Innovations in battery chemistry aim to reduce reliance on scarce or environmentally problematic materials. Advancements in manufacturing processes focus on improving energy efficiency and reducing waste generation. Additionally, the transition to renewable energy sources for powering production facilities can significantly decrease the carbon footprint of battery manufacturing.
As the demand for batteries continues to grow, addressing these environmental challenges becomes increasingly crucial. Sustainable practices throughout the battery lifecycle, from raw material sourcing to end-of-life management, are necessary to ensure that the benefits of battery technologies do not come at an unacceptable environmental cost.
The energy-intensive nature of battery production contributes to its environmental footprint. The fabrication of electrode materials and the assembly of battery cells require substantial amounts of electricity, often sourced from fossil fuels in many regions. This reliance on non-renewable energy sources during production partially offsets the environmental benefits of battery-powered technologies in their use phase.
Chemical processes involved in battery production generate hazardous waste and emissions. The synthesis of electrode materials and electrolytes involves the use of toxic solvents and reagents, which can pose risks to worker health and local ecosystems if not properly managed. Proper disposal and treatment of these chemical byproducts are crucial to mitigate environmental harm.
Water usage is another critical environmental concern in battery production. The manufacturing process requires large volumes of water for cooling, cleaning, and chemical processes. In water-stressed regions, this high demand can exacerbate local water scarcity issues and impact surrounding communities and ecosystems.
The environmental impact extends to the end-of-life stage of batteries. Improper disposal of spent batteries can lead to soil and water contamination due to the leaching of heavy metals and toxic compounds. Developing efficient recycling technologies and implementing robust recycling programs are essential to reduce the overall environmental footprint of battery production and use.
Efforts to mitigate these environmental impacts are ongoing. Innovations in battery chemistry aim to reduce reliance on scarce or environmentally problematic materials. Advancements in manufacturing processes focus on improving energy efficiency and reducing waste generation. Additionally, the transition to renewable energy sources for powering production facilities can significantly decrease the carbon footprint of battery manufacturing.
As the demand for batteries continues to grow, addressing these environmental challenges becomes increasingly crucial. Sustainable practices throughout the battery lifecycle, from raw material sourcing to end-of-life management, are necessary to ensure that the benefits of battery technologies do not come at an unacceptable environmental cost.
Safety Regulations in Battery Manufacturing
Safety regulations in battery manufacturing are crucial for protecting workers, consumers, and the environment from potential hazards associated with battery production and use. These regulations cover various aspects of the manufacturing process, including the handling of battery acid and its interaction with different electrode materials.
One of the primary concerns in battery manufacturing is the safe handling of battery acid, typically sulfuric acid in lead-acid batteries. Regulations mandate the use of appropriate personal protective equipment (PPE) for workers, including acid-resistant gloves, goggles, and protective clothing. Proper ventilation systems are required to prevent the accumulation of harmful fumes and gases generated during the interaction between battery acid and electrode materials.
Storage and transportation of battery acid are subject to strict guidelines. Manufacturers must use corrosion-resistant containers and implement spill containment measures to prevent accidental releases. Emergency response plans and procedures for handling acid spills are mandatory, including the provision of neutralizing agents and proper disposal methods for contaminated materials.
The interaction between battery acid and electrode materials is a critical focus of safety regulations. Manufacturers must ensure that electrode materials are compatible with the specific acid used and can withstand prolonged exposure without degradation. Regular testing and quality control measures are required to verify the integrity of electrode materials and prevent premature failure or leakage.
Regulations also address the potential environmental impact of battery manufacturing. Proper waste management and recycling procedures are mandated to minimize the release of hazardous materials into the environment. This includes the safe disposal of spent battery acid and the recycling of electrode materials to recover valuable metals and reduce environmental contamination.
Worker training is a key component of safety regulations in battery manufacturing. Employees must receive comprehensive instruction on the proper handling of battery acid, the use of PPE, and emergency response procedures. Regular safety audits and inspections are required to ensure compliance with regulations and identify potential hazards in the manufacturing process.
As battery technology evolves, safety regulations must adapt to address new challenges. For instance, the increasing use of lithium-ion batteries has led to the development of specific safety standards for their production, focusing on thermal management and the prevention of thermal runaway events. Ongoing research into the interaction between battery acid and novel electrode materials continues to inform and shape safety regulations in the industry.
One of the primary concerns in battery manufacturing is the safe handling of battery acid, typically sulfuric acid in lead-acid batteries. Regulations mandate the use of appropriate personal protective equipment (PPE) for workers, including acid-resistant gloves, goggles, and protective clothing. Proper ventilation systems are required to prevent the accumulation of harmful fumes and gases generated during the interaction between battery acid and electrode materials.
Storage and transportation of battery acid are subject to strict guidelines. Manufacturers must use corrosion-resistant containers and implement spill containment measures to prevent accidental releases. Emergency response plans and procedures for handling acid spills are mandatory, including the provision of neutralizing agents and proper disposal methods for contaminated materials.
The interaction between battery acid and electrode materials is a critical focus of safety regulations. Manufacturers must ensure that electrode materials are compatible with the specific acid used and can withstand prolonged exposure without degradation. Regular testing and quality control measures are required to verify the integrity of electrode materials and prevent premature failure or leakage.
Regulations also address the potential environmental impact of battery manufacturing. Proper waste management and recycling procedures are mandated to minimize the release of hazardous materials into the environment. This includes the safe disposal of spent battery acid and the recycling of electrode materials to recover valuable metals and reduce environmental contamination.
Worker training is a key component of safety regulations in battery manufacturing. Employees must receive comprehensive instruction on the proper handling of battery acid, the use of PPE, and emergency response procedures. Regular safety audits and inspections are required to ensure compliance with regulations and identify potential hazards in the manufacturing process.
As battery technology evolves, safety regulations must adapt to address new challenges. For instance, the increasing use of lithium-ion batteries has led to the development of specific safety standards for their production, focusing on thermal management and the prevention of thermal runaway events. Ongoing research into the interaction between battery acid and novel electrode materials continues to inform and shape safety regulations in the industry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!