How Fuel Additives Impact Battery Acid Electrolyte Stability
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fuel Additive Impact on Electrolytes: Background and Objectives
The impact of fuel additives on battery acid electrolyte stability has become a critical area of research in the automotive and energy storage industries. This technological domain has evolved significantly over the past few decades, driven by the increasing demand for more efficient and durable battery systems in various applications, particularly in electric and hybrid vehicles.
Historically, battery acid electrolytes have been susceptible to degradation and instability issues, which can significantly affect battery performance and lifespan. The introduction of fuel additives into this equation has added a new layer of complexity to the challenge of maintaining electrolyte stability. These additives, originally designed to enhance fuel efficiency and engine performance, have been found to have unintended consequences on battery systems when they come into contact with electrolytes.
The primary objective of this technical research is to comprehensively examine the mechanisms by which fuel additives interact with and impact battery acid electrolyte stability. This involves investigating the chemical reactions that occur between various types of fuel additives and common electrolyte compositions, as well as analyzing the resulting effects on battery performance, longevity, and safety.
Furthermore, this research aims to identify potential solutions and innovative approaches to mitigate any negative impacts of fuel additives on electrolyte stability. This may include the development of new electrolyte formulations that are more resistant to additive-induced degradation, or the creation of protective measures to prevent direct contact between additives and electrolytes.
Another key goal is to establish a set of best practices and guidelines for the use of fuel additives in vehicles with advanced battery systems. This will require a thorough understanding of the trade-offs between fuel performance enhancement and battery system integrity, as well as the development of testing protocols to assess the compatibility of new fuel additives with existing and emerging battery technologies.
The technological trajectory in this field is expected to lead towards more integrated approaches to vehicle design, where fuel systems and battery systems are developed in tandem, with careful consideration of their potential interactions. This holistic approach could pave the way for significant advancements in both fuel efficiency and battery performance, ultimately contributing to more sustainable and reliable transportation solutions.
Historically, battery acid electrolytes have been susceptible to degradation and instability issues, which can significantly affect battery performance and lifespan. The introduction of fuel additives into this equation has added a new layer of complexity to the challenge of maintaining electrolyte stability. These additives, originally designed to enhance fuel efficiency and engine performance, have been found to have unintended consequences on battery systems when they come into contact with electrolytes.
The primary objective of this technical research is to comprehensively examine the mechanisms by which fuel additives interact with and impact battery acid electrolyte stability. This involves investigating the chemical reactions that occur between various types of fuel additives and common electrolyte compositions, as well as analyzing the resulting effects on battery performance, longevity, and safety.
Furthermore, this research aims to identify potential solutions and innovative approaches to mitigate any negative impacts of fuel additives on electrolyte stability. This may include the development of new electrolyte formulations that are more resistant to additive-induced degradation, or the creation of protective measures to prevent direct contact between additives and electrolytes.
Another key goal is to establish a set of best practices and guidelines for the use of fuel additives in vehicles with advanced battery systems. This will require a thorough understanding of the trade-offs between fuel performance enhancement and battery system integrity, as well as the development of testing protocols to assess the compatibility of new fuel additives with existing and emerging battery technologies.
The technological trajectory in this field is expected to lead towards more integrated approaches to vehicle design, where fuel systems and battery systems are developed in tandem, with careful consideration of their potential interactions. This holistic approach could pave the way for significant advancements in both fuel efficiency and battery performance, ultimately contributing to more sustainable and reliable transportation solutions.
Market Analysis of Fuel Additives and Battery Technologies
The fuel additives and battery technologies market has experienced significant growth in recent years, driven by increasing demand for improved fuel efficiency and the rapid expansion of electric vehicle (EV) adoption. The global fuel additives market was valued at $7.2 billion in 2020 and is projected to reach $9.8 billion by 2025, growing at a CAGR of 6.3%. This growth is primarily attributed to the rising demand for cleaner and more efficient fuels, stringent environmental regulations, and the need to enhance engine performance.
On the battery technologies front, the market has witnessed exponential growth, largely propelled by the EV revolution. The global lithium-ion battery market, a key component in EV batteries, was valued at $36.7 billion in 2020 and is expected to reach $129.3 billion by 2027, with a CAGR of 18.0%. This remarkable growth is fueled by increasing EV sales, government initiatives promoting clean energy, and advancements in battery technology.
The intersection of fuel additives and battery technologies presents an intriguing area of study, particularly in the context of electrolyte stability. As the automotive industry transitions towards electrification, there is growing interest in understanding how fuel additives may impact battery performance and longevity. This is especially relevant for hybrid vehicles, which utilize both internal combustion engines and electric powertrains.
The market for fuel additives specifically designed to enhance battery electrolyte stability is still in its nascent stages but shows promising potential. Major players in both the fuel additives and battery technology sectors are investing in research and development to create innovative solutions that address this emerging need. Companies like BASF, Lubrizol, and Ingevity are exploring new formulations that can potentially improve battery life and performance while maintaining optimal fuel efficiency.
In the EV battery market, manufacturers are focusing on developing more stable and efficient electrolytes to enhance battery performance and safety. This has led to increased demand for advanced electrolyte additives, creating new opportunities for fuel additive companies to diversify their product portfolios. The global battery electrolyte market is projected to grow from $4.7 billion in 2020 to $8.3 billion by 2025, at a CAGR of 12.0%.
As the automotive industry continues to evolve, the convergence of fuel additives and battery technologies is expected to play a crucial role in shaping future mobility solutions. This presents significant opportunities for companies operating in both sectors to collaborate and innovate, driving further market growth and technological advancements.
On the battery technologies front, the market has witnessed exponential growth, largely propelled by the EV revolution. The global lithium-ion battery market, a key component in EV batteries, was valued at $36.7 billion in 2020 and is expected to reach $129.3 billion by 2027, with a CAGR of 18.0%. This remarkable growth is fueled by increasing EV sales, government initiatives promoting clean energy, and advancements in battery technology.
The intersection of fuel additives and battery technologies presents an intriguing area of study, particularly in the context of electrolyte stability. As the automotive industry transitions towards electrification, there is growing interest in understanding how fuel additives may impact battery performance and longevity. This is especially relevant for hybrid vehicles, which utilize both internal combustion engines and electric powertrains.
The market for fuel additives specifically designed to enhance battery electrolyte stability is still in its nascent stages but shows promising potential. Major players in both the fuel additives and battery technology sectors are investing in research and development to create innovative solutions that address this emerging need. Companies like BASF, Lubrizol, and Ingevity are exploring new formulations that can potentially improve battery life and performance while maintaining optimal fuel efficiency.
In the EV battery market, manufacturers are focusing on developing more stable and efficient electrolytes to enhance battery performance and safety. This has led to increased demand for advanced electrolyte additives, creating new opportunities for fuel additive companies to diversify their product portfolios. The global battery electrolyte market is projected to grow from $4.7 billion in 2020 to $8.3 billion by 2025, at a CAGR of 12.0%.
As the automotive industry continues to evolve, the convergence of fuel additives and battery technologies is expected to play a crucial role in shaping future mobility solutions. This presents significant opportunities for companies operating in both sectors to collaborate and innovate, driving further market growth and technological advancements.
Current Challenges in Electrolyte Stability
The stability of battery acid electrolytes is a critical factor in the performance and longevity of lead-acid batteries. However, the introduction of fuel additives has presented new challenges to maintaining this stability. One of the primary issues is the contamination of the electrolyte by these additives, which can occur through various mechanisms such as vapor intrusion or direct contact during refueling processes.
Fuel additives, designed to enhance engine performance and reduce emissions, often contain organic compounds that can react with the sulfuric acid electrolyte. These reactions can lead to the formation of complex molecules that alter the electrolyte's chemical composition and physical properties. The resulting changes can significantly impact the battery's efficiency, capacity, and overall lifespan.
Another challenge is the potential for increased corrosion of battery components due to the presence of certain fuel additives. Some additives contain corrosive elements that, when introduced to the electrolyte, can accelerate the degradation of lead plates and other internal structures. This corrosion not only reduces the battery's performance but also shortens its operational life.
The formation of unwanted byproducts is a further concern in electrolyte stability. When fuel additives interact with the electrolyte, they may produce substances that can coat the battery plates, impeding the electrochemical reactions necessary for proper battery function. This phenomenon, known as sulfation, can significantly decrease the battery's ability to hold a charge and deliver power effectively.
Temperature fluctuations exacerbate these challenges, as higher temperatures can accelerate the chemical reactions between fuel additives and the electrolyte. This can lead to more rapid degradation of the electrolyte and increased gas generation within the battery, potentially causing safety hazards and further reducing battery life.
The automotive industry faces difficulties in developing standardized testing methods to evaluate the long-term effects of various fuel additives on electrolyte stability. The wide range of additives available and the complex interactions between these substances and battery components make it challenging to create comprehensive testing protocols that accurately predict real-world performance.
Lastly, there is a growing need for advanced electrolyte formulations that can withstand the presence of fuel additives without compromising battery performance. Developing such formulations requires extensive research and testing, balancing the need for stability with other critical battery characteristics such as conductivity and energy density.
Fuel additives, designed to enhance engine performance and reduce emissions, often contain organic compounds that can react with the sulfuric acid electrolyte. These reactions can lead to the formation of complex molecules that alter the electrolyte's chemical composition and physical properties. The resulting changes can significantly impact the battery's efficiency, capacity, and overall lifespan.
Another challenge is the potential for increased corrosion of battery components due to the presence of certain fuel additives. Some additives contain corrosive elements that, when introduced to the electrolyte, can accelerate the degradation of lead plates and other internal structures. This corrosion not only reduces the battery's performance but also shortens its operational life.
The formation of unwanted byproducts is a further concern in electrolyte stability. When fuel additives interact with the electrolyte, they may produce substances that can coat the battery plates, impeding the electrochemical reactions necessary for proper battery function. This phenomenon, known as sulfation, can significantly decrease the battery's ability to hold a charge and deliver power effectively.
Temperature fluctuations exacerbate these challenges, as higher temperatures can accelerate the chemical reactions between fuel additives and the electrolyte. This can lead to more rapid degradation of the electrolyte and increased gas generation within the battery, potentially causing safety hazards and further reducing battery life.
The automotive industry faces difficulties in developing standardized testing methods to evaluate the long-term effects of various fuel additives on electrolyte stability. The wide range of additives available and the complex interactions between these substances and battery components make it challenging to create comprehensive testing protocols that accurately predict real-world performance.
Lastly, there is a growing need for advanced electrolyte formulations that can withstand the presence of fuel additives without compromising battery performance. Developing such formulations requires extensive research and testing, balancing the need for stability with other critical battery characteristics such as conductivity and energy density.
Existing Solutions for Enhancing Electrolyte Stability
01 Additives for electrolyte stability
Various additives can be incorporated into battery acid electrolytes to enhance their stability. These additives may include organic compounds, inorganic salts, or polymers that can improve the chemical and thermal stability of the electrolyte, reduce decomposition, and extend battery life.- Additives for electrolyte stability enhancement: Various additives can be incorporated into battery acid electrolytes to improve their stability. These additives may include organic compounds, inorganic salts, or polymers that help prevent decomposition of the electrolyte, reduce side reactions, and maintain the electrolyte's performance over time. Such additives can also contribute to extending the battery's overall lifespan and improving its safety characteristics.
- Temperature control for electrolyte stability: Maintaining optimal temperature conditions is crucial for battery acid electrolyte stability. Implementing effective thermal management systems can help prevent electrolyte degradation caused by extreme temperatures. This may involve the use of cooling systems, insulation materials, or heat-dissipating structures to keep the electrolyte within a stable temperature range, thereby preserving its chemical composition and performance characteristics.
- Electrolyte composition optimization: Optimizing the composition of battery acid electrolytes can significantly improve their stability. This may involve adjusting the concentration of acid, incorporating stabilizing agents, or using alternative electrolyte formulations. By carefully balancing the electrolyte components, it is possible to enhance its resistance to degradation, improve ionic conductivity, and maintain consistent performance throughout the battery's lifecycle.
- Electrode surface treatment for improved electrolyte stability: Treating the surfaces of battery electrodes can contribute to enhanced electrolyte stability. Various surface modification techniques, such as coating, etching, or functionalization, can be applied to electrode materials to reduce unwanted reactions with the electrolyte. This approach can help minimize electrolyte decomposition, prevent the formation of harmful byproducts, and maintain the electrolyte's integrity over extended periods of use.
- Advanced separator materials for electrolyte stability: The use of advanced separator materials can play a crucial role in maintaining battery acid electrolyte stability. Innovative separator designs with improved chemical and mechanical properties can help prevent electrolyte degradation by reducing unwanted interactions between electrodes and electrolyte. These separators may incorporate specialized coatings, nanostructures, or composite materials that enhance their ability to maintain electrolyte stability while ensuring efficient ion transport.
02 Electrolyte composition optimization
Optimizing the composition of the electrolyte can significantly improve its stability. This may involve adjusting the concentration of acid, adding supporting electrolytes, or incorporating specific solvents to create a more stable and efficient electrolyte system for battery applications.Expand Specific Solutions03 Temperature control mechanisms
Implementing temperature control mechanisms can help maintain electrolyte stability. This may include the use of thermal management systems, heat-resistant materials, or temperature-responsive additives to prevent degradation of the electrolyte under extreme temperature conditions.Expand Specific Solutions04 Surface modification of electrodes
Modifying the surface of battery electrodes can enhance the stability of the acid electrolyte. This may involve coating the electrodes with protective layers, using specific surface treatments, or incorporating nanostructures to reduce unwanted reactions between the electrolyte and electrode materials.Expand Specific Solutions05 Advanced separator technologies
Developing and implementing advanced separator technologies can contribute to electrolyte stability. This may include the use of novel materials, composite structures, or functionalized separators that can effectively prevent electrolyte degradation, reduce ion migration, and improve overall battery performance.Expand Specific Solutions
Key Players in Fuel Additive and Battery Industries
The fuel additive impact on battery acid electrolyte stability is an emerging field with growing market potential. The industry is in its early development stage, characterized by ongoing research and technological advancements. Market size is expanding as automotive and energy storage sectors increasingly focus on battery performance and longevity. Companies like Toyota Motor Corp., LG Chem Ltd., and Contemporary Amperex Technology Co., Ltd. are at the forefront of this technology, investing in R&D to improve battery efficiency and durability. The technology's maturity is still evolving, with major players like Hyundai Motor Co., Ltd. and Samsung SDI Co., Ltd. actively working on innovative solutions to enhance electrolyte stability and overall battery performance.
Toyota Motor Corp.
Technical Solution: Toyota has invested heavily in developing fuel additives and electrolyte stabilizers for next-generation battery technologies. Their research focuses on silicon-based anodes and solid-state batteries, which require specialized electrolyte additives to maintain stability[5]. Toyota's approach includes the use of fluorinated ether compounds and boron-based additives to form protective layers on electrode surfaces, reducing unwanted side reactions and improving cycle life[6]. The company has also explored the use of lithium salt additives, such as lithium bis(fluorosulfonyl)imide (LiFSI), to enhance ionic conductivity and stabilize the electrolyte-electrode interface[7]. Toyota's efforts extend to developing additives that can mitigate the effects of moisture contamination in battery systems, a critical factor in maintaining long-term stability[8].
Strengths: Extensive resources for R&D, integration with vehicle development, and a strong focus on practical applications. Weaknesses: Slower commercialization process due to rigorous automotive safety standards.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced electrolyte additives to enhance battery acid electrolyte stability. Their research focuses on fluorinated compounds and organic sulfur-containing additives that form stable solid electrolyte interphase (SEI) layers[1]. These additives, such as vinylene carbonate (VC) and fluoroethylene carbonate (FEC), significantly improve the cycling performance and thermal stability of lithium-ion batteries[2]. LG Chem's proprietary electrolyte formulations incorporate multiple additives in precise ratios to synergistically enhance battery performance and longevity[3]. The company has also explored the use of ionic liquids as electrolyte additives to improve high-temperature stability and reduce flammability[4].
Strengths: Extensive R&D capabilities, proprietary additive formulations, and proven track record in commercial battery production. Weaknesses: High production costs for advanced additives and potential intellectual property constraints.
Core Innovations in Electrolyte-Fuel Additive Interactions
Electrolyte additive, and battery electrolyte comprising same and secondary battery comprising same
PatentWO2024096450A1
Innovation
- A novel electrolyte additive, represented by specific compounds such as vinylene carbonate and lithium difluorophosphate, is introduced to form a stable film on the anode and cathode, suppressing side reactions, reducing charge/discharge resistance, and scavenging hydrofluoric acid, thereby enhancing battery performance and lifespan.
Electrolyte additive, electrolyte, and lithium-ion battery
PatentInactiveEP4290636A1
Innovation
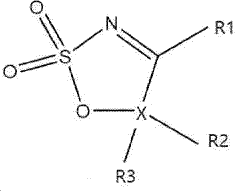
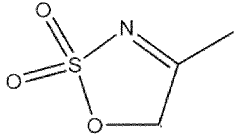
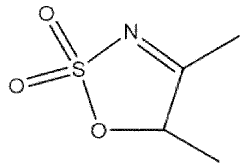
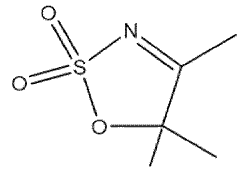
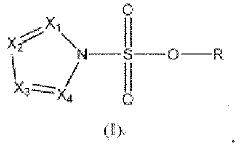
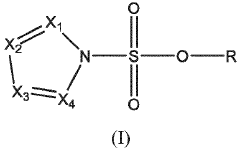
- A nitrogen-containing five-membered heterocyclic sulfonate compound is used as an electrolyte additive, forming a stable SEI film and inhibiting acidity and chromaticity rise by complexing with PF5, enhancing compatibility with graphite and ionic conductivity.
Environmental Impact of Fuel Additives on Battery Systems
The environmental impact of fuel additives on battery systems is a critical consideration in the ongoing development of sustainable energy solutions. Fuel additives, while primarily designed to enhance engine performance and efficiency, can have significant downstream effects on battery systems, particularly in hybrid and electric vehicles.
One of the primary concerns is the potential for fuel additives to contaminate battery acid electrolytes. This contamination can occur through various pathways, including vapor transmission and direct contact during maintenance procedures. When fuel additives interact with battery electrolytes, they can alter the chemical composition and stability of the solution, potentially leading to reduced battery performance and lifespan.
The most common fuel additives, such as detergents, corrosion inhibitors, and octane boosters, contain complex chemical compounds that may react with the sulfuric acid typically found in lead-acid batteries. These reactions can produce byproducts that accumulate in the battery over time, affecting the electrolyte's ability to facilitate efficient ion transfer between electrodes.
Furthermore, some fuel additives may increase the volatility of the electrolyte, leading to accelerated water loss and concentration changes. This can result in increased battery internal resistance and reduced capacity, ultimately impacting the overall efficiency of the vehicle's electrical system.
Environmental concerns also extend to the disposal and recycling of batteries exposed to fuel additive contamination. The presence of these additives may complicate recycling processes and potentially introduce harmful substances into the environment if not properly managed.
Research has shown that certain fuel additives can contribute to the formation of sulfation on battery plates, a process that reduces the battery's ability to accept and hold a charge. This not only affects the battery's performance but also shortens its operational life, leading to more frequent battery replacements and increased environmental waste.
To mitigate these environmental impacts, ongoing research is focused on developing more compatible fuel additives and improving battery designs to resist contamination. Additionally, advancements in battery technology, such as the development of solid-state batteries, may provide solutions that are less susceptible to the negative effects of fuel additives.
As the automotive industry continues to shift towards electrification, understanding and addressing the environmental impact of fuel additives on battery systems becomes increasingly important. This knowledge will be crucial in designing more sustainable and efficient energy storage solutions for the future of transportation.
One of the primary concerns is the potential for fuel additives to contaminate battery acid electrolytes. This contamination can occur through various pathways, including vapor transmission and direct contact during maintenance procedures. When fuel additives interact with battery electrolytes, they can alter the chemical composition and stability of the solution, potentially leading to reduced battery performance and lifespan.
The most common fuel additives, such as detergents, corrosion inhibitors, and octane boosters, contain complex chemical compounds that may react with the sulfuric acid typically found in lead-acid batteries. These reactions can produce byproducts that accumulate in the battery over time, affecting the electrolyte's ability to facilitate efficient ion transfer between electrodes.
Furthermore, some fuel additives may increase the volatility of the electrolyte, leading to accelerated water loss and concentration changes. This can result in increased battery internal resistance and reduced capacity, ultimately impacting the overall efficiency of the vehicle's electrical system.
Environmental concerns also extend to the disposal and recycling of batteries exposed to fuel additive contamination. The presence of these additives may complicate recycling processes and potentially introduce harmful substances into the environment if not properly managed.
Research has shown that certain fuel additives can contribute to the formation of sulfation on battery plates, a process that reduces the battery's ability to accept and hold a charge. This not only affects the battery's performance but also shortens its operational life, leading to more frequent battery replacements and increased environmental waste.
To mitigate these environmental impacts, ongoing research is focused on developing more compatible fuel additives and improving battery designs to resist contamination. Additionally, advancements in battery technology, such as the development of solid-state batteries, may provide solutions that are less susceptible to the negative effects of fuel additives.
As the automotive industry continues to shift towards electrification, understanding and addressing the environmental impact of fuel additives on battery systems becomes increasingly important. This knowledge will be crucial in designing more sustainable and efficient energy storage solutions for the future of transportation.
Regulatory Framework for Fuel Additives and Battery Technologies
The regulatory framework governing fuel additives and battery technologies is a complex landscape that intersects multiple jurisdictions and agencies. In the United States, the Environmental Protection Agency (EPA) plays a central role in regulating fuel additives under the Clean Air Act. The EPA maintains a list of registered fuel additives and requires manufacturers to provide extensive data on their products' composition and potential environmental impacts. This process ensures that additives do not contribute to increased emissions or engine damage.
Concurrently, the Department of Energy (DOE) oversees research and development in battery technologies, including those used in electric vehicles. The DOE's Vehicle Technologies Office sets performance targets and safety standards for advanced battery systems, influencing the direction of industry innovation.
On the international stage, the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation impacts both fuel additives and battery components. REACH requires companies to register chemical substances and provide safety data, affecting the global supply chain for these technologies.
The United Nations Economic Commission for Europe (UNECE) has established the Global Technical Regulation on Electric Vehicle Safety (GTR 20), which addresses battery safety in electric vehicles. This regulation aims to harmonize safety requirements across different countries, facilitating international trade and technology transfer.
In the context of battery acid electrolyte stability, regulatory bodies such as the Occupational Safety and Health Administration (OSHA) in the U.S. set guidelines for the handling and storage of battery acids. These regulations are crucial for ensuring workplace safety in industries that manufacture or use lead-acid batteries.
The interplay between fuel additive regulations and battery technology standards becomes particularly relevant as the automotive industry transitions towards electrification. Regulatory frameworks are evolving to address the potential interactions between traditional fuel systems and electric powertrains in hybrid vehicles. This includes considerations for the compatibility of fuel additives with materials used in battery systems and the potential for cross-contamination.
As emerging technologies blur the lines between conventional fuels and electric power sources, regulatory bodies are faced with the challenge of updating existing frameworks. This may involve creating new categories of regulations that address the unique characteristics of hybrid and transitional technologies, ensuring that safety and environmental standards keep pace with technological advancements.
Concurrently, the Department of Energy (DOE) oversees research and development in battery technologies, including those used in electric vehicles. The DOE's Vehicle Technologies Office sets performance targets and safety standards for advanced battery systems, influencing the direction of industry innovation.
On the international stage, the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation impacts both fuel additives and battery components. REACH requires companies to register chemical substances and provide safety data, affecting the global supply chain for these technologies.
The United Nations Economic Commission for Europe (UNECE) has established the Global Technical Regulation on Electric Vehicle Safety (GTR 20), which addresses battery safety in electric vehicles. This regulation aims to harmonize safety requirements across different countries, facilitating international trade and technology transfer.
In the context of battery acid electrolyte stability, regulatory bodies such as the Occupational Safety and Health Administration (OSHA) in the U.S. set guidelines for the handling and storage of battery acids. These regulations are crucial for ensuring workplace safety in industries that manufacture or use lead-acid batteries.
The interplay between fuel additive regulations and battery technology standards becomes particularly relevant as the automotive industry transitions towards electrification. Regulatory frameworks are evolving to address the potential interactions between traditional fuel systems and electric powertrains in hybrid vehicles. This includes considerations for the compatibility of fuel additives with materials used in battery systems and the potential for cross-contamination.
As emerging technologies blur the lines between conventional fuels and electric power sources, regulatory bodies are faced with the challenge of updating existing frameworks. This may involve creating new categories of regulations that address the unique characteristics of hybrid and transitional technologies, ensuring that safety and environmental standards keep pace with technological advancements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!