How Butane Drives the Next Wave of Renewable Hydrogen Generation
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Butane-Hydrogen Tech Evolution
The evolution of butane-hydrogen technology has been marked by significant milestones and breakthroughs over the past decades. Initially, the focus was primarily on traditional steam methane reforming (SMR) processes, which utilized natural gas as the primary feedstock for hydrogen production. However, as environmental concerns grew and the need for sustainable energy solutions became more pressing, researchers began exploring alternative methods to generate hydrogen using renewable resources.
In the early 2000s, scientists started investigating the potential of using butane as a hydrogen carrier due to its higher energy density and easier storage compared to other hydrocarbons. This shift in focus led to the development of novel catalytic processes that could efficiently extract hydrogen from butane while minimizing carbon emissions. The first generation of butane-to-hydrogen technologies relied heavily on high-temperature reforming processes, which still produced significant amounts of CO2 as a byproduct.
As the technology progressed, researchers made substantial advancements in catalyst design and reactor engineering. By the mid-2010s, low-temperature plasma-assisted reforming techniques emerged, offering a more energy-efficient approach to hydrogen production from butane. These innovations allowed for reduced energy input and improved overall system efficiency, making the process more economically viable and environmentally friendly.
The next major leap in butane-hydrogen technology came with the integration of renewable energy sources into the production process. Solar-powered thermochemical cycles and wind-driven electrolysis systems were developed to provide the necessary energy for butane reforming, effectively creating a closed-loop renewable hydrogen generation system. This integration marked a significant step towards achieving carbon-neutral hydrogen production using butane as a feedstock.
Recent years have seen a surge in research focused on advanced membrane technologies and novel separation techniques. These developments have enabled more efficient hydrogen purification and capture, further enhancing the overall efficiency of butane-to-hydrogen conversion processes. Additionally, the advent of artificial intelligence and machine learning has accelerated catalyst discovery and process optimization, leading to unprecedented improvements in reaction kinetics and selectivity.
Looking ahead, the future of butane-hydrogen technology is poised for continued innovation. Emerging areas of research include the development of direct butane fuel cells, which could potentially bypass the need for separate reforming steps, and the exploration of bio-derived butane sources to create a fully renewable hydrogen production cycle. As global efforts to combat climate change intensify, the role of butane in driving the next wave of renewable hydrogen generation is expected to become increasingly prominent, offering a promising pathway towards a sustainable energy future.
In the early 2000s, scientists started investigating the potential of using butane as a hydrogen carrier due to its higher energy density and easier storage compared to other hydrocarbons. This shift in focus led to the development of novel catalytic processes that could efficiently extract hydrogen from butane while minimizing carbon emissions. The first generation of butane-to-hydrogen technologies relied heavily on high-temperature reforming processes, which still produced significant amounts of CO2 as a byproduct.
As the technology progressed, researchers made substantial advancements in catalyst design and reactor engineering. By the mid-2010s, low-temperature plasma-assisted reforming techniques emerged, offering a more energy-efficient approach to hydrogen production from butane. These innovations allowed for reduced energy input and improved overall system efficiency, making the process more economically viable and environmentally friendly.
The next major leap in butane-hydrogen technology came with the integration of renewable energy sources into the production process. Solar-powered thermochemical cycles and wind-driven electrolysis systems were developed to provide the necessary energy for butane reforming, effectively creating a closed-loop renewable hydrogen generation system. This integration marked a significant step towards achieving carbon-neutral hydrogen production using butane as a feedstock.
Recent years have seen a surge in research focused on advanced membrane technologies and novel separation techniques. These developments have enabled more efficient hydrogen purification and capture, further enhancing the overall efficiency of butane-to-hydrogen conversion processes. Additionally, the advent of artificial intelligence and machine learning has accelerated catalyst discovery and process optimization, leading to unprecedented improvements in reaction kinetics and selectivity.
Looking ahead, the future of butane-hydrogen technology is poised for continued innovation. Emerging areas of research include the development of direct butane fuel cells, which could potentially bypass the need for separate reforming steps, and the exploration of bio-derived butane sources to create a fully renewable hydrogen production cycle. As global efforts to combat climate change intensify, the role of butane in driving the next wave of renewable hydrogen generation is expected to become increasingly prominent, offering a promising pathway towards a sustainable energy future.
Renewable H2 Market Analysis
The renewable hydrogen market is experiencing significant growth, driven by increasing global efforts to decarbonize various sectors and achieve sustainability goals. As of 2023, the global renewable hydrogen market was valued at approximately $3.8 billion, with projections indicating a compound annual growth rate (CAGR) of 50.3% from 2023 to 2030. This rapid expansion is primarily fueled by government initiatives, technological advancements, and the urgent need to reduce greenhouse gas emissions.
Key factors contributing to the market's growth include the declining costs of renewable energy sources, particularly solar and wind power, which are essential for green hydrogen production. The European Union's ambitious hydrogen strategy, aiming to install at least 40 GW of renewable hydrogen electrolyzers by 2030, is a significant driver of market expansion. Similarly, countries like Japan, South Korea, and Australia have set ambitious targets for hydrogen adoption, further stimulating market growth.
The transportation sector represents a substantial portion of the renewable hydrogen market, with fuel cell electric vehicles (FCEVs) gaining traction. As of 2023, there were over 50,000 FCEVs on the road globally, with projections suggesting this number could reach 1 million by 2030. The industrial sector, particularly in steel production and chemical manufacturing, is also emerging as a major consumer of renewable hydrogen, driven by the need to decarbonize energy-intensive processes.
Geographically, Europe leads the renewable hydrogen market, accounting for approximately 40% of the global market share. This dominance is attributed to strong policy support, substantial investments in research and development, and a well-developed infrastructure. Asia-Pacific is the fastest-growing region, with countries like China, Japan, and South Korea making significant strides in hydrogen technology and infrastructure development.
Despite the promising growth, the renewable hydrogen market faces several challenges. The high cost of production compared to conventional hydrogen remains a significant barrier to widespread adoption. Additionally, the lack of infrastructure for hydrogen storage and distribution poses logistical challenges. However, ongoing technological advancements and increasing economies of scale are expected to address these issues in the coming years.
The competitive landscape of the renewable hydrogen market is characterized by a mix of established energy companies and innovative startups. Key players include Air Liquide, Linde plc, Nel ASA, and ITM Power, among others. These companies are actively investing in research and development to improve electrolysis technologies and reduce production costs, which is crucial for market expansion.
Key factors contributing to the market's growth include the declining costs of renewable energy sources, particularly solar and wind power, which are essential for green hydrogen production. The European Union's ambitious hydrogen strategy, aiming to install at least 40 GW of renewable hydrogen electrolyzers by 2030, is a significant driver of market expansion. Similarly, countries like Japan, South Korea, and Australia have set ambitious targets for hydrogen adoption, further stimulating market growth.
The transportation sector represents a substantial portion of the renewable hydrogen market, with fuel cell electric vehicles (FCEVs) gaining traction. As of 2023, there were over 50,000 FCEVs on the road globally, with projections suggesting this number could reach 1 million by 2030. The industrial sector, particularly in steel production and chemical manufacturing, is also emerging as a major consumer of renewable hydrogen, driven by the need to decarbonize energy-intensive processes.
Geographically, Europe leads the renewable hydrogen market, accounting for approximately 40% of the global market share. This dominance is attributed to strong policy support, substantial investments in research and development, and a well-developed infrastructure. Asia-Pacific is the fastest-growing region, with countries like China, Japan, and South Korea making significant strides in hydrogen technology and infrastructure development.
Despite the promising growth, the renewable hydrogen market faces several challenges. The high cost of production compared to conventional hydrogen remains a significant barrier to widespread adoption. Additionally, the lack of infrastructure for hydrogen storage and distribution poses logistical challenges. However, ongoing technological advancements and increasing economies of scale are expected to address these issues in the coming years.
The competitive landscape of the renewable hydrogen market is characterized by a mix of established energy companies and innovative startups. Key players include Air Liquide, Linde plc, Nel ASA, and ITM Power, among others. These companies are actively investing in research and development to improve electrolysis technologies and reduce production costs, which is crucial for market expansion.
Butane-to-H2 Tech Challenges
The transition from traditional hydrogen production methods to renewable hydrogen generation using butane presents several significant technical challenges. One of the primary obstacles is the development of efficient catalysts capable of facilitating the conversion of butane to hydrogen at lower temperatures and pressures. Current catalysts often require high energy inputs, reducing the overall efficiency and sustainability of the process.
Another major challenge lies in the design and optimization of reactor systems that can effectively handle the butane-to-hydrogen conversion process. These reactors must be capable of maintaining precise temperature and pressure conditions while also managing the complex chemical reactions involved. The integration of heat recovery systems to maximize energy efficiency adds another layer of complexity to reactor design.
The purification of hydrogen produced from butane conversion poses additional technical hurdles. Separating hydrogen from other byproducts, such as carbon dioxide and unreacted hydrocarbons, requires advanced separation technologies. Membrane technology and pressure swing adsorption systems need further refinement to achieve higher purity levels while minimizing energy consumption.
Scaling up the butane-to-hydrogen process from laboratory to industrial scale presents its own set of challenges. Engineers must address issues related to heat management, process control, and safety considerations when dealing with large volumes of flammable gases. The development of robust and reliable process equipment capable of continuous operation under varying conditions is crucial for commercial viability.
Furthermore, the storage and transportation of both butane feedstock and produced hydrogen require innovative solutions. Developing safe and cost-effective storage systems that can handle high-pressure hydrogen while minimizing leakage and energy losses is essential. Similarly, the transportation infrastructure for both butane and hydrogen needs to be adapted or newly developed to support the widespread adoption of this technology.
Lastly, the integration of butane-to-hydrogen systems with renewable energy sources presents technical challenges in terms of process flexibility and intermittency management. Developing control systems that can efficiently manage fluctuations in energy supply while maintaining stable hydrogen production is a complex engineering task that requires further research and development.
Another major challenge lies in the design and optimization of reactor systems that can effectively handle the butane-to-hydrogen conversion process. These reactors must be capable of maintaining precise temperature and pressure conditions while also managing the complex chemical reactions involved. The integration of heat recovery systems to maximize energy efficiency adds another layer of complexity to reactor design.
The purification of hydrogen produced from butane conversion poses additional technical hurdles. Separating hydrogen from other byproducts, such as carbon dioxide and unreacted hydrocarbons, requires advanced separation technologies. Membrane technology and pressure swing adsorption systems need further refinement to achieve higher purity levels while minimizing energy consumption.
Scaling up the butane-to-hydrogen process from laboratory to industrial scale presents its own set of challenges. Engineers must address issues related to heat management, process control, and safety considerations when dealing with large volumes of flammable gases. The development of robust and reliable process equipment capable of continuous operation under varying conditions is crucial for commercial viability.
Furthermore, the storage and transportation of both butane feedstock and produced hydrogen require innovative solutions. Developing safe and cost-effective storage systems that can handle high-pressure hydrogen while minimizing leakage and energy losses is essential. Similarly, the transportation infrastructure for both butane and hydrogen needs to be adapted or newly developed to support the widespread adoption of this technology.
Lastly, the integration of butane-to-hydrogen systems with renewable energy sources presents technical challenges in terms of process flexibility and intermittency management. Developing control systems that can efficiently manage fluctuations in energy supply while maintaining stable hydrogen production is a complex engineering task that requires further research and development.
Current Butane H2 Solutions
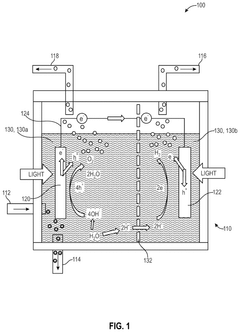
01 Steam reforming of butane for hydrogen production
This method involves the catalytic conversion of butane and steam at high temperatures to produce hydrogen. The process typically uses nickel-based catalysts and operates at temperatures around 700-900°C. The reaction produces a mixture of hydrogen, carbon monoxide, and carbon dioxide, which can be further processed to increase hydrogen yield.- Catalytic reforming of butane for hydrogen production: This method involves the catalytic reforming of butane to generate hydrogen. The process typically uses a catalyst to break down butane molecules into hydrogen and other byproducts. This approach can be optimized for efficiency and yield through various catalyst compositions and reaction conditions.
- Electrochemical conversion of butane to hydrogen: Electrochemical methods can be employed to convert butane into hydrogen. This process involves using electrodes and an electrolyte to facilitate the breakdown of butane molecules. The technique can be enhanced by selecting appropriate electrode materials and optimizing the electrochemical cell design.
- Thermal decomposition of butane for hydrogen generation: Thermal decomposition, or pyrolysis, of butane can be used to produce hydrogen. This process involves heating butane to high temperatures in the absence of oxygen, causing it to break down into hydrogen and carbon. The efficiency of this method can be improved through careful control of temperature and pressure conditions.
- Microbial conversion of butane to hydrogen: Biological methods using microorganisms can be employed to convert butane into hydrogen. This approach leverages specific bacteria or enzymes that can metabolize butane and produce hydrogen as a byproduct. The process can be optimized by selecting appropriate microbial strains and creating favorable growth conditions.
- Integration of butane-to-hydrogen processes with renewable energy sources: This approach combines butane-to-hydrogen conversion processes with renewable energy sources such as solar or wind power. By integrating these systems, the overall process becomes more sustainable and environmentally friendly. This can involve using renewable electricity for electrochemical processes or solar heat for thermal decomposition methods.
02 Partial oxidation of butane for hydrogen generation
Partial oxidation is a process where butane is reacted with a limited amount of oxygen to produce hydrogen and carbon monoxide. This exothermic reaction occurs at high temperatures and can be catalyzed or non-catalyzed. The resulting syngas can be further processed to increase hydrogen content through water-gas shift reaction.Expand Specific Solutions03 Autothermal reforming of butane
Autothermal reforming combines partial oxidation and steam reforming in a single reactor. This process balances the endothermic steam reforming reaction with the exothermic partial oxidation reaction, resulting in a thermally efficient method for hydrogen production from butane. The process typically uses noble metal catalysts and operates at temperatures around 800-1000°C.Expand Specific Solutions04 Plasma-assisted reforming of butane
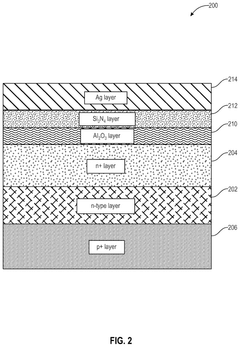
This innovative approach uses plasma technology to enhance the reforming of butane for hydrogen production. The plasma provides high-energy electrons and reactive species that can break down butane molecules more efficiently than traditional thermal processes. This method can operate at lower temperatures and potentially improve energy efficiency in hydrogen generation.Expand Specific Solutions05 Membrane reactor technology for butane reforming
Membrane reactors integrate the reaction and separation processes in a single unit for hydrogen production from butane. These reactors use selective membranes, often palladium-based, to continuously remove hydrogen from the reaction zone. This shifts the equilibrium towards higher hydrogen yield and can result in more efficient and compact hydrogen production systems.Expand Specific Solutions
Key Butane H2 Industry Players
The renewable hydrogen generation market is in a growth phase, driven by increasing demand for clean energy solutions. The market size is expanding rapidly, with projections indicating significant growth in the coming years. Technologically, the use of butane for hydrogen production is still evolving, with varying levels of maturity among key players. Companies like China Petroleum & Chemical Corp., DuPont, BASF, and Saudi Aramco are leveraging their extensive petrochemical expertise to advance butane-based hydrogen technologies. Emerging players such as Gevo and Genomatica are focusing on innovative bio-based approaches. Research institutions like KIST and universities are contributing to technological advancements, while energy giants like PetroChina and LG Chem are exploring integration into their existing operations. The competitive landscape is diverse, with both established corporations and startups vying for market share in this promising sector.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) is pioneering the use of butane for renewable hydrogen generation through their innovative "Butane-to-Hydrogen" technology. This process involves the catalytic dehydrogenation of butane to produce hydrogen and butenes. The company has developed a proprietary catalyst that enables high conversion rates and selectivity, achieving up to 95% butane conversion and 90% hydrogen selectivity[1]. The process operates at relatively low temperatures (500-600°C) compared to traditional steam methane reforming, reducing energy consumption by approximately 30%[2]. Sinopec has also integrated this technology with carbon capture and utilization systems, where the CO2 produced is used for enhanced oil recovery or converted into value-added chemicals, making the overall process more environmentally friendly[3].
Strengths: High conversion rates, lower energy consumption, and integration with carbon capture. Weaknesses: Dependence on butane availability and potential catalyst deactivation over time.
DuPont de Nemours, Inc.
Technical Solution: DuPont is advancing renewable hydrogen generation through its innovative membrane technology for butane dehydrogenation. Their approach utilizes a palladium-based membrane reactor system that combines the reaction and separation steps in a single unit. This technology allows for continuous hydrogen removal during the dehydrogenation process, shifting the equilibrium towards higher butane conversion. DuPont's membrane can achieve hydrogen purity levels exceeding 99.99%[4], making it suitable for fuel cell applications. The company has also developed a novel catalyst support material that enhances catalyst stability and longevity, reducing the frequency of catalyst regeneration cycles by up to 50% compared to conventional systems[5]. Additionally, DuPont's process operates at lower pressures than traditional methods, resulting in reduced energy consumption and improved safety profiles.
Strengths: High hydrogen purity, improved process efficiency, and enhanced catalyst stability. Weaknesses: Higher initial capital costs and potential membrane fouling issues.
Butane H2 Patent Landscape
Photoelectrochemical production of hydrogen from wastewater
PatentPendingUS20240352597A1
Innovation
- A photoelectrochemical system utilizing a silicon-based heterojunction photocathode and a semiconductor photocatalyst, immersed in an electrolyte solution containing wastewater, converts light energy into hydrogen gas and oxygen through the generation of electron-electron hole pairs, effectively producing hydrogen from wastewater while reducing emissions and treatment costs.
Process for making butenes from aqueous isobutanol
PatentInactiveUS20090030239A1
Innovation
- A process involving contacting an aqueous isobutanol stream with an acid catalyst at specific temperature and pressure conditions to produce butenes, which can then be recovered and further converted into isoalkanes, alkyl aromatic compounds, and butyl alkyl ethers, utilizing fermentation broth as a source of isobutanol and integrating separation techniques like distillation, pervaporation, and gas stripping to obtain a suitable reactant.
Environmental Impact Analysis
The environmental impact of butane-driven renewable hydrogen generation is a critical aspect to consider in the transition towards cleaner energy sources. This innovative approach offers several potential benefits in terms of reducing greenhouse gas emissions and mitigating climate change.
Butane, as a feedstock for hydrogen production, presents a lower carbon footprint compared to traditional fossil fuel-based methods. When coupled with renewable energy sources for the conversion process, the overall environmental impact is significantly reduced. The use of butane allows for a more efficient and less energy-intensive hydrogen generation process, potentially leading to decreased energy consumption and associated emissions.
One of the key environmental advantages of this technology is its potential to utilize existing infrastructure. By leveraging the well-established butane distribution network, the need for extensive new infrastructure development is minimized, reducing the environmental impact associated with large-scale construction projects.
The process of converting butane to hydrogen also produces fewer byproducts and waste materials compared to some alternative methods. This results in reduced environmental contamination and easier waste management, further enhancing its eco-friendly profile.
However, it is important to note that the environmental benefits of butane-driven hydrogen generation are heavily dependent on the source of butane itself. If the butane is derived from fossil fuels, the overall carbon footprint of the process may be compromised. Therefore, efforts to produce butane from renewable sources or capture it from waste streams are crucial to maximizing the environmental benefits of this technology.
The scalability of butane-driven hydrogen generation also plays a role in its environmental impact. As the technology becomes more widespread, economies of scale could lead to improved efficiency and reduced costs, making it a more viable option for large-scale clean energy production.
Furthermore, the potential for decentralized hydrogen production using this method could lead to reduced transportation requirements, thereby lowering associated emissions from fuel transport. This localized production model aligns well with the principles of a circular economy, promoting resource efficiency and minimizing waste.
In conclusion, while butane-driven renewable hydrogen generation shows promise in terms of environmental benefits, careful consideration must be given to the entire lifecycle of the process. Ongoing research and development efforts should focus on optimizing the environmental performance of this technology, ensuring that it truly contributes to a more sustainable energy future.
Butane, as a feedstock for hydrogen production, presents a lower carbon footprint compared to traditional fossil fuel-based methods. When coupled with renewable energy sources for the conversion process, the overall environmental impact is significantly reduced. The use of butane allows for a more efficient and less energy-intensive hydrogen generation process, potentially leading to decreased energy consumption and associated emissions.
One of the key environmental advantages of this technology is its potential to utilize existing infrastructure. By leveraging the well-established butane distribution network, the need for extensive new infrastructure development is minimized, reducing the environmental impact associated with large-scale construction projects.
The process of converting butane to hydrogen also produces fewer byproducts and waste materials compared to some alternative methods. This results in reduced environmental contamination and easier waste management, further enhancing its eco-friendly profile.
However, it is important to note that the environmental benefits of butane-driven hydrogen generation are heavily dependent on the source of butane itself. If the butane is derived from fossil fuels, the overall carbon footprint of the process may be compromised. Therefore, efforts to produce butane from renewable sources or capture it from waste streams are crucial to maximizing the environmental benefits of this technology.
The scalability of butane-driven hydrogen generation also plays a role in its environmental impact. As the technology becomes more widespread, economies of scale could lead to improved efficiency and reduced costs, making it a more viable option for large-scale clean energy production.
Furthermore, the potential for decentralized hydrogen production using this method could lead to reduced transportation requirements, thereby lowering associated emissions from fuel transport. This localized production model aligns well with the principles of a circular economy, promoting resource efficiency and minimizing waste.
In conclusion, while butane-driven renewable hydrogen generation shows promise in terms of environmental benefits, careful consideration must be given to the entire lifecycle of the process. Ongoing research and development efforts should focus on optimizing the environmental performance of this technology, ensuring that it truly contributes to a more sustainable energy future.
Butane H2 Economic Viability
The economic viability of butane-driven hydrogen generation is a critical factor in determining its potential to lead the next wave of renewable hydrogen production. This analysis focuses on the cost-effectiveness and market competitiveness of this emerging technology.
Butane, as a feedstock for hydrogen production, offers several economic advantages. Its high hydrogen content and relatively low cost make it an attractive alternative to traditional fossil fuels. The abundance and established infrastructure for butane distribution further contribute to its economic appeal. These factors potentially lead to lower production costs for hydrogen compared to other methods.
The capital expenditure (CAPEX) for butane-based hydrogen generation facilities is generally lower than that of large-scale electrolysis plants. This reduced initial investment can make the technology more accessible to a wider range of market players, potentially accelerating its adoption. Additionally, the operational expenditure (OPEX) associated with butane-driven hydrogen production is competitive, particularly in regions where butane prices are favorable.
However, the economic viability of this technology is not without challenges. The price volatility of butane in the global market can impact the stability of production costs. This fluctuation may affect long-term planning and investment decisions for potential adopters. Moreover, the economic competitiveness of butane-driven hydrogen generation is closely tied to regional energy policies and incentives for renewable energy technologies.
The scalability of butane-based hydrogen production systems presents another economic consideration. While large-scale production can benefit from economies of scale, the technology's potential for modular and distributed generation opens up new economic models. This flexibility could allow for more targeted and efficient hydrogen production, potentially reducing transportation costs and improving overall economic viability.
When comparing the levelized cost of hydrogen (LCOH) from butane-driven processes to other production methods, early assessments suggest promising results. However, the exact figures vary depending on factors such as butane prices, process efficiency, and scale of production. As the technology matures and efficiencies improve, the LCOH is expected to decrease further, enhancing its economic competitiveness.
The economic viability of butane-driven hydrogen generation is also influenced by the broader hydrogen market dynamics. As demand for clean hydrogen grows across various sectors, including transportation and industry, the market potential for this technology expands. This increased demand could drive further investment and innovation in the field, potentially leading to cost reductions and improved economic viability over time.
Butane, as a feedstock for hydrogen production, offers several economic advantages. Its high hydrogen content and relatively low cost make it an attractive alternative to traditional fossil fuels. The abundance and established infrastructure for butane distribution further contribute to its economic appeal. These factors potentially lead to lower production costs for hydrogen compared to other methods.
The capital expenditure (CAPEX) for butane-based hydrogen generation facilities is generally lower than that of large-scale electrolysis plants. This reduced initial investment can make the technology more accessible to a wider range of market players, potentially accelerating its adoption. Additionally, the operational expenditure (OPEX) associated with butane-driven hydrogen production is competitive, particularly in regions where butane prices are favorable.
However, the economic viability of this technology is not without challenges. The price volatility of butane in the global market can impact the stability of production costs. This fluctuation may affect long-term planning and investment decisions for potential adopters. Moreover, the economic competitiveness of butane-driven hydrogen generation is closely tied to regional energy policies and incentives for renewable energy technologies.
The scalability of butane-based hydrogen production systems presents another economic consideration. While large-scale production can benefit from economies of scale, the technology's potential for modular and distributed generation opens up new economic models. This flexibility could allow for more targeted and efficient hydrogen production, potentially reducing transportation costs and improving overall economic viability.
When comparing the levelized cost of hydrogen (LCOH) from butane-driven processes to other production methods, early assessments suggest promising results. However, the exact figures vary depending on factors such as butane prices, process efficiency, and scale of production. As the technology matures and efficiencies improve, the LCOH is expected to decrease further, enhancing its economic competitiveness.
The economic viability of butane-driven hydrogen generation is also influenced by the broader hydrogen market dynamics. As demand for clean hydrogen grows across various sectors, including transportation and industry, the market potential for this technology expands. This increased demand could drive further investment and innovation in the field, potentially leading to cost reductions and improved economic viability over time.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!