How Does Perchloric Acid Affect Biomolecule Stability?
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid and Biomolecules: Background and Objectives
Perchloric acid, a powerful oxidizing agent and strong acid, has been a subject of significant interest in biochemistry and molecular biology due to its unique properties and effects on biomolecules. The study of perchloric acid's impact on biomolecule stability is crucial for understanding its potential applications and limitations in various scientific and industrial processes.
The primary objective of this technical research report is to comprehensively examine the interactions between perchloric acid and various biomolecules, including proteins, nucleic acids, lipids, and carbohydrates. By exploring these interactions, we aim to elucidate the mechanisms by which perchloric acid affects the stability, structure, and function of these essential biological components.
Historically, perchloric acid has been utilized in numerous biochemical applications, such as protein precipitation, nucleic acid extraction, and sample preparation for various analytical techniques. Its strong oxidizing properties and ability to form stable salts have made it a valuable tool in many laboratory procedures. However, these same properties also raise concerns about its potential to alter or damage biomolecules, necessitating a thorough investigation of its effects.
Recent advancements in analytical techniques and molecular biology have enabled researchers to delve deeper into the molecular-level interactions between perchloric acid and biomolecules. This has led to a growing body of knowledge regarding the acid's impact on protein denaturation, nucleic acid hydrolysis, lipid peroxidation, and carbohydrate degradation.
Understanding the stability of biomolecules in the presence of perchloric acid is not only crucial for optimizing existing laboratory protocols but also for developing new applications in fields such as proteomics, genomics, and metabolomics. Additionally, this knowledge is vital for assessing potential risks associated with the use of perchloric acid in industrial processes and environmental contexts.
The technological evolution in this field has been driven by the need for more precise control over biomolecule stability during various experimental procedures. This has led to the development of modified protocols, alternative reagents, and novel analytical methods that aim to mitigate the potentially detrimental effects of perchloric acid while harnessing its beneficial properties.
As we explore the current state of research on perchloric acid's effects on biomolecule stability, we will examine the latest findings, identify key challenges, and propose potential avenues for future investigation. This comprehensive analysis will provide valuable insights for researchers, industry professionals, and policymakers working in fields where the interaction between perchloric acid and biomolecules is of paramount importance.
The primary objective of this technical research report is to comprehensively examine the interactions between perchloric acid and various biomolecules, including proteins, nucleic acids, lipids, and carbohydrates. By exploring these interactions, we aim to elucidate the mechanisms by which perchloric acid affects the stability, structure, and function of these essential biological components.
Historically, perchloric acid has been utilized in numerous biochemical applications, such as protein precipitation, nucleic acid extraction, and sample preparation for various analytical techniques. Its strong oxidizing properties and ability to form stable salts have made it a valuable tool in many laboratory procedures. However, these same properties also raise concerns about its potential to alter or damage biomolecules, necessitating a thorough investigation of its effects.
Recent advancements in analytical techniques and molecular biology have enabled researchers to delve deeper into the molecular-level interactions between perchloric acid and biomolecules. This has led to a growing body of knowledge regarding the acid's impact on protein denaturation, nucleic acid hydrolysis, lipid peroxidation, and carbohydrate degradation.
Understanding the stability of biomolecules in the presence of perchloric acid is not only crucial for optimizing existing laboratory protocols but also for developing new applications in fields such as proteomics, genomics, and metabolomics. Additionally, this knowledge is vital for assessing potential risks associated with the use of perchloric acid in industrial processes and environmental contexts.
The technological evolution in this field has been driven by the need for more precise control over biomolecule stability during various experimental procedures. This has led to the development of modified protocols, alternative reagents, and novel analytical methods that aim to mitigate the potentially detrimental effects of perchloric acid while harnessing its beneficial properties.
As we explore the current state of research on perchloric acid's effects on biomolecule stability, we will examine the latest findings, identify key challenges, and propose potential avenues for future investigation. This comprehensive analysis will provide valuable insights for researchers, industry professionals, and policymakers working in fields where the interaction between perchloric acid and biomolecules is of paramount importance.
Market Analysis for Perchloric Acid in Biochemical Research
The market for perchloric acid in biochemical research is experiencing steady growth, driven by its unique properties and applications in various analytical techniques. Perchloric acid's strong oxidizing capabilities make it valuable for protein precipitation, extraction of nucleic acids, and sample preparation in mass spectrometry. These applications have led to increased demand in pharmaceutical research, proteomics, and genomics studies.
The global biochemical reagents market, which includes perchloric acid, is projected to expand significantly in the coming years. This growth is primarily attributed to the rising investments in life sciences research, advancements in biotechnology, and the increasing prevalence of chronic diseases necessitating more extensive biochemical studies. Perchloric acid's role in these research areas positions it as a key component in this market expansion.
Geographically, North America and Europe dominate the market for perchloric acid in biochemical research, owing to their well-established research infrastructure and substantial funding for life sciences. However, the Asia-Pacific region is emerging as a rapidly growing market, fueled by increasing government initiatives to boost biotechnology and pharmaceutical research in countries like China and India.
The market is characterized by a mix of large chemical manufacturers and specialized biochemical suppliers. Key players are focusing on developing high-purity grades of perchloric acid to meet the stringent requirements of advanced analytical techniques. This trend is expected to drive innovation in product formulations and packaging to ensure stability and safety during transportation and storage.
Despite its utility, the market for perchloric acid faces challenges related to safety concerns and regulatory restrictions. Its explosive nature when combined with organic compounds necessitates strict handling protocols, potentially limiting its widespread adoption. However, these challenges also present opportunities for companies to develop safer formulations or alternative reagents that can match perchloric acid's effectiveness in biochemical applications.
The increasing focus on personalized medicine and biomarker discovery is expected to further boost the demand for perchloric acid in proteomics research. Additionally, the growing field of metabolomics, which often requires efficient extraction and purification of metabolites from complex biological samples, is likely to contribute to the market's growth.
The global biochemical reagents market, which includes perchloric acid, is projected to expand significantly in the coming years. This growth is primarily attributed to the rising investments in life sciences research, advancements in biotechnology, and the increasing prevalence of chronic diseases necessitating more extensive biochemical studies. Perchloric acid's role in these research areas positions it as a key component in this market expansion.
Geographically, North America and Europe dominate the market for perchloric acid in biochemical research, owing to their well-established research infrastructure and substantial funding for life sciences. However, the Asia-Pacific region is emerging as a rapidly growing market, fueled by increasing government initiatives to boost biotechnology and pharmaceutical research in countries like China and India.
The market is characterized by a mix of large chemical manufacturers and specialized biochemical suppliers. Key players are focusing on developing high-purity grades of perchloric acid to meet the stringent requirements of advanced analytical techniques. This trend is expected to drive innovation in product formulations and packaging to ensure stability and safety during transportation and storage.
Despite its utility, the market for perchloric acid faces challenges related to safety concerns and regulatory restrictions. Its explosive nature when combined with organic compounds necessitates strict handling protocols, potentially limiting its widespread adoption. However, these challenges also present opportunities for companies to develop safer formulations or alternative reagents that can match perchloric acid's effectiveness in biochemical applications.
The increasing focus on personalized medicine and biomarker discovery is expected to further boost the demand for perchloric acid in proteomics research. Additionally, the growing field of metabolomics, which often requires efficient extraction and purification of metabolites from complex biological samples, is likely to contribute to the market's growth.
Current Challenges in Biomolecule Stability Studies
The field of biomolecule stability studies faces several significant challenges that hinder progress and limit the accuracy of research outcomes. One of the primary obstacles is the complexity of biological systems, which makes it difficult to isolate and study individual biomolecules without disrupting their natural environment. This complexity often leads to inconsistent results and challenges in reproducing experimental findings across different laboratories.
Another major challenge is the sensitivity of biomolecules to environmental factors. Temperature, pH, ionic strength, and the presence of other molecules can all significantly impact biomolecule stability. Researchers struggle to maintain consistent conditions throughout their experiments, especially when studying biomolecules over extended periods. This sensitivity also complicates the storage and handling of biological samples, potentially introducing artifacts into the data.
The lack of standardized protocols for stability studies presents another hurdle. Different research groups often employ varied methodologies, making it challenging to compare results across studies. This inconsistency hampers the development of a unified understanding of biomolecule stability and slows down the progress in the field.
Furthermore, the limitations of current analytical techniques pose significant challenges. Many methods used to assess biomolecule stability are invasive or destructive, potentially altering the very properties they aim to measure. Non-invasive techniques often lack the sensitivity or resolution required to detect subtle changes in biomolecule structure or function.
The time-dependent nature of stability studies also presents difficulties. Long-term studies are resource-intensive and prone to experimental errors, while short-term studies may not accurately predict long-term stability. Researchers struggle to find a balance between these competing factors, often leading to compromises in experimental design.
Additionally, the diverse nature of biomolecules, ranging from small metabolites to large proteins and nucleic acids, requires a wide array of specialized techniques and expertise. This diversity makes it challenging for researchers to develop comprehensive stability studies that encompass all relevant biomolecule classes.
Lastly, the impact of chemical agents, such as perchloric acid, on biomolecule stability remains a complex issue. While these agents are often used in sample preparation and analysis, their effects on biomolecule structure and function are not always fully understood or accounted for in stability studies. This knowledge gap can lead to misinterpretation of results and hinder the development of accurate stability models.
Another major challenge is the sensitivity of biomolecules to environmental factors. Temperature, pH, ionic strength, and the presence of other molecules can all significantly impact biomolecule stability. Researchers struggle to maintain consistent conditions throughout their experiments, especially when studying biomolecules over extended periods. This sensitivity also complicates the storage and handling of biological samples, potentially introducing artifacts into the data.
The lack of standardized protocols for stability studies presents another hurdle. Different research groups often employ varied methodologies, making it challenging to compare results across studies. This inconsistency hampers the development of a unified understanding of biomolecule stability and slows down the progress in the field.
Furthermore, the limitations of current analytical techniques pose significant challenges. Many methods used to assess biomolecule stability are invasive or destructive, potentially altering the very properties they aim to measure. Non-invasive techniques often lack the sensitivity or resolution required to detect subtle changes in biomolecule structure or function.
The time-dependent nature of stability studies also presents difficulties. Long-term studies are resource-intensive and prone to experimental errors, while short-term studies may not accurately predict long-term stability. Researchers struggle to find a balance between these competing factors, often leading to compromises in experimental design.
Additionally, the diverse nature of biomolecules, ranging from small metabolites to large proteins and nucleic acids, requires a wide array of specialized techniques and expertise. This diversity makes it challenging for researchers to develop comprehensive stability studies that encompass all relevant biomolecule classes.
Lastly, the impact of chemical agents, such as perchloric acid, on biomolecule stability remains a complex issue. While these agents are often used in sample preparation and analysis, their effects on biomolecule structure and function are not always fully understood or accounted for in stability studies. This knowledge gap can lead to misinterpretation of results and hinder the development of accurate stability models.
Existing Protocols for Perchloric Acid Usage in Biomolecule Studies
01 Storage and handling of perchloric acid
Proper storage and handling techniques are crucial for maintaining the stability of perchloric acid. This includes using specialized containers, controlling temperature and humidity, and implementing safety measures to prevent contamination or decomposition.- Storage and handling of perchloric acid: Proper storage and handling techniques are crucial for maintaining the stability of perchloric acid. This includes using specialized containers, controlling temperature and humidity, and implementing safety measures to prevent contamination or decomposition.
- Stabilization through additives: The addition of certain compounds can enhance the stability of perchloric acid. These additives may include antioxidants, pH regulators, or other chemical stabilizers that help prevent decomposition and maintain the acid's properties over time.
- Purification methods for improved stability: Various purification techniques can be employed to remove impurities and increase the stability of perchloric acid. These methods may include distillation, recrystallization, or advanced separation processes that help maintain the acid's purity and stability.
- Monitoring and control systems: Implementing advanced monitoring and control systems can help maintain the stability of perchloric acid during storage and use. These systems may include sensors for temperature, pressure, and chemical composition, as well as automated safety mechanisms to prevent degradation or accidents.
- Stabilization through concentration adjustment: Adjusting the concentration of perchloric acid can significantly impact its stability. Certain concentration ranges may offer improved stability, and techniques for precise concentration control and maintenance can be employed to enhance the acid's long-term stability.
02 Stabilization through additives
The addition of certain compounds can enhance the stability of perchloric acid. These additives may include antioxidants, pH regulators, or other chemical stabilizers that help prevent decomposition and maintain the acid's properties over time.Expand Specific Solutions03 Purification methods for improved stability
Various purification techniques can be employed to remove impurities and increase the stability of perchloric acid. These methods may include distillation, recrystallization, or advanced separation processes to obtain high-purity perchloric acid with enhanced stability.Expand Specific Solutions04 Monitoring and control systems
Implementing advanced monitoring and control systems can help maintain the stability of perchloric acid during storage and use. These systems may include sensors for temperature, pressure, and chemical composition, as well as automated safety mechanisms to prevent degradation or accidents.Expand Specific Solutions05 Stabilization through environmental control
Controlling the environmental conditions surrounding perchloric acid can significantly impact its stability. This may involve regulating factors such as light exposure, temperature fluctuations, and atmospheric composition to minimize degradation and maintain the acid's properties.Expand Specific Solutions
Key Players in Biochemical Reagents and Research
The competitive landscape for research on perchloric acid's impact on biomolecule stability is in an early development stage, with a relatively small but growing market. The technology is still emerging, with varying levels of maturity across different applications. Key players include academic institutions like Zhejiang University, Auburn University, and Louisiana State University, alongside industry leaders such as Ecolab USA, Inc. and Henkel AG & Co. KGaA. These organizations are exploring diverse aspects of perchloric acid's interactions with biomolecules, from fundamental research to potential industrial applications. The field shows promise for expansion as the importance of understanding biomolecule stability in various chemical environments continues to grow.
Zhejiang University
Technical Solution: Zhejiang University has developed a multi-faceted approach to investigate the impact of perchloric acid on biomolecule stability. Their research team has focused on the effects of perchloric acid on various biomolecules, including proteins, nucleic acids, and lipids. They have employed a combination of biophysical techniques, such as circular dichroism (CD) spectroscopy and fluorescence spectroscopy, to analyze conformational changes in biomolecules[3]. The university's researchers have discovered that perchloric acid can disrupt hydrogen bonding and electrostatic interactions in proteins, leading to altered secondary and tertiary structures. They have also identified specific amino acid residues that are particularly susceptible to perchloric acid-induced modifications[4]. Additionally, their studies on nucleic acids have revealed that perchloric acid can cause base pair mismatches and strand separation in DNA, potentially affecting genetic stability.
Strengths: Comprehensive analysis of perchloric acid effects on multiple types of biomolecules. Use of diverse biophysical techniques for detailed structural analysis. Weaknesses: Possible limitations in studying complex biomolecular interactions in the presence of perchloric acid. Challenges in extrapolating in vitro findings to physiological conditions.
Auburn University
Technical Solution: Auburn University has developed an innovative approach to study the effects of perchloric acid on biomolecule stability, focusing particularly on enzyme activity and protein-ligand interactions. Their research team has utilized a combination of enzymatic assays, isothermal titration calorimetry (ITC), and molecular dynamics simulations to investigate the impact of perchloric acid on biomolecular function[5]. They have found that perchloric acid can significantly alter enzyme kinetics, often leading to decreased catalytic efficiency. The university's researchers have also observed that perchloric acid can disrupt protein-ligand binding affinities, potentially affecting drug-target interactions and cellular signaling pathways[6]. Their molecular dynamics simulations have provided insights into the atomic-level changes in protein structure and dynamics induced by perchloric acid exposure, revealing specific regions of proteins that are most susceptible to acid-induced conformational changes.
Strengths: Integration of experimental and computational approaches for a comprehensive understanding of perchloric acid effects. Focus on functional consequences of acid-induced structural changes. Weaknesses: Limited investigation of other biomolecules beyond proteins and enzymes. Potential challenges in validating computational predictions experimentally.
Innovative Techniques for Assessing Biomolecule Stability
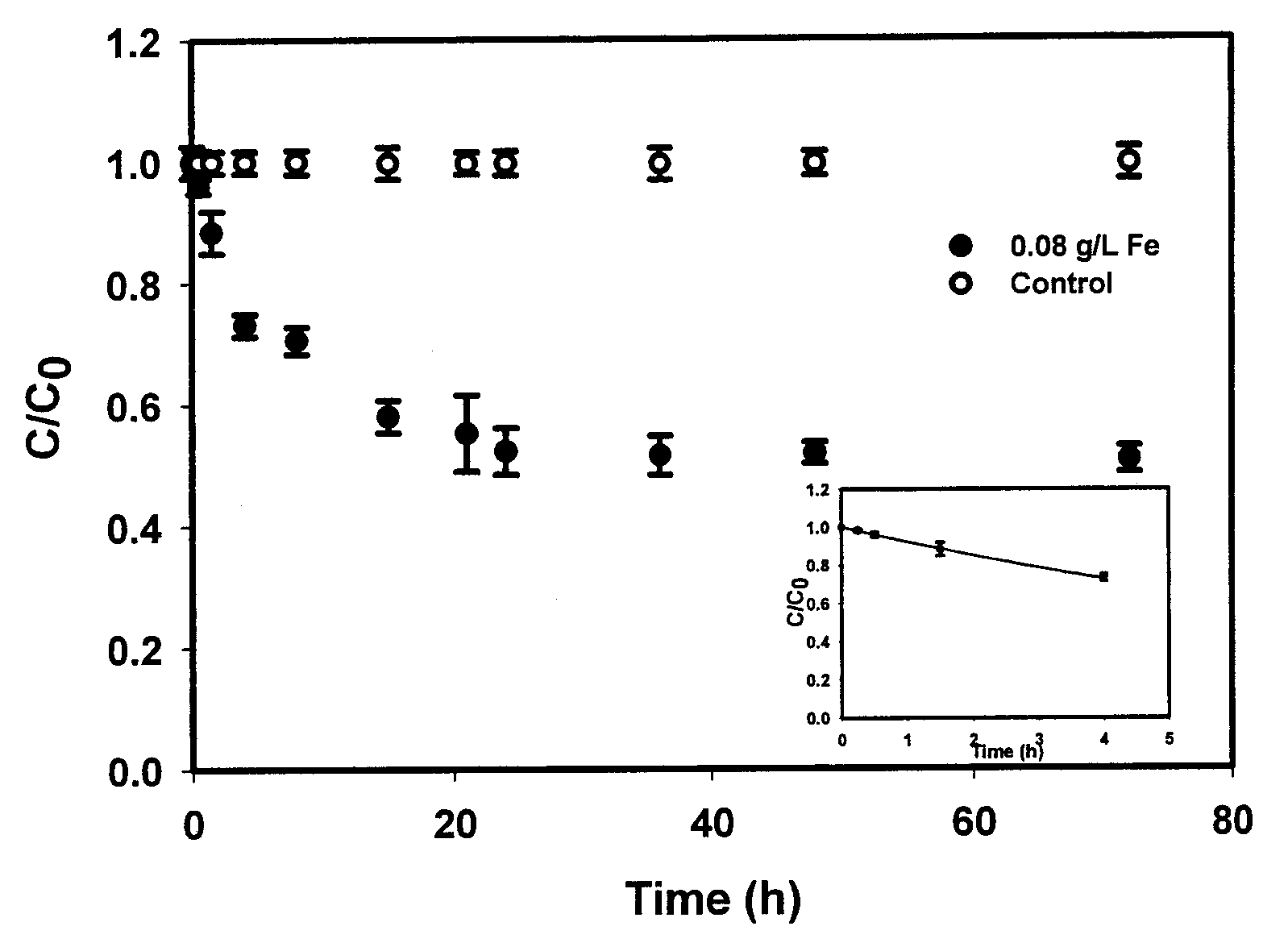
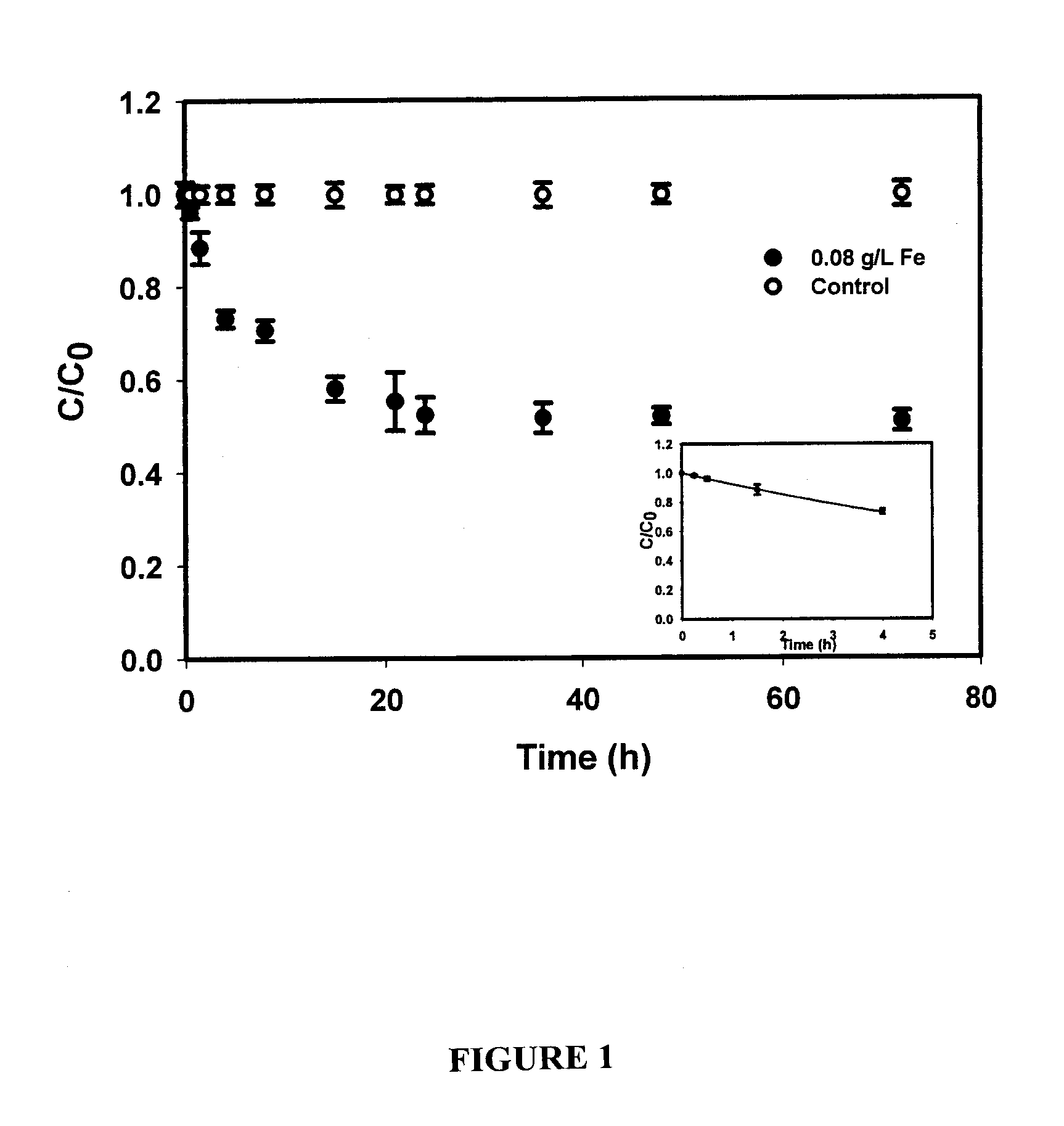
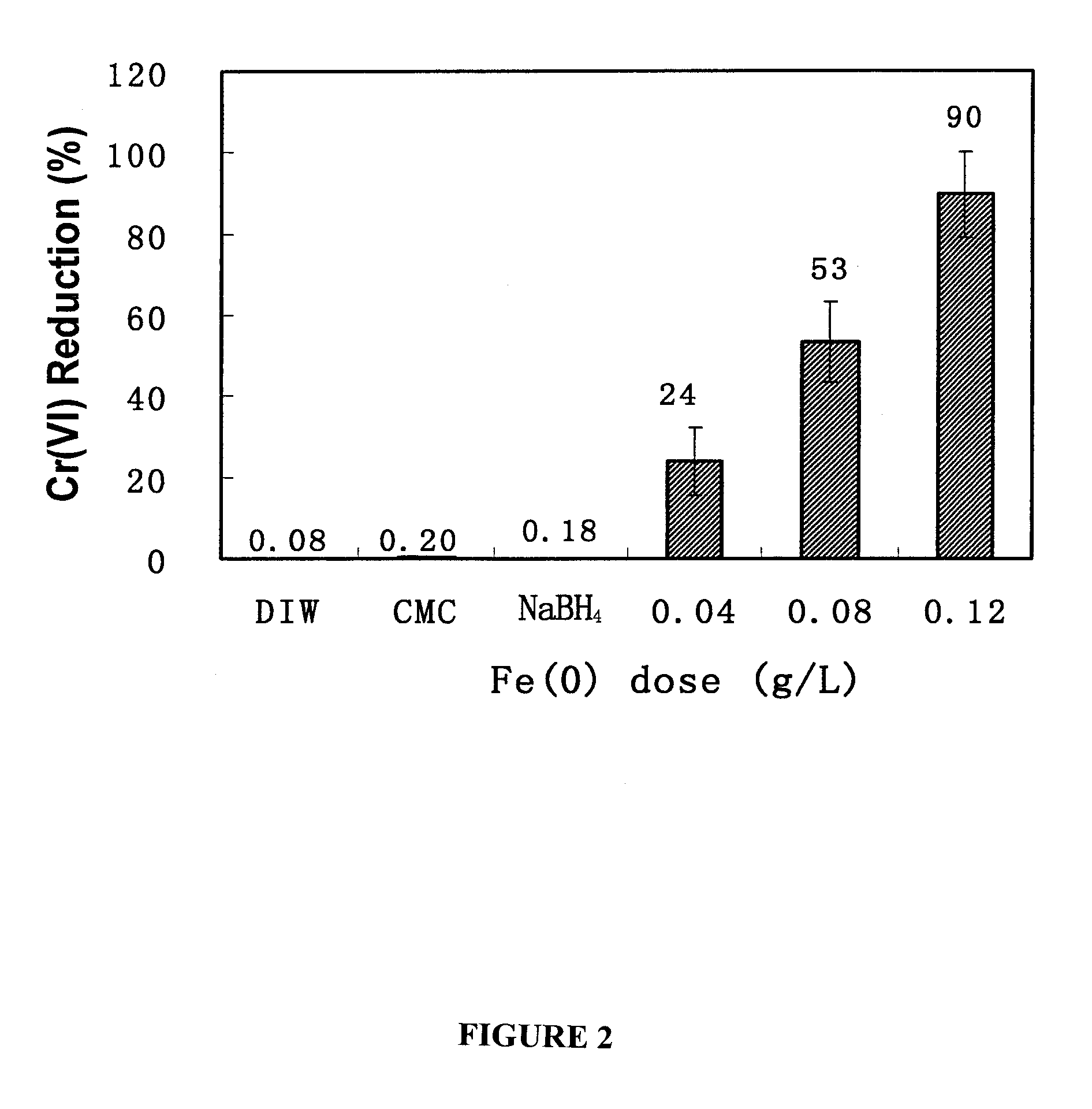
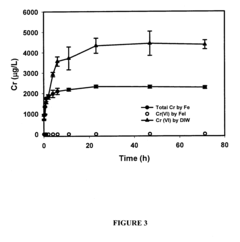
In situ remediation of inorganic contaminants using stabilized zero-valent iron nanoparticles
PatentInactiveUS7635236B2
Innovation
- The use of stabilized zero-valent iron nanoparticles, facilitated by polysaccharides like starch or cellulose as stabilizers, allows for controlled size, dispersibility, and reactivity, enabling effective in situ remediation of these toxins in water, soil, and brine by transforming them into less toxic forms.
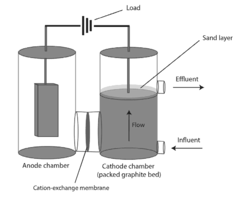
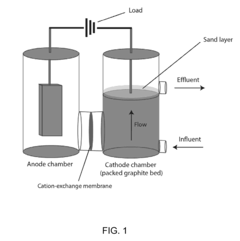
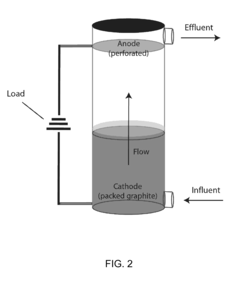
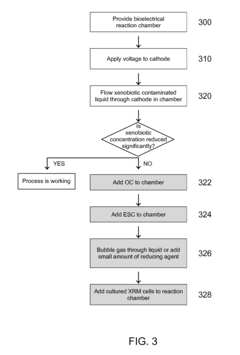
Bioelectrical treatment of xenobiotics
PatentInactiveUS20100108522A1
Innovation
- A bioelectrical reactor system that uses an electric current to provide electrons for microbial perchlorate reduction, eliminating the need for chemical electron donors and utilizing a cathode as an electron donor, thereby reducing biofouling and corrosion issues while maintaining long-term operation.
Safety Regulations for Handling Perchloric Acid in Labs
The handling of perchloric acid in laboratory settings requires strict adherence to safety regulations due to its highly reactive and potentially explosive nature. These regulations are designed to protect personnel, equipment, and facilities from the hazards associated with perchloric acid use.
Personal protective equipment (PPE) is paramount when working with perchloric acid. Laboratory personnel must wear chemical-resistant gloves, a lab coat, and safety goggles or a face shield. In cases where splashing or aerosolization is possible, additional protective gear such as a chemical-resistant apron and closed-toe shoes are mandatory.
Proper storage of perchloric acid is critical to prevent accidents. It must be stored in a cool, dry area away from organic materials, reducing agents, and other incompatible substances. Glass or other inert containers should be used, and secondary containment is required to capture potential spills. Regular inspections of storage areas are necessary to ensure container integrity and detect any signs of degradation or leakage.
Ventilation is a key safety consideration when working with perchloric acid. All operations involving the acid must be conducted in a designated perchloric acid fume hood equipped with a wash-down system. These specialized hoods are designed to prevent the accumulation of explosive perchlorate salts in the ductwork and must be regularly maintained and cleaned.
Waste disposal procedures for perchloric acid are stringent. The acid must never be disposed of down the drain or mixed with other chemical waste streams. Instead, it should be neutralized carefully and disposed of according to local regulations, typically through a certified chemical waste disposal service.
Emergency response protocols must be in place and well-communicated to all laboratory personnel. This includes the location and proper use of safety showers, eyewash stations, and spill kits specifically designed for perchloric acid. Regular safety drills and training sessions should be conducted to ensure all staff are prepared for potential incidents.
Documentation and training are essential components of safety regulations. Detailed standard operating procedures (SOPs) for handling perchloric acid must be developed, regularly reviewed, and made readily available to all users. Comprehensive training programs should be implemented, covering the properties of perchloric acid, proper handling techniques, emergency procedures, and the use of safety equipment.
Risk assessments should be conducted prior to any new procedures involving perchloric acid. These assessments help identify potential hazards and establish appropriate control measures. Regular audits of laboratory practices and safety equipment are necessary to maintain compliance with safety regulations and identify areas for improvement.
Personal protective equipment (PPE) is paramount when working with perchloric acid. Laboratory personnel must wear chemical-resistant gloves, a lab coat, and safety goggles or a face shield. In cases where splashing or aerosolization is possible, additional protective gear such as a chemical-resistant apron and closed-toe shoes are mandatory.
Proper storage of perchloric acid is critical to prevent accidents. It must be stored in a cool, dry area away from organic materials, reducing agents, and other incompatible substances. Glass or other inert containers should be used, and secondary containment is required to capture potential spills. Regular inspections of storage areas are necessary to ensure container integrity and detect any signs of degradation or leakage.
Ventilation is a key safety consideration when working with perchloric acid. All operations involving the acid must be conducted in a designated perchloric acid fume hood equipped with a wash-down system. These specialized hoods are designed to prevent the accumulation of explosive perchlorate salts in the ductwork and must be regularly maintained and cleaned.
Waste disposal procedures for perchloric acid are stringent. The acid must never be disposed of down the drain or mixed with other chemical waste streams. Instead, it should be neutralized carefully and disposed of according to local regulations, typically through a certified chemical waste disposal service.
Emergency response protocols must be in place and well-communicated to all laboratory personnel. This includes the location and proper use of safety showers, eyewash stations, and spill kits specifically designed for perchloric acid. Regular safety drills and training sessions should be conducted to ensure all staff are prepared for potential incidents.
Documentation and training are essential components of safety regulations. Detailed standard operating procedures (SOPs) for handling perchloric acid must be developed, regularly reviewed, and made readily available to all users. Comprehensive training programs should be implemented, covering the properties of perchloric acid, proper handling techniques, emergency procedures, and the use of safety equipment.
Risk assessments should be conducted prior to any new procedures involving perchloric acid. These assessments help identify potential hazards and establish appropriate control measures. Regular audits of laboratory practices and safety equipment are necessary to maintain compliance with safety regulations and identify areas for improvement.
Environmental Impact of Perchloric Acid in Research
The use of perchloric acid in research laboratories has significant environmental implications that warrant careful consideration. Perchloric acid is a strong oxidizing agent and a powerful dehydrating substance, which makes it valuable for various scientific applications. However, its reactive nature also poses potential risks to the environment if not properly managed.
When released into the environment, perchloric acid can have detrimental effects on soil and water ecosystems. It can alter the pH of soil and water bodies, potentially disrupting the delicate balance of microbial communities and affecting plant growth. The high oxidizing potential of perchloric acid can lead to the degradation of organic matter in soil, reducing its fertility and impacting agricultural productivity in affected areas.
In aquatic environments, perchloric acid can be particularly harmful. It can cause rapid changes in water chemistry, leading to stress or mortality in aquatic organisms. Fish and other aquatic life may experience respiratory difficulties due to the oxidizing effects of perchlorate ions on gill tissues. Furthermore, the persistence of perchlorate in water systems can result in long-term ecological impacts, as it may bioaccumulate in the food chain.
The atmospheric release of perchloric acid vapors during research activities can contribute to air pollution. These vapors can react with other atmospheric compounds, potentially forming secondary pollutants and contributing to smog formation in urban areas. Additionally, perchloric acid can react with metals and organic materials in the environment, leading to the formation of explosive perchlorates, which pose safety hazards.
Proper disposal of perchloric acid and its waste products is crucial to mitigate environmental risks. Improper disposal can lead to contamination of groundwater and surface water sources, potentially affecting drinking water supplies. The persistence of perchlorate in the environment is a significant concern, as it can remain stable for extended periods, leading to long-term environmental and health impacts.
Research institutions must implement strict protocols for the handling, storage, and disposal of perchloric acid to minimize its environmental footprint. This includes using fume hoods equipped with wash-down systems, implementing spill containment measures, and ensuring proper neutralization and disposal of perchloric acid waste. Additionally, alternatives to perchloric acid should be considered where possible to reduce its usage and associated environmental risks.
Monitoring and remediation efforts are essential in areas where perchloric acid contamination has occurred. Advanced treatment technologies, such as ion exchange and biological reduction, can be employed to remove perchlorate from contaminated water sources. However, these processes can be costly and time-consuming, emphasizing the importance of prevention and responsible use in research settings.
When released into the environment, perchloric acid can have detrimental effects on soil and water ecosystems. It can alter the pH of soil and water bodies, potentially disrupting the delicate balance of microbial communities and affecting plant growth. The high oxidizing potential of perchloric acid can lead to the degradation of organic matter in soil, reducing its fertility and impacting agricultural productivity in affected areas.
In aquatic environments, perchloric acid can be particularly harmful. It can cause rapid changes in water chemistry, leading to stress or mortality in aquatic organisms. Fish and other aquatic life may experience respiratory difficulties due to the oxidizing effects of perchlorate ions on gill tissues. Furthermore, the persistence of perchlorate in water systems can result in long-term ecological impacts, as it may bioaccumulate in the food chain.
The atmospheric release of perchloric acid vapors during research activities can contribute to air pollution. These vapors can react with other atmospheric compounds, potentially forming secondary pollutants and contributing to smog formation in urban areas. Additionally, perchloric acid can react with metals and organic materials in the environment, leading to the formation of explosive perchlorates, which pose safety hazards.
Proper disposal of perchloric acid and its waste products is crucial to mitigate environmental risks. Improper disposal can lead to contamination of groundwater and surface water sources, potentially affecting drinking water supplies. The persistence of perchlorate in the environment is a significant concern, as it can remain stable for extended periods, leading to long-term environmental and health impacts.
Research institutions must implement strict protocols for the handling, storage, and disposal of perchloric acid to minimize its environmental footprint. This includes using fume hoods equipped with wash-down systems, implementing spill containment measures, and ensuring proper neutralization and disposal of perchloric acid waste. Additionally, alternatives to perchloric acid should be considered where possible to reduce its usage and associated environmental risks.
Monitoring and remediation efforts are essential in areas where perchloric acid contamination has occurred. Advanced treatment technologies, such as ion exchange and biological reduction, can be employed to remove perchlorate from contaminated water sources. However, these processes can be costly and time-consuming, emphasizing the importance of prevention and responsible use in research settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!