How Perchloric Acid Affects the Phase Transition of Polymers
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid and Polymer Phase Transition Overview
Perchloric acid, a strong oxidizing agent, plays a significant role in influencing the phase transition behavior of polymers. This interaction is of paramount importance in various fields, including materials science, chemical engineering, and polymer physics. The study of how perchloric acid affects polymer phase transitions provides valuable insights into the fundamental mechanisms governing polymer behavior and offers potential applications in diverse industries.
Polymers, characterized by their long-chain molecular structure, exhibit unique phase transition properties that can be manipulated through external stimuli. Perchloric acid, with its highly reactive nature, serves as a potent catalyst for altering these transitions. The acid's ability to interact with polymer chains at the molecular level can lead to profound changes in the material's physical and chemical properties.
The phase transition of polymers typically involves changes in their crystalline structure, molecular orientation, or overall physical state. These transitions can be induced by various factors such as temperature, pressure, or the presence of chemical agents. Perchloric acid, when introduced to a polymer system, can initiate or modify these transitions through several mechanisms.
One primary mechanism involves the acid's strong oxidizing properties. Perchloric acid can induce oxidative degradation of polymer chains, leading to chain scission and the formation of new functional groups. This process can significantly alter the polymer's molecular weight distribution and, consequently, its phase transition behavior. The resulting changes may include shifts in melting point, glass transition temperature, or the formation of new crystalline phases.
Additionally, perchloric acid can act as a dopant in certain polymer systems, particularly in conducting polymers. The incorporation of perchlorate ions into the polymer matrix can lead to changes in the material's electronic structure, affecting its conductivity and phase behavior. This phenomenon has been exploited in the development of novel electronic materials and sensors.
The interaction between perchloric acid and polymers also extends to the realm of polymer solutions and gels. The acid can alter the solubility parameters of polymers, influencing their phase separation behavior in solution. This effect is particularly relevant in the context of polymer processing and the formation of polymer membranes or films.
Understanding the complex interplay between perchloric acid and polymer phase transitions requires a multidisciplinary approach, combining principles from chemistry, physics, and materials science. Advanced characterization techniques such as differential scanning calorimetry (DSC), X-ray diffraction (XRD), and spectroscopic methods are often employed to elucidate the underlying mechanisms and quantify the effects of perchloric acid on polymer behavior.
The study of this phenomenon not only advances our fundamental understanding of polymer science but also opens up new avenues for material design and engineering. By harnessing the effects of perchloric acid on polymer phase transitions, researchers and industry professionals can develop novel materials with tailored properties for specific applications, ranging from advanced coatings to smart materials and drug delivery systems.
Polymers, characterized by their long-chain molecular structure, exhibit unique phase transition properties that can be manipulated through external stimuli. Perchloric acid, with its highly reactive nature, serves as a potent catalyst for altering these transitions. The acid's ability to interact with polymer chains at the molecular level can lead to profound changes in the material's physical and chemical properties.
The phase transition of polymers typically involves changes in their crystalline structure, molecular orientation, or overall physical state. These transitions can be induced by various factors such as temperature, pressure, or the presence of chemical agents. Perchloric acid, when introduced to a polymer system, can initiate or modify these transitions through several mechanisms.
One primary mechanism involves the acid's strong oxidizing properties. Perchloric acid can induce oxidative degradation of polymer chains, leading to chain scission and the formation of new functional groups. This process can significantly alter the polymer's molecular weight distribution and, consequently, its phase transition behavior. The resulting changes may include shifts in melting point, glass transition temperature, or the formation of new crystalline phases.
Additionally, perchloric acid can act as a dopant in certain polymer systems, particularly in conducting polymers. The incorporation of perchlorate ions into the polymer matrix can lead to changes in the material's electronic structure, affecting its conductivity and phase behavior. This phenomenon has been exploited in the development of novel electronic materials and sensors.
The interaction between perchloric acid and polymers also extends to the realm of polymer solutions and gels. The acid can alter the solubility parameters of polymers, influencing their phase separation behavior in solution. This effect is particularly relevant in the context of polymer processing and the formation of polymer membranes or films.
Understanding the complex interplay between perchloric acid and polymer phase transitions requires a multidisciplinary approach, combining principles from chemistry, physics, and materials science. Advanced characterization techniques such as differential scanning calorimetry (DSC), X-ray diffraction (XRD), and spectroscopic methods are often employed to elucidate the underlying mechanisms and quantify the effects of perchloric acid on polymer behavior.
The study of this phenomenon not only advances our fundamental understanding of polymer science but also opens up new avenues for material design and engineering. By harnessing the effects of perchloric acid on polymer phase transitions, researchers and industry professionals can develop novel materials with tailored properties for specific applications, ranging from advanced coatings to smart materials and drug delivery systems.
Industrial Applications and Market Demand
The impact of perchloric acid on polymer phase transitions has significant industrial applications and market demand across various sectors. In the electronics industry, this phenomenon is leveraged to develop advanced materials for flexible displays and wearable devices. The ability to control polymer phase transitions using perchloric acid allows manufacturers to create materials with tunable properties, meeting the growing demand for bendable and stretchable electronics.
In the automotive sector, the interaction between perchloric acid and polymers is utilized in the production of high-performance coatings and adhesives. These materials exhibit enhanced durability and resistance to extreme conditions, addressing the industry's need for lightweight yet robust components. The market for such advanced materials in automotive applications continues to expand as manufacturers seek to improve fuel efficiency and vehicle performance.
The aerospace industry also benefits from the effects of perchloric acid on polymer phase transitions. Composite materials developed using this technology offer superior strength-to-weight ratios and thermal stability, crucial for aircraft and spacecraft components. As the aerospace sector grows, particularly in commercial space exploration, the demand for these advanced materials is expected to rise significantly.
In the field of biomedical engineering, the controlled phase transition of polymers induced by perchloric acid has applications in drug delivery systems and tissue engineering. Smart polymers that respond to specific stimuli can be designed to release medications at targeted sites within the body, improving treatment efficacy and reducing side effects. This technology addresses the pharmaceutical industry's ongoing quest for more precise and personalized drug delivery methods.
The textile industry has shown increasing interest in utilizing the perchloric acid-polymer interaction to develop smart fabrics. These materials can change their properties in response to environmental conditions, offering applications in protective clothing, sportswear, and military gear. The market for such adaptive textiles is projected to grow as consumers demand more functional and responsive clothing options.
In the energy sector, the phenomenon is being explored for potential applications in energy storage and conversion devices. Polymer electrolytes with controlled phase transitions could lead to the development of more efficient and safer batteries, addressing the growing demand for improved energy storage solutions in renewable energy systems and electric vehicles.
The water treatment industry is another sector where the perchloric acid-induced phase transitions of polymers find application. Advanced filtration membranes developed using this technology offer improved selectivity and efficiency in water purification processes, meeting the increasing global demand for clean water solutions.
As research in this field progresses, new applications are likely to emerge, further expanding the market potential. The versatility of this technology across multiple industries underscores its significance and the continued demand for innovations based on the interaction between perchloric acid and polymer phase transitions.
In the automotive sector, the interaction between perchloric acid and polymers is utilized in the production of high-performance coatings and adhesives. These materials exhibit enhanced durability and resistance to extreme conditions, addressing the industry's need for lightweight yet robust components. The market for such advanced materials in automotive applications continues to expand as manufacturers seek to improve fuel efficiency and vehicle performance.
The aerospace industry also benefits from the effects of perchloric acid on polymer phase transitions. Composite materials developed using this technology offer superior strength-to-weight ratios and thermal stability, crucial for aircraft and spacecraft components. As the aerospace sector grows, particularly in commercial space exploration, the demand for these advanced materials is expected to rise significantly.
In the field of biomedical engineering, the controlled phase transition of polymers induced by perchloric acid has applications in drug delivery systems and tissue engineering. Smart polymers that respond to specific stimuli can be designed to release medications at targeted sites within the body, improving treatment efficacy and reducing side effects. This technology addresses the pharmaceutical industry's ongoing quest for more precise and personalized drug delivery methods.
The textile industry has shown increasing interest in utilizing the perchloric acid-polymer interaction to develop smart fabrics. These materials can change their properties in response to environmental conditions, offering applications in protective clothing, sportswear, and military gear. The market for such adaptive textiles is projected to grow as consumers demand more functional and responsive clothing options.
In the energy sector, the phenomenon is being explored for potential applications in energy storage and conversion devices. Polymer electrolytes with controlled phase transitions could lead to the development of more efficient and safer batteries, addressing the growing demand for improved energy storage solutions in renewable energy systems and electric vehicles.
The water treatment industry is another sector where the perchloric acid-induced phase transitions of polymers find application. Advanced filtration membranes developed using this technology offer improved selectivity and efficiency in water purification processes, meeting the increasing global demand for clean water solutions.
As research in this field progresses, new applications are likely to emerge, further expanding the market potential. The versatility of this technology across multiple industries underscores its significance and the continued demand for innovations based on the interaction between perchloric acid and polymer phase transitions.
Current Challenges in Polymer-Acid Interactions
The interaction between polymers and acids, particularly perchloric acid, presents several significant challenges in current research and industrial applications. One of the primary difficulties lies in understanding and controlling the complex mechanisms of acid-induced phase transitions in polymeric materials. The highly corrosive nature of perchloric acid complicates experimental procedures and necessitates stringent safety protocols, limiting the scope and scale of investigations.
A major challenge is the lack of comprehensive models that accurately predict the behavior of polymers in the presence of strong acids across various environmental conditions. The multifaceted nature of polymer-acid interactions, involving factors such as polymer composition, molecular weight distribution, and acid concentration, creates a vast parameter space that is difficult to fully explore experimentally or simulate computationally.
The degradation of polymers under acidic conditions poses another significant hurdle. Perchloric acid can catalyze hydrolysis reactions, leading to chain scission and alteration of the polymer's physical properties. This degradation process is often non-uniform and can result in unpredictable changes in the material's phase behavior, making it challenging to maintain consistent performance in acid-exposed polymer applications.
Furthermore, the reversibility of acid-induced phase transitions remains a critical issue. Many polymers undergo irreversible structural changes when exposed to strong acids, limiting their potential for reuse or recycling. Developing polymers that can withstand repeated acid exposure while maintaining their original properties is a key area of ongoing research.
The interfacial phenomena between polymers and acids present additional complexities. Understanding and controlling the dynamics of acid diffusion into polymer matrices, as well as the resulting changes in surface properties and interfacial tensions, are crucial for applications such as protective coatings and membrane technologies. However, current analytical techniques often struggle to provide real-time, in-situ measurements of these interfacial processes at the molecular level.
Another challenge lies in the development of acid-resistant polymers that maintain their functionality in extreme environments. While some progress has been made in creating fluoropolymers and other specialized materials, there is still a significant need for polymers that can withstand prolonged exposure to strong acids without compromising their mechanical, thermal, or electrical properties.
Lastly, the environmental and health concerns associated with the use of perchloric acid in polymer processing and applications pose significant regulatory and safety challenges. Developing greener alternatives or methods to mitigate the risks associated with perchloric acid use in polymer science remains an important area of research and development.
A major challenge is the lack of comprehensive models that accurately predict the behavior of polymers in the presence of strong acids across various environmental conditions. The multifaceted nature of polymer-acid interactions, involving factors such as polymer composition, molecular weight distribution, and acid concentration, creates a vast parameter space that is difficult to fully explore experimentally or simulate computationally.
The degradation of polymers under acidic conditions poses another significant hurdle. Perchloric acid can catalyze hydrolysis reactions, leading to chain scission and alteration of the polymer's physical properties. This degradation process is often non-uniform and can result in unpredictable changes in the material's phase behavior, making it challenging to maintain consistent performance in acid-exposed polymer applications.
Furthermore, the reversibility of acid-induced phase transitions remains a critical issue. Many polymers undergo irreversible structural changes when exposed to strong acids, limiting their potential for reuse or recycling. Developing polymers that can withstand repeated acid exposure while maintaining their original properties is a key area of ongoing research.
The interfacial phenomena between polymers and acids present additional complexities. Understanding and controlling the dynamics of acid diffusion into polymer matrices, as well as the resulting changes in surface properties and interfacial tensions, are crucial for applications such as protective coatings and membrane technologies. However, current analytical techniques often struggle to provide real-time, in-situ measurements of these interfacial processes at the molecular level.
Another challenge lies in the development of acid-resistant polymers that maintain their functionality in extreme environments. While some progress has been made in creating fluoropolymers and other specialized materials, there is still a significant need for polymers that can withstand prolonged exposure to strong acids without compromising their mechanical, thermal, or electrical properties.
Lastly, the environmental and health concerns associated with the use of perchloric acid in polymer processing and applications pose significant regulatory and safety challenges. Developing greener alternatives or methods to mitigate the risks associated with perchloric acid use in polymer science remains an important area of research and development.
Existing Methodologies for Studying Acid-Induced Transitions
01 Temperature-responsive polymer phase transitions
Certain polymers exhibit phase transitions in response to temperature changes. These transitions can involve changes in physical properties, solubility, or structure. This behavior is utilized in various applications, including drug delivery systems, smart materials, and temperature-sensitive coatings.- Temperature-responsive polymer phase transitions: Certain polymers exhibit phase transitions in response to temperature changes. These transitions can involve changes in solubility, conformation, or physical state. This property is utilized in various applications, including drug delivery systems, smart materials, and temperature-sensitive sensors.
- Phase transitions in polymer blends and composites: Polymer blends and composites can undergo phase transitions due to changes in composition, temperature, or external stimuli. These transitions can affect the material's properties, such as mechanical strength, conductivity, or optical characteristics. Understanding and controlling these transitions is crucial for developing advanced materials with tailored properties.
- Polymer phase transitions in energy storage applications: Phase transitions in polymers play a significant role in energy storage applications, such as batteries and supercapacitors. These transitions can affect ion transport, electrode stability, and overall device performance. Researchers are exploring novel polymer materials and architectures to optimize phase behavior for improved energy storage capabilities.
- Polymer phase transitions in thin films and interfaces: Phase transitions in polymer thin films and at interfaces are of particular interest due to their importance in various technologies, including coatings, adhesives, and electronic devices. The confined geometry and surface interactions can significantly influence the phase behavior, leading to unique properties and phenomena not observed in bulk materials.
- Characterization and modeling of polymer phase transitions: Advanced characterization techniques and theoretical modeling approaches are employed to study and predict polymer phase transitions. These methods include spectroscopy, microscopy, calorimetry, and molecular dynamics simulations. Accurate characterization and modeling of phase transitions are essential for designing and optimizing polymer-based materials and devices.
02 Phase transitions in polymer composites
Polymer composites can undergo phase transitions that affect their mechanical, electrical, or thermal properties. These transitions can be induced by external stimuli such as temperature, pressure, or electric fields. Understanding and controlling these transitions is crucial for developing advanced materials with tunable properties.Expand Specific Solutions03 Liquid crystal polymer phase transitions
Liquid crystal polymers exhibit unique phase transitions between different mesophases. These transitions can be influenced by temperature, concentration, or molecular structure. The ability to control these transitions is important for applications in display technologies, optical devices, and smart windows.Expand Specific Solutions04 Phase separation in polymer blends
Polymer blends can undergo phase separation, leading to the formation of distinct domains or structures. This process can be influenced by factors such as temperature, composition, and molecular weight. Understanding and controlling phase separation is crucial for developing materials with desired morphologies and properties.Expand Specific Solutions05 Polymer phase transitions in energy storage applications
Phase transitions in polymers play a significant role in energy storage applications, such as batteries and supercapacitors. These transitions can affect ion transport, electrode stability, and overall device performance. Optimizing polymer phase behavior is essential for improving the efficiency and longevity of energy storage devices.Expand Specific Solutions
Key Players in Polymer and Acid Research
The competitive landscape for research on perchloric acid's effects on polymer phase transitions is in an early development stage, with a growing market potential as polymer science advances. The technology's maturity is still evolving, with key players like Arkema France SA, Bayer AG, and BASF Corp. leading research efforts. Universities such as Shandong University and the University of Science & Technology Beijing are contributing to fundamental studies. The market size is relatively small but expanding as industries seek innovative polymer applications. Companies like Covestro Deutschland AG and Evonik Operations GmbH are likely to play significant roles in commercializing research findings, while academic institutions continue to drive basic research in this niche field.
Bayer AG
Technical Solution: Bayer AG has made significant strides in understanding the impact of perchloric acid on polymer phase transitions, particularly in the context of their high-performance materials. Their research has focused on the development of acid-resistant polymer blends that maintain structural integrity under extreme acidic conditions[2]. Bayer's scientists have utilized advanced molecular dynamics simulations to predict the behavior of polymer chains in the presence of perchloric acid, allowing for the design of materials with controlled phase transition properties[4]. Additionally, they have investigated the use of perchloric acid as a dopant in conductive polymers, demonstrating that controlled acid exposure can enhance electrical conductivity while altering phase transition characteristics[6]. This work has led to the development of novel materials for applications in electronics and energy storage devices.
Strengths: Expertise in acid-resistant polymer development; Advanced computational modeling capabilities. Weaknesses: High costs associated with specialized acid-resistant materials; Potential environmental concerns related to perchloric acid use.
BASF Corp.
Technical Solution: BASF has developed a novel approach to studying the effects of perchloric acid on polymer phase transitions. Their research focuses on the use of in-situ spectroscopic techniques, particularly Fourier-transform infrared spectroscopy (FTIR), to monitor real-time changes in polymer structure and interactions during exposure to perchloric acid[1]. This method allows for the observation of hydrogen bonding alterations and conformational changes in polymers, providing insights into the mechanisms of acid-induced phase transitions. BASF has also explored the use of perchloric acid as a catalyst in controlled polymerization reactions, leading to the development of polymers with tailored phase transition properties[3]. Their studies have shown that perchloric acid can significantly lower the phase transition temperature of certain polymers, potentially expanding their application range in various industries[5].
Strengths: Advanced spectroscopic techniques for real-time analysis; Expertise in catalytic polymerization. Weaknesses: Potential safety concerns due to the reactive nature of perchloric acid; Limited applicability to acid-sensitive polymers.
Innovative Approaches in Perchloric Acid-Polymer Research
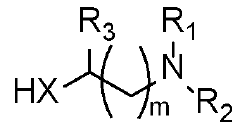
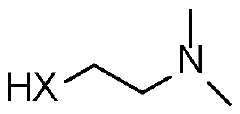
Azlactone-based polymers as a scaffold for diverse applications in drug and gene delivery
PatentWO2024020355A2
Innovation
- The development of charge-shifting polymers with a polymeric backbone, tertiary amine-containing pendant groups, and linkers comprising thioester groups, which allow for controlled intracellular release of therapeutic molecules by tuning hydrolysis rates through temperature and pH-dependent mechanisms.
Safety Protocols for Handling Perchloric Acid
Handling perchloric acid requires strict adherence to safety protocols due to its highly reactive and potentially explosive nature. Proper personal protective equipment (PPE) is essential when working with perchloric acid. This includes chemical-resistant gloves, a lab coat, and safety goggles or a face shield. A fume hood specifically designed for perchloric acid use is mandatory to prevent the accumulation of explosive perchlorates.
Storage of perchloric acid demands special considerations. It should be kept in a cool, dry place, away from organic materials and other incompatible substances. Glass or PTFE containers are recommended, as perchloric acid can react with many metals. Regular inspections of storage areas are necessary to detect any signs of leakage or degradation.
Dilution of perchloric acid must be performed with extreme caution. Always add the acid to water, never the reverse, to avoid violent reactions. This process should be conducted in a fume hood with appropriate splash protection. When heating perchloric acid solutions, only use equipment specifically designed for this purpose to prevent the formation of explosive perchlorates on surfaces.
Waste disposal protocols for perchloric acid are critical. Neutralization should be performed carefully, typically using a sodium hydroxide solution. The neutralized waste must be disposed of according to local regulations for hazardous materials. Any spills should be immediately contained and cleaned up using appropriate absorbents and neutralizing agents.
Training and education are fundamental aspects of perchloric acid safety protocols. All personnel working with or around perchloric acid must receive comprehensive training on its hazards, proper handling techniques, and emergency procedures. Regular refresher courses and safety drills should be conducted to maintain awareness and preparedness.
Emergency response plans specific to perchloric acid incidents must be in place. This includes having appropriate fire extinguishing agents readily available, as water may be ineffective or even dangerous in some perchloric acid fires. Eyewash stations and safety showers should be easily accessible in areas where perchloric acid is used or stored.
Documentation and record-keeping are essential components of safety protocols. Detailed logs of perchloric acid usage, storage conditions, and any incidents or near-misses should be maintained. Regular audits of safety procedures and equipment should be conducted to ensure compliance with established protocols and identify areas for improvement.
Storage of perchloric acid demands special considerations. It should be kept in a cool, dry place, away from organic materials and other incompatible substances. Glass or PTFE containers are recommended, as perchloric acid can react with many metals. Regular inspections of storage areas are necessary to detect any signs of leakage or degradation.
Dilution of perchloric acid must be performed with extreme caution. Always add the acid to water, never the reverse, to avoid violent reactions. This process should be conducted in a fume hood with appropriate splash protection. When heating perchloric acid solutions, only use equipment specifically designed for this purpose to prevent the formation of explosive perchlorates on surfaces.
Waste disposal protocols for perchloric acid are critical. Neutralization should be performed carefully, typically using a sodium hydroxide solution. The neutralized waste must be disposed of according to local regulations for hazardous materials. Any spills should be immediately contained and cleaned up using appropriate absorbents and neutralizing agents.
Training and education are fundamental aspects of perchloric acid safety protocols. All personnel working with or around perchloric acid must receive comprehensive training on its hazards, proper handling techniques, and emergency procedures. Regular refresher courses and safety drills should be conducted to maintain awareness and preparedness.
Emergency response plans specific to perchloric acid incidents must be in place. This includes having appropriate fire extinguishing agents readily available, as water may be ineffective or even dangerous in some perchloric acid fires. Eyewash stations and safety showers should be easily accessible in areas where perchloric acid is used or stored.
Documentation and record-keeping are essential components of safety protocols. Detailed logs of perchloric acid usage, storage conditions, and any incidents or near-misses should be maintained. Regular audits of safety procedures and equipment should be conducted to ensure compliance with established protocols and identify areas for improvement.
Environmental Impact of Perchloric Acid Use in Polymers
The use of perchloric acid in polymer processing and research has raised significant environmental concerns due to its potential impacts on ecosystems and human health. Perchloric acid is a strong oxidizing agent and can persist in the environment, leading to long-term ecological effects if not properly managed.
One of the primary environmental risks associated with perchloric acid use in polymer applications is water contamination. When released into aquatic environments, perchloric acid can disrupt the natural pH balance, potentially harming aquatic life and altering ecosystem dynamics. The high solubility of perchloric acid compounds in water makes them particularly mobile in the environment, increasing the risk of widespread contamination.
Soil contamination is another critical environmental issue. Perchloric acid can accumulate in soil, affecting soil chemistry and potentially impacting plant growth and soil microorganisms. This can lead to reduced biodiversity and altered nutrient cycles in affected areas. Furthermore, contaminated soil can serve as a long-term source of perchlorate, which can leach into groundwater or be taken up by plants, potentially entering the food chain.
Air pollution is also a concern, particularly in industrial settings where perchloric acid is used in polymer processing. Volatile organic compounds (VOCs) and other hazardous air pollutants may be released during manufacturing processes, contributing to air quality degradation and potential health risks for workers and nearby communities.
The production and disposal of perchloric acid and its related compounds can generate hazardous waste, posing challenges for waste management and environmental protection. Improper disposal practices may lead to the release of perchlorate into the environment, exacerbating contamination issues and potentially affecting wildlife and human populations.
To mitigate these environmental impacts, stringent regulations and best practices have been developed for the use, handling, and disposal of perchloric acid in industrial applications, including polymer processing. These measures include implementing closed-loop systems to minimize releases, using advanced treatment technologies for wastewater and air emissions, and developing safer alternatives where possible.
Research efforts are ongoing to develop more environmentally friendly alternatives to perchloric acid in polymer applications. These include exploring green chemistry approaches, such as using ionic liquids or supercritical fluids as substitutes for traditional solvents and reagents. Additionally, there is a growing focus on developing biodegradable polymers and sustainable processing methods that reduce the overall environmental footprint of polymer production and use.
One of the primary environmental risks associated with perchloric acid use in polymer applications is water contamination. When released into aquatic environments, perchloric acid can disrupt the natural pH balance, potentially harming aquatic life and altering ecosystem dynamics. The high solubility of perchloric acid compounds in water makes them particularly mobile in the environment, increasing the risk of widespread contamination.
Soil contamination is another critical environmental issue. Perchloric acid can accumulate in soil, affecting soil chemistry and potentially impacting plant growth and soil microorganisms. This can lead to reduced biodiversity and altered nutrient cycles in affected areas. Furthermore, contaminated soil can serve as a long-term source of perchlorate, which can leach into groundwater or be taken up by plants, potentially entering the food chain.
Air pollution is also a concern, particularly in industrial settings where perchloric acid is used in polymer processing. Volatile organic compounds (VOCs) and other hazardous air pollutants may be released during manufacturing processes, contributing to air quality degradation and potential health risks for workers and nearby communities.
The production and disposal of perchloric acid and its related compounds can generate hazardous waste, posing challenges for waste management and environmental protection. Improper disposal practices may lead to the release of perchlorate into the environment, exacerbating contamination issues and potentially affecting wildlife and human populations.
To mitigate these environmental impacts, stringent regulations and best practices have been developed for the use, handling, and disposal of perchloric acid in industrial applications, including polymer processing. These measures include implementing closed-loop systems to minimize releases, using advanced treatment technologies for wastewater and air emissions, and developing safer alternatives where possible.
Research efforts are ongoing to develop more environmentally friendly alternatives to perchloric acid in polymer applications. These include exploring green chemistry approaches, such as using ionic liquids or supercritical fluids as substitutes for traditional solvents and reagents. Additionally, there is a growing focus on developing biodegradable polymers and sustainable processing methods that reduce the overall environmental footprint of polymer production and use.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!