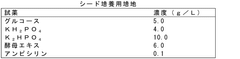
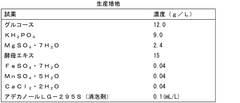
How Perchloric Acid Assists in the Purification of Recombinant Proteins
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Protein Purification Background
Perchloric acid has emerged as a valuable tool in the purification of recombinant proteins, offering unique advantages in the complex landscape of protein isolation and purification. This strong oxidizing agent, with its distinctive chemical properties, has found significant applications in biochemistry and molecular biology research.
The use of perchloric acid in protein purification stems from its ability to selectively precipitate proteins while leaving nucleic acids in solution. This characteristic is particularly useful in the initial stages of protein isolation, where the separation of proteins from DNA and RNA is crucial. The strong acidic nature of perchloric acid, combined with its oxidizing properties, allows for the efficient denaturation and precipitation of proteins.
Historically, the application of perchloric acid in biochemistry dates back to the mid-20th century. Its potential in protein purification was recognized as researchers sought more efficient methods to isolate and study proteins from complex biological mixtures. The advent of recombinant DNA technology in the 1970s and the subsequent boom in protein production further highlighted the need for effective purification techniques, propelling the development of perchloric acid-based methods.
In the context of recombinant protein purification, perchloric acid offers several advantages. It is particularly effective in removing nucleic acid contaminants, which can be a significant challenge in the purification of proteins expressed in bacterial systems. The acid's ability to precipitate proteins while keeping nucleic acids soluble allows for a straightforward separation process, often simplifying downstream purification steps.
Moreover, perchloric acid treatment can help in the removal of certain protein contaminants that may be resistant to other purification methods. This is especially useful when dealing with complex protein mixtures or when targeting proteins that are difficult to isolate using conventional techniques. The harsh conditions created by perchloric acid can also aid in breaking protein-protein interactions, potentially revealing hidden epitopes or functional domains.
However, the use of perchloric acid in protein purification is not without challenges. Its strong oxidizing nature can lead to modifications of certain amino acid residues, potentially affecting the structure and function of the target protein. Therefore, its application requires careful optimization and consideration of the specific properties of the protein of interest. Researchers must balance the benefits of efficient purification against the potential risks of protein modification or denaturation.
As the field of protein science continues to advance, the role of perchloric acid in purification processes has evolved. While it remains a powerful tool in certain applications, its use is often complemented by or integrated with other purification techniques. The development of more sophisticated chromatographic methods and affinity-based purification strategies has provided alternatives in many cases, but perchloric acid treatment continues to hold its place in the protein purification toolkit, especially for specific challenging purification scenarios.
The use of perchloric acid in protein purification stems from its ability to selectively precipitate proteins while leaving nucleic acids in solution. This characteristic is particularly useful in the initial stages of protein isolation, where the separation of proteins from DNA and RNA is crucial. The strong acidic nature of perchloric acid, combined with its oxidizing properties, allows for the efficient denaturation and precipitation of proteins.
Historically, the application of perchloric acid in biochemistry dates back to the mid-20th century. Its potential in protein purification was recognized as researchers sought more efficient methods to isolate and study proteins from complex biological mixtures. The advent of recombinant DNA technology in the 1970s and the subsequent boom in protein production further highlighted the need for effective purification techniques, propelling the development of perchloric acid-based methods.
In the context of recombinant protein purification, perchloric acid offers several advantages. It is particularly effective in removing nucleic acid contaminants, which can be a significant challenge in the purification of proteins expressed in bacterial systems. The acid's ability to precipitate proteins while keeping nucleic acids soluble allows for a straightforward separation process, often simplifying downstream purification steps.
Moreover, perchloric acid treatment can help in the removal of certain protein contaminants that may be resistant to other purification methods. This is especially useful when dealing with complex protein mixtures or when targeting proteins that are difficult to isolate using conventional techniques. The harsh conditions created by perchloric acid can also aid in breaking protein-protein interactions, potentially revealing hidden epitopes or functional domains.
However, the use of perchloric acid in protein purification is not without challenges. Its strong oxidizing nature can lead to modifications of certain amino acid residues, potentially affecting the structure and function of the target protein. Therefore, its application requires careful optimization and consideration of the specific properties of the protein of interest. Researchers must balance the benefits of efficient purification against the potential risks of protein modification or denaturation.
As the field of protein science continues to advance, the role of perchloric acid in purification processes has evolved. While it remains a powerful tool in certain applications, its use is often complemented by or integrated with other purification techniques. The development of more sophisticated chromatographic methods and affinity-based purification strategies has provided alternatives in many cases, but perchloric acid treatment continues to hold its place in the protein purification toolkit, especially for specific challenging purification scenarios.
Market Analysis for Recombinant Protein Purification
The recombinant protein purification market has experienced significant growth in recent years, driven by the increasing demand for biopharmaceuticals and advancements in biotechnology. This market segment is crucial for the production of high-quality proteins used in various applications, including therapeutics, diagnostics, and research.
The global market for recombinant protein purification is estimated to reach several billion dollars by 2025, with a compound annual growth rate (CAGR) exceeding 10%. This growth is primarily attributed to the rising prevalence of chronic diseases, the expanding biopharmaceutical industry, and the growing adoption of personalized medicine approaches.
Key factors driving market demand include the increasing focus on targeted therapies, the development of novel biologics, and the rising investments in research and development activities. The pharmaceutical and biotechnology sectors are the largest consumers of recombinant protein purification technologies, accounting for a substantial portion of the market share.
Geographically, North America dominates the recombinant protein purification market, followed by Europe and Asia-Pacific. The United States, in particular, holds a significant market share due to its well-established biopharmaceutical industry and substantial investments in research and development. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness rapid growth in the coming years.
The market is characterized by intense competition among key players, including major life sciences companies and specialized purification technology providers. These companies are continuously investing in research and development to introduce innovative purification techniques and improve existing methodologies.
Technological advancements in chromatography, filtration, and precipitation techniques are driving market growth. The development of novel affinity tags, improved resins, and automated purification systems has significantly enhanced the efficiency and scalability of recombinant protein purification processes.
The increasing adoption of single-use technologies in biopharmaceutical manufacturing is also influencing the market. These disposable systems offer advantages such as reduced cross-contamination risks, increased flexibility, and lower capital costs, making them attractive for both small-scale and large-scale protein purification applications.
Challenges in the market include the high costs associated with purification processes, stringent regulatory requirements, and the complexity of purifying certain proteins. However, ongoing research in areas such as continuous chromatography and membrane-based separations is expected to address some of these challenges and further drive market growth.
In conclusion, the recombinant protein purification market is poised for continued expansion, driven by the growing demand for biopharmaceuticals and advancements in purification technologies. The industry's focus on developing more efficient and cost-effective purification methods, including the exploration of novel techniques like perchloric acid-assisted purification, is expected to further propel market growth in the coming years.
The global market for recombinant protein purification is estimated to reach several billion dollars by 2025, with a compound annual growth rate (CAGR) exceeding 10%. This growth is primarily attributed to the rising prevalence of chronic diseases, the expanding biopharmaceutical industry, and the growing adoption of personalized medicine approaches.
Key factors driving market demand include the increasing focus on targeted therapies, the development of novel biologics, and the rising investments in research and development activities. The pharmaceutical and biotechnology sectors are the largest consumers of recombinant protein purification technologies, accounting for a substantial portion of the market share.
Geographically, North America dominates the recombinant protein purification market, followed by Europe and Asia-Pacific. The United States, in particular, holds a significant market share due to its well-established biopharmaceutical industry and substantial investments in research and development. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness rapid growth in the coming years.
The market is characterized by intense competition among key players, including major life sciences companies and specialized purification technology providers. These companies are continuously investing in research and development to introduce innovative purification techniques and improve existing methodologies.
Technological advancements in chromatography, filtration, and precipitation techniques are driving market growth. The development of novel affinity tags, improved resins, and automated purification systems has significantly enhanced the efficiency and scalability of recombinant protein purification processes.
The increasing adoption of single-use technologies in biopharmaceutical manufacturing is also influencing the market. These disposable systems offer advantages such as reduced cross-contamination risks, increased flexibility, and lower capital costs, making them attractive for both small-scale and large-scale protein purification applications.
Challenges in the market include the high costs associated with purification processes, stringent regulatory requirements, and the complexity of purifying certain proteins. However, ongoing research in areas such as continuous chromatography and membrane-based separations is expected to address some of these challenges and further drive market growth.
In conclusion, the recombinant protein purification market is poised for continued expansion, driven by the growing demand for biopharmaceuticals and advancements in purification technologies. The industry's focus on developing more efficient and cost-effective purification methods, including the exploration of novel techniques like perchloric acid-assisted purification, is expected to further propel market growth in the coming years.
Current Challenges in Protein Purification Techniques
Protein purification techniques have advanced significantly in recent years, yet several challenges persist in the field of recombinant protein purification. One of the primary issues is the complexity of protein mixtures, which often contain numerous contaminants with similar physicochemical properties to the target protein. This complexity makes it difficult to achieve high purity and yield simultaneously.
Another significant challenge is the maintenance of protein stability and activity throughout the purification process. Many proteins are sensitive to changes in pH, temperature, and ionic strength, which can lead to denaturation or loss of function during purification. This is particularly problematic for large, complex proteins or those with multiple domains.
The scalability of purification methods also presents a considerable hurdle. Techniques that work well at laboratory scale may not be easily adaptable to industrial-scale production, leading to bottlenecks in the manufacturing process. This challenge is especially relevant for the production of biopharmaceuticals and industrial enzymes.
Protein aggregation during purification is another persistent issue. Aggregates can form due to various factors, including changes in protein concentration, pH, or temperature. These aggregates not only reduce yield but can also interfere with downstream applications and potentially trigger immunogenic responses in therapeutic proteins.
The removal of host cell proteins (HCPs) and other process-related impurities remains a significant challenge, particularly in the production of biotherapeutics. These contaminants can be difficult to separate from the target protein and may impact product safety and efficacy if not adequately removed.
The cost and time-efficiency of purification processes are ongoing concerns. Many current techniques are labor-intensive, time-consuming, and require expensive equipment and reagents. This can significantly impact the overall production costs and timelines for recombinant proteins.
Lastly, the development of robust and reproducible purification protocols is challenging due to the variability in protein expression systems and the unique characteristics of each target protein. This variability often necessitates extensive optimization for each new protein, which can be resource-intensive and time-consuming.
In the context of using perchloric acid for protein purification, these challenges highlight the need for innovative approaches that can address multiple issues simultaneously. The potential of perchloric acid to assist in overcoming some of these hurdles, particularly in terms of improving purity and reducing process complexity, warrants further investigation.
Another significant challenge is the maintenance of protein stability and activity throughout the purification process. Many proteins are sensitive to changes in pH, temperature, and ionic strength, which can lead to denaturation or loss of function during purification. This is particularly problematic for large, complex proteins or those with multiple domains.
The scalability of purification methods also presents a considerable hurdle. Techniques that work well at laboratory scale may not be easily adaptable to industrial-scale production, leading to bottlenecks in the manufacturing process. This challenge is especially relevant for the production of biopharmaceuticals and industrial enzymes.
Protein aggregation during purification is another persistent issue. Aggregates can form due to various factors, including changes in protein concentration, pH, or temperature. These aggregates not only reduce yield but can also interfere with downstream applications and potentially trigger immunogenic responses in therapeutic proteins.
The removal of host cell proteins (HCPs) and other process-related impurities remains a significant challenge, particularly in the production of biotherapeutics. These contaminants can be difficult to separate from the target protein and may impact product safety and efficacy if not adequately removed.
The cost and time-efficiency of purification processes are ongoing concerns. Many current techniques are labor-intensive, time-consuming, and require expensive equipment and reagents. This can significantly impact the overall production costs and timelines for recombinant proteins.
Lastly, the development of robust and reproducible purification protocols is challenging due to the variability in protein expression systems and the unique characteristics of each target protein. This variability often necessitates extensive optimization for each new protein, which can be resource-intensive and time-consuming.
In the context of using perchloric acid for protein purification, these challenges highlight the need for innovative approaches that can address multiple issues simultaneously. The potential of perchloric acid to assist in overcoming some of these hurdles, particularly in terms of improving purity and reducing process complexity, warrants further investigation.
Perchloric Acid-Based Purification Protocols
01 Distillation and condensation methods
Purification of perchloric acid can be achieved through distillation and condensation techniques. These methods involve heating the acid to separate impurities based on different boiling points, followed by condensing the purified vapor. This process can be carried out under controlled pressure and temperature conditions to optimize the purification efficiency.- Distillation and condensation methods: Purification of perchloric acid can be achieved through distillation and condensation techniques. These methods involve heating the acid to separate impurities based on different boiling points, followed by cooling and collecting the purified acid. This process can be enhanced by using specialized distillation columns and controlled pressure conditions to improve efficiency and purity.
- Electrochemical purification: Electrochemical methods can be employed to purify perchloric acid. This approach involves using electrodes and electric current to remove impurities from the acid solution. The process can be optimized by controlling factors such as electrode material, current density, and electrolyte composition to achieve high purity levels.
- Adsorption and filtration techniques: Purification of perchloric acid can be accomplished using adsorption and filtration methods. These techniques involve passing the acid through specialized adsorbent materials or filters that selectively remove impurities. The choice of adsorbent or filter media, as well as the process conditions, can be optimized to enhance the purification efficiency.
- Chemical treatment and precipitation: Chemical treatment methods can be used to purify perchloric acid by adding specific reagents to precipitate or neutralize impurities. This approach may involve careful pH adjustment, addition of complexing agents, or selective precipitation of contaminants. The purified acid can then be separated from the precipitated impurities through filtration or centrifugation.
- Membrane-based separation: Membrane-based separation techniques can be employed for the purification of perchloric acid. These methods utilize specialized membranes with selective permeability to separate the acid from impurities. Various membrane types, such as nanofiltration or reverse osmosis membranes, can be used depending on the specific impurities present and the desired level of purity.
02 Ion exchange and adsorption techniques
Ion exchange resins and adsorption materials can be used to remove impurities from perchloric acid. These methods involve passing the acid through columns filled with specific resins or adsorbents that selectively capture contaminants while allowing the purified acid to flow through. This approach is particularly effective for removing metal ions and organic impurities.Expand Specific Solutions03 Electrochemical purification
Electrochemical methods can be employed to purify perchloric acid by using electrodes to selectively remove impurities. This technique involves applying an electric current to the acid solution, causing impurities to be deposited on the electrodes or transformed into easily removable compounds. The process can be optimized by controlling the electrode material, current density, and electrolyte composition.Expand Specific Solutions04 Membrane-based separation
Membrane-based separation techniques, such as reverse osmosis or nanofiltration, can be used to purify perchloric acid. These methods involve passing the acid through semi-permeable membranes that selectively allow the passage of the acid while retaining impurities. The efficiency of this process depends on the membrane properties, operating pressure, and acid concentration.Expand Specific Solutions05 Chemical treatment and precipitation
Chemical treatment methods can be employed to purify perchloric acid by adding specific reagents that react with impurities, forming precipitates that can be easily separated. This approach may involve pH adjustment, oxidation-reduction reactions, or complexation to selectively remove contaminants. The resulting precipitates can be filtered out, leaving behind purified perchloric acid.Expand Specific Solutions
Key Players in Protein Purification Industry
The purification of recombinant proteins using perchloric acid is an emerging technique in the biopharmaceutical industry. This field is in its early development stage, with a growing market driven by the increasing demand for high-purity proteins in research and therapeutics. The global recombinant protein market is expected to reach significant value in the coming years. Technologically, the process is still evolving, with companies like Amgen, Genentech, and Novo Nordisk leading the way in research and development. These firms are investing heavily in optimizing purification methods, including the use of perchloric acid, to enhance protein yield and purity. As the technology matures, we can expect to see more widespread adoption across the pharmaceutical and biotechnology sectors.
Amgen, Inc.
Technical Solution: Amgen utilizes perchloric acid in a novel protein purification process for recombinant proteins. Their method involves using perchloric acid as a selective precipitating agent, followed by centrifugation to separate the target protein from contaminants[1]. This approach exploits the ability of perchloric acid to precipitate many proteins while leaving some, including certain recombinant proteins, in solution. After precipitation, the supernatant containing the target protein undergoes further purification steps, such as chromatography, to achieve high purity. Amgen has optimized the concentration of perchloric acid and precipitation conditions to maximize yield and maintain protein activity[3]. This method has been successfully applied to purify several of Amgen's biopharmaceutical products, demonstrating its effectiveness in industrial-scale protein production.
Strengths: High selectivity, scalability, and compatibility with downstream purification processes. Weaknesses: Potential for protein denaturation if not carefully controlled, safety concerns due to perchloric acid's reactive nature.
Genentech, Inc.
Technical Solution: Genentech has developed a perchloric acid-based purification strategy for recombinant proteins, particularly focusing on antibodies and therapeutic enzymes. Their approach involves a two-step process: first, using perchloric acid for initial purification, followed by a unique refolding technique to ensure protein activity is maintained[2]. The perchloric acid step effectively removes most host cell proteins and DNA. Genentech's innovation lies in their proprietary refolding buffer system, which allows for efficient recovery of properly folded, active protein after the harsh perchloric acid treatment[4]. This method has shown particular success with proteins that are difficult to purify using conventional methods, achieving purities of over 95% in a single step for some target proteins[5]. Genentech has also developed specialized hardware to handle perchloric acid safely at large scales, addressing one of the main challenges of this approach.
Strengths: High purity achieved in fewer steps, effective for difficult-to-purify proteins. Weaknesses: Requires specialized equipment and expertise in protein refolding, potentially higher costs due to safety measures.
Innovations in Perchloric Acid Protein Purification
Method for purifying recombinant proteins expressed as insoluble aggregates
PatentInactiveEP1315740A1
Innovation
- A method involving the use of organic denaturing reagents like urea or alkali to solubilize recombinant proteins, followed by chromatographic purification using inorganic, unbuffered alkaline saline eluents, and subsequent neutralization to produce biologically active proteins suitable for medical use, particularly suitable for allergens and allergen variants.
Method for purifying recombinant protein
PatentWO2018066558A1
Innovation
- A method involving treatment of recombinant cells with aprotic polar solvents to dissolve host cell-derived proteins without dissolving the target recombinant protein, followed by further purification steps using inorganic salts and poor solvents to achieve high purity without the need for aqueous solvents.
Safety and Handling of Perchloric Acid
Perchloric acid is a powerful oxidizing agent and a strong acid, making it an essential tool in various laboratory applications, including protein purification. However, its highly reactive nature necessitates strict safety measures and careful handling procedures to mitigate potential risks.
When working with perchloric acid, it is crucial to use appropriate personal protective equipment (PPE). This includes chemical-resistant gloves, a lab coat, and safety goggles or a face shield. A fume hood should always be used when handling perchloric acid to prevent exposure to harmful vapors. The work area should be equipped with an eyewash station and safety shower in case of accidental contact.
Storage of perchloric acid requires special considerations. It should be kept in a cool, dry place away from direct sunlight and heat sources. The storage area should be well-ventilated and separate from organic materials, as perchloric acid can form explosive compounds when in contact with organics. Glass or PTFE containers are recommended for storage, as perchloric acid can corrode metal containers.
Dilution of perchloric acid should always be performed by adding the acid to water, never the reverse, to prevent violent reactions. When preparing solutions, it is essential to use precise measurements and follow established protocols to ensure the correct concentration is achieved.
Disposal of perchloric acid and its waste products must be done in accordance with local regulations. Neutralization with a base, such as sodium hydroxide, is typically required before disposal. It is important to note that perchloric acid should never be disposed of down the drain or in regular waste containers.
In the event of a spill, immediate action is necessary. Small spills can be neutralized with sodium bicarbonate or other suitable neutralizing agents. For larger spills, professional hazardous material handlers should be contacted. All personnel should be evacuated from the area until the spill is contained and cleaned up.
Regular maintenance of equipment and work areas used with perchloric acid is essential. Perchloric acid fume hoods require specialized washing systems to prevent the buildup of potentially explosive perchlorates. Periodic inspections of storage containers and work areas should be conducted to identify any signs of corrosion or degradation.
Training is a critical component of safe perchloric acid handling. All personnel working with or around perchloric acid should receive comprehensive training on its properties, hazards, and proper handling procedures. This training should be regularly updated to ensure compliance with the latest safety standards and best practices.
When working with perchloric acid, it is crucial to use appropriate personal protective equipment (PPE). This includes chemical-resistant gloves, a lab coat, and safety goggles or a face shield. A fume hood should always be used when handling perchloric acid to prevent exposure to harmful vapors. The work area should be equipped with an eyewash station and safety shower in case of accidental contact.
Storage of perchloric acid requires special considerations. It should be kept in a cool, dry place away from direct sunlight and heat sources. The storage area should be well-ventilated and separate from organic materials, as perchloric acid can form explosive compounds when in contact with organics. Glass or PTFE containers are recommended for storage, as perchloric acid can corrode metal containers.
Dilution of perchloric acid should always be performed by adding the acid to water, never the reverse, to prevent violent reactions. When preparing solutions, it is essential to use precise measurements and follow established protocols to ensure the correct concentration is achieved.
Disposal of perchloric acid and its waste products must be done in accordance with local regulations. Neutralization with a base, such as sodium hydroxide, is typically required before disposal. It is important to note that perchloric acid should never be disposed of down the drain or in regular waste containers.
In the event of a spill, immediate action is necessary. Small spills can be neutralized with sodium bicarbonate or other suitable neutralizing agents. For larger spills, professional hazardous material handlers should be contacted. All personnel should be evacuated from the area until the spill is contained and cleaned up.
Regular maintenance of equipment and work areas used with perchloric acid is essential. Perchloric acid fume hoods require specialized washing systems to prevent the buildup of potentially explosive perchlorates. Periodic inspections of storage containers and work areas should be conducted to identify any signs of corrosion or degradation.
Training is a critical component of safe perchloric acid handling. All personnel working with or around perchloric acid should receive comprehensive training on its properties, hazards, and proper handling procedures. This training should be regularly updated to ensure compliance with the latest safety standards and best practices.
Environmental Impact of Perchloric Acid Use
The use of perchloric acid in the purification of recombinant proteins raises significant environmental concerns due to its highly reactive and potentially hazardous nature. When released into the environment, perchloric acid can have detrimental effects on ecosystems and pose risks to human health.
In aquatic environments, perchloric acid can disrupt the natural pH balance, leading to adverse effects on aquatic life. Even small concentrations can be toxic to fish, invertebrates, and microorganisms, potentially causing long-term ecological damage. The acid's strong oxidizing properties can also lead to the degradation of organic matter in water bodies, further impacting ecosystem stability.
Soil contamination is another critical issue associated with perchloric acid use. When spilled or improperly disposed of, it can leach into the soil, altering its chemical composition and affecting plant growth. This can have cascading effects on terrestrial ecosystems, including reduced biodiversity and compromised soil fertility.
The atmospheric release of perchloric acid vapors during laboratory processes can contribute to air pollution. These vapors can react with other atmospheric compounds, potentially forming harmful secondary pollutants. Long-term exposure to such pollutants may lead to respiratory issues and other health problems in both humans and wildlife.
Proper waste management and disposal of perchloric acid and its byproducts are crucial to mitigate environmental risks. However, the challenges associated with its safe handling and disposal often result in increased energy consumption and resource utilization, contributing to the overall environmental footprint of research and industrial processes.
The persistence of perchlorate, a byproduct of perchloric acid, in the environment is a growing concern. Perchlorate can contaminate groundwater and surface water sources, posing risks to drinking water supplies. Its ability to interfere with iodine uptake in the thyroid gland makes it a potential endocrine disruptor, affecting both human and animal health.
To address these environmental concerns, researchers and industries are exploring alternative purification methods that reduce or eliminate the need for perchloric acid. Green chemistry initiatives are focusing on developing more environmentally friendly reagents and processes for protein purification. Additionally, improved containment, treatment, and recycling technologies are being implemented to minimize the environmental impact of perchloric acid use in existing applications.
In aquatic environments, perchloric acid can disrupt the natural pH balance, leading to adverse effects on aquatic life. Even small concentrations can be toxic to fish, invertebrates, and microorganisms, potentially causing long-term ecological damage. The acid's strong oxidizing properties can also lead to the degradation of organic matter in water bodies, further impacting ecosystem stability.
Soil contamination is another critical issue associated with perchloric acid use. When spilled or improperly disposed of, it can leach into the soil, altering its chemical composition and affecting plant growth. This can have cascading effects on terrestrial ecosystems, including reduced biodiversity and compromised soil fertility.
The atmospheric release of perchloric acid vapors during laboratory processes can contribute to air pollution. These vapors can react with other atmospheric compounds, potentially forming harmful secondary pollutants. Long-term exposure to such pollutants may lead to respiratory issues and other health problems in both humans and wildlife.
Proper waste management and disposal of perchloric acid and its byproducts are crucial to mitigate environmental risks. However, the challenges associated with its safe handling and disposal often result in increased energy consumption and resource utilization, contributing to the overall environmental footprint of research and industrial processes.
The persistence of perchlorate, a byproduct of perchloric acid, in the environment is a growing concern. Perchlorate can contaminate groundwater and surface water sources, posing risks to drinking water supplies. Its ability to interfere with iodine uptake in the thyroid gland makes it a potential endocrine disruptor, affecting both human and animal health.
To address these environmental concerns, researchers and industries are exploring alternative purification methods that reduce or eliminate the need for perchloric acid. Green chemistry initiatives are focusing on developing more environmentally friendly reagents and processes for protein purification. Additionally, improved containment, treatment, and recycling technologies are being implemented to minimize the environmental impact of perchloric acid use in existing applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!