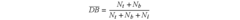How Perchloric Acid Catalyzes the Synthesis of Hyperbranched Polymers
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Catalysis in Polymer Synthesis
Perchloric acid has emerged as a powerful catalyst in the synthesis of hyperbranched polymers, revolutionizing the field of polymer chemistry. This strong acid plays a crucial role in promoting the polymerization process, leading to the formation of highly branched macromolecules with unique properties and applications. The catalytic action of perchloric acid in hyperbranched polymer synthesis has been a subject of intense research and development over the past few decades.
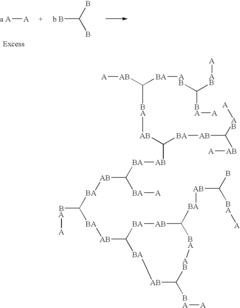
The mechanism by which perchloric acid catalyzes the synthesis of hyperbranched polymers involves several key steps. Initially, the acid protonates the functional groups of the monomer units, activating them for subsequent reactions. This protonation step is critical in initiating the polymerization process and controlling the branching pattern of the resulting polymer. The strong acidic nature of perchloric acid ensures efficient protonation, even at low catalyst concentrations.
Following the activation of monomers, perchloric acid facilitates the formation of covalent bonds between the activated species. This step is characterized by rapid and selective reactions, leading to the growth of polymer chains with multiple branching points. The catalyst's ability to maintain its activity throughout the polymerization process contributes to the formation of highly branched structures with a high degree of functionality.
One of the key advantages of using perchloric acid as a catalyst in hyperbranched polymer synthesis is its ability to promote controlled polymerization. The acid's strong proton-donating capacity allows for precise control over the reaction kinetics, enabling the synthesis of polymers with predetermined molecular weights and branching densities. This level of control is essential for tailoring the properties of the resulting hyperbranched polymers to specific applications.
Furthermore, perchloric acid catalysis often results in hyperbranched polymers with narrow molecular weight distributions. This characteristic is attributed to the catalyst's ability to maintain a consistent reaction environment throughout the polymerization process. The uniformity in molecular weight distribution contributes to the reproducibility of polymer properties, which is crucial for industrial applications.
The catalytic efficiency of perchloric acid in hyperbranched polymer synthesis is also influenced by reaction conditions such as temperature, solvent choice, and monomer concentration. Optimizing these parameters in conjunction with the catalyst concentration allows for fine-tuning of the polymerization process, resulting in hyperbranched polymers with desired architectures and functionalities.
In recent years, researchers have explored modifications to the perchloric acid catalysis system to enhance its performance and expand its applicability. These modifications include the use of supported perchloric acid catalysts, which offer advantages in terms of catalyst recovery and reusability. Additionally, the combination of perchloric acid with co-catalysts or additives has shown promise in further improving the control over polymer structure and properties.
The mechanism by which perchloric acid catalyzes the synthesis of hyperbranched polymers involves several key steps. Initially, the acid protonates the functional groups of the monomer units, activating them for subsequent reactions. This protonation step is critical in initiating the polymerization process and controlling the branching pattern of the resulting polymer. The strong acidic nature of perchloric acid ensures efficient protonation, even at low catalyst concentrations.
Following the activation of monomers, perchloric acid facilitates the formation of covalent bonds between the activated species. This step is characterized by rapid and selective reactions, leading to the growth of polymer chains with multiple branching points. The catalyst's ability to maintain its activity throughout the polymerization process contributes to the formation of highly branched structures with a high degree of functionality.
One of the key advantages of using perchloric acid as a catalyst in hyperbranched polymer synthesis is its ability to promote controlled polymerization. The acid's strong proton-donating capacity allows for precise control over the reaction kinetics, enabling the synthesis of polymers with predetermined molecular weights and branching densities. This level of control is essential for tailoring the properties of the resulting hyperbranched polymers to specific applications.
Furthermore, perchloric acid catalysis often results in hyperbranched polymers with narrow molecular weight distributions. This characteristic is attributed to the catalyst's ability to maintain a consistent reaction environment throughout the polymerization process. The uniformity in molecular weight distribution contributes to the reproducibility of polymer properties, which is crucial for industrial applications.
The catalytic efficiency of perchloric acid in hyperbranched polymer synthesis is also influenced by reaction conditions such as temperature, solvent choice, and monomer concentration. Optimizing these parameters in conjunction with the catalyst concentration allows for fine-tuning of the polymerization process, resulting in hyperbranched polymers with desired architectures and functionalities.
In recent years, researchers have explored modifications to the perchloric acid catalysis system to enhance its performance and expand its applicability. These modifications include the use of supported perchloric acid catalysts, which offer advantages in terms of catalyst recovery and reusability. Additionally, the combination of perchloric acid with co-catalysts or additives has shown promise in further improving the control over polymer structure and properties.
Market Demand for Hyperbranched Polymers
The market demand for hyperbranched polymers has been steadily increasing due to their unique properties and versatile applications across various industries. These highly branched macromolecules offer exceptional characteristics such as low viscosity, high solubility, and numerous functional end groups, making them attractive for a wide range of applications.
In the coatings industry, hyperbranched polymers have gained significant traction as additives and modifiers. Their ability to enhance scratch resistance, improve adhesion, and reduce viscosity in coating formulations has led to increased adoption in automotive, industrial, and protective coatings. The growing emphasis on environmentally friendly products has further boosted the demand for hyperbranched polymers in water-based and low-VOC coating systems.
The electronics sector has also shown a rising interest in hyperbranched polymers. These materials are being increasingly used in the production of printed circuit boards, electronic packaging, and as dielectric materials in microelectronics. Their low shrinkage, high thermal stability, and excellent electrical properties make them ideal for these applications, driving market growth in the electronics industry.
In the medical and pharmaceutical fields, hyperbranched polymers are finding applications in drug delivery systems, tissue engineering, and medical devices. Their biocompatibility, biodegradability, and ability to encapsulate and release drugs in a controlled manner have led to increased research and development activities, potentially opening up new market opportunities.
The packaging industry is another sector where hyperbranched polymers are gaining traction. Their barrier properties, recyclability, and potential to reduce material usage align well with the growing demand for sustainable packaging solutions. This has led to increased interest from food and beverage companies looking to improve their packaging performance while reducing environmental impact.
As environmental regulations become more stringent, the demand for hyperbranched polymers in water treatment applications is also on the rise. Their ability to act as effective flocculants and chelating agents for heavy metal removal has opened up new market opportunities in industrial wastewater treatment and municipal water purification processes.
The global market for hyperbranched polymers is expected to continue its growth trajectory, driven by ongoing research and development efforts to expand their applications and improve their performance. As industries seek innovative materials to address challenges in sustainability, efficiency, and performance, hyperbranched polymers are well-positioned to meet these evolving market demands.
In the coatings industry, hyperbranched polymers have gained significant traction as additives and modifiers. Their ability to enhance scratch resistance, improve adhesion, and reduce viscosity in coating formulations has led to increased adoption in automotive, industrial, and protective coatings. The growing emphasis on environmentally friendly products has further boosted the demand for hyperbranched polymers in water-based and low-VOC coating systems.
The electronics sector has also shown a rising interest in hyperbranched polymers. These materials are being increasingly used in the production of printed circuit boards, electronic packaging, and as dielectric materials in microelectronics. Their low shrinkage, high thermal stability, and excellent electrical properties make them ideal for these applications, driving market growth in the electronics industry.
In the medical and pharmaceutical fields, hyperbranched polymers are finding applications in drug delivery systems, tissue engineering, and medical devices. Their biocompatibility, biodegradability, and ability to encapsulate and release drugs in a controlled manner have led to increased research and development activities, potentially opening up new market opportunities.
The packaging industry is another sector where hyperbranched polymers are gaining traction. Their barrier properties, recyclability, and potential to reduce material usage align well with the growing demand for sustainable packaging solutions. This has led to increased interest from food and beverage companies looking to improve their packaging performance while reducing environmental impact.
As environmental regulations become more stringent, the demand for hyperbranched polymers in water treatment applications is also on the rise. Their ability to act as effective flocculants and chelating agents for heavy metal removal has opened up new market opportunities in industrial wastewater treatment and municipal water purification processes.
The global market for hyperbranched polymers is expected to continue its growth trajectory, driven by ongoing research and development efforts to expand their applications and improve their performance. As industries seek innovative materials to address challenges in sustainability, efficiency, and performance, hyperbranched polymers are well-positioned to meet these evolving market demands.
Current Challenges in Hyperbranched Polymer Synthesis
The synthesis of hyperbranched polymers has gained significant attention in recent years due to their unique properties and potential applications. However, several challenges persist in their production, particularly when using perchloric acid as a catalyst. One of the primary issues is controlling the degree of branching and molecular weight distribution. The highly reactive nature of perchloric acid often leads to rapid and uncontrolled polymerization, resulting in polymers with inconsistent structures and properties.
Another significant challenge is the prevention of gelation during the synthesis process. The high reactivity of perchloric acid can cause excessive crosslinking, leading to the formation of insoluble gels rather than the desired soluble hyperbranched polymers. This issue is particularly pronounced when working with monomers that have multiple reactive sites, as the probability of unwanted side reactions increases.
The selectivity of the catalytic process also presents a considerable hurdle. Perchloric acid, being a strong acid, can catalyze various side reactions, including the formation of cyclic structures or linear polymer chains. These undesired products can significantly affect the final properties of the hyperbranched polymer and reduce the overall efficiency of the synthesis.
Furthermore, the handling and safety concerns associated with perchloric acid pose substantial challenges in industrial-scale production. Its corrosive and potentially explosive nature necessitates stringent safety measures and specialized equipment, which can increase production costs and complexity.
The removal of residual perchloric acid from the final product is another critical issue. Trace amounts of the catalyst can affect the stability and performance of the hyperbranched polymer in its intended applications. Developing efficient purification methods that do not compromise the polymer's structure or properties remains a significant challenge.
Additionally, achieving reproducibility in the synthesis process is often difficult due to the sensitivity of the reaction to various parameters such as temperature, concentration, and reaction time. Small variations in these conditions can lead to significant differences in the final product, making it challenging to maintain consistent quality across batches.
Lastly, the environmental impact of using perchloric acid in polymer synthesis is a growing concern. Developing more sustainable and environmentally friendly catalytic systems that can match or exceed the efficiency of perchloric acid is an ongoing challenge in the field of hyperbranched polymer synthesis.
Another significant challenge is the prevention of gelation during the synthesis process. The high reactivity of perchloric acid can cause excessive crosslinking, leading to the formation of insoluble gels rather than the desired soluble hyperbranched polymers. This issue is particularly pronounced when working with monomers that have multiple reactive sites, as the probability of unwanted side reactions increases.
The selectivity of the catalytic process also presents a considerable hurdle. Perchloric acid, being a strong acid, can catalyze various side reactions, including the formation of cyclic structures or linear polymer chains. These undesired products can significantly affect the final properties of the hyperbranched polymer and reduce the overall efficiency of the synthesis.
Furthermore, the handling and safety concerns associated with perchloric acid pose substantial challenges in industrial-scale production. Its corrosive and potentially explosive nature necessitates stringent safety measures and specialized equipment, which can increase production costs and complexity.
The removal of residual perchloric acid from the final product is another critical issue. Trace amounts of the catalyst can affect the stability and performance of the hyperbranched polymer in its intended applications. Developing efficient purification methods that do not compromise the polymer's structure or properties remains a significant challenge.
Additionally, achieving reproducibility in the synthesis process is often difficult due to the sensitivity of the reaction to various parameters such as temperature, concentration, and reaction time. Small variations in these conditions can lead to significant differences in the final product, making it challenging to maintain consistent quality across batches.
Lastly, the environmental impact of using perchloric acid in polymer synthesis is a growing concern. Developing more sustainable and environmentally friendly catalytic systems that can match or exceed the efficiency of perchloric acid is an ongoing challenge in the field of hyperbranched polymer synthesis.
Existing Catalytic Solutions for Hyperbranched Polymers
01 Synthesis methods for hyperbranched polymers
Various methods are employed for synthesizing hyperbranched polymers, including step-growth polymerization, chain-growth polymerization, and ring-opening polymerization. These techniques allow for the creation of highly branched macromolecules with unique properties and functionalities.- Synthesis methods for hyperbranched polymers: Various methods are employed for synthesizing hyperbranched polymers, including step-growth polymerization, chain-growth polymerization, and ring-opening polymerization. These techniques allow for the creation of highly branched macromolecules with unique properties and structures.
- Functionalization of hyperbranched polymers: Hyperbranched polymers can be functionalized with various end groups or reactive sites to tailor their properties for specific applications. This process involves introducing functional groups at the terminal units or within the polymer structure, enhancing their versatility in different fields.
- Applications of hyperbranched polymers in electronics: Hyperbranched polymers find applications in electronic devices, such as organic light-emitting diodes (OLEDs) and photovoltaic cells. Their unique structure and properties make them suitable for use as charge transport materials, encapsulants, or as components in flexible electronics.
- Hyperbranched polymers in drug delivery systems: The synthesis of hyperbranched polymers for drug delivery applications involves creating structures that can encapsulate or conjugate with drug molecules. These polymers offer advantages such as improved drug solubility, controlled release, and enhanced bioavailability.
- Sustainable synthesis of hyperbranched polymers: Research is focused on developing environmentally friendly methods for synthesizing hyperbranched polymers. This includes using renewable resources as starting materials, employing green chemistry principles, and designing biodegradable hyperbranched structures for various applications.
02 Functionalization of hyperbranched polymers
Hyperbranched polymers can be functionalized with various end groups or reactive sites to tailor their properties for specific applications. This includes the incorporation of specific chemical moieties or the modification of existing functional groups to enhance performance in areas such as adhesion, coating, or drug delivery.Expand Specific Solutions03 Application of hyperbranched polymers in electronic devices
Hyperbranched polymers find applications in electronic devices due to their unique structural and functional properties. They can be used as dielectric materials, encapsulants, or in the fabrication of semiconductor devices, offering advantages such as improved thermal stability and electrical insulation.Expand Specific Solutions04 Hyperbranched polymers in biomedical applications
The synthesis of hyperbranched polymers for biomedical applications focuses on creating materials with biocompatibility and biodegradability. These polymers can be used in drug delivery systems, tissue engineering scaffolds, or as imaging agents, leveraging their multifunctional nature and controlled degradation properties.Expand Specific Solutions05 Sustainable synthesis of hyperbranched polymers
Research is directed towards developing sustainable methods for synthesizing hyperbranched polymers, including the use of renewable resources and environmentally friendly processes. This approach aims to reduce the environmental impact of polymer production while maintaining the desired structural and functional properties.Expand Specific Solutions
Key Players in Hyperbranched Polymer Industry
The synthesis of hyperbranched polymers using perchloric acid as a catalyst represents an emerging field in polymer chemistry, currently in its early development stage. The market for these specialized polymers is growing, driven by their unique properties and potential applications in various industries. While the market size is still relatively small, it is expected to expand significantly in the coming years. The technology is in a transitional phase from laboratory research to industrial application, with companies like BASF, DuPont, and Wanhua Chemical Group leading the way in research and development. These firms are investing in advancing the technology, focusing on optimizing synthesis processes and exploring new applications. However, the technology's maturity level varies across different companies, with some still in the experimental stage while others are moving towards commercialization.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has made significant advancements in the perchloric acid-catalyzed synthesis of hyperbranched polymers, particularly for applications in the oil and gas industry. Their research focuses on developing hyperbranched polymers with enhanced thermal stability and chemical resistance. Sinopec's method utilizes a controlled feed approach, where monomers are gradually introduced to the reaction mixture containing perchloric acid, allowing for better control over the polymerization kinetics[7]. They have also explored the use of supercritical CO2 as a reaction medium, which has shown promise in improving polymer properties and reducing environmental impact[8].
Strengths: Specialized polymers for harsh environments, potential for integration with existing petrochemical processes. Weaknesses: Limited applicability outside the oil and gas sector, potential regulatory challenges due to environmental concerns.
BASF SE
Technical Solution: BASF SE has developed a novel approach for synthesizing hyperbranched polymers using perchloric acid as a catalyst. Their method involves a controlled polymerization process where perchloric acid acts as both an initiator and a catalyst. The acid catalyzes the ring-opening polymerization of cyclic monomers, leading to highly branched structures. BASF's technique allows for precise control over the degree of branching and molecular weight distribution[1]. They have optimized reaction conditions, including temperature and monomer concentration, to achieve desired polymer properties. Additionally, BASF has implemented in-situ spectroscopic monitoring to track the polymerization progress and adjust parameters in real-time[3].
Strengths: Precise control over polymer architecture, scalable process, and versatile application potential. Weaknesses: Potential safety concerns due to perchloric acid handling, and possible limitations in certain end-use applications due to residual acid.
Core Innovations in Perchloric Acid Catalysis
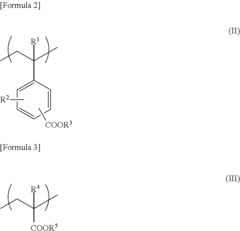
Hyperbranched polymer synthesizing method, hyperbranched polymer, resist composition, semiconductor integrated circuit, and semiconductor integrated circuit fabrication method
PatentInactiveUS20110101503A1
Innovation
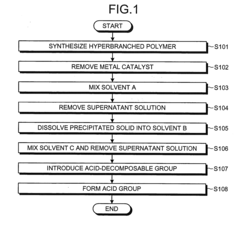
- A method involving living radical polymerization with controlled metal catalyst removal, ultrapure water washing, and liquid-liquid extraction to stabilize and scale up core-shell hyperbranched polymer synthesis, using a specific volume ratio of ultrapure water to organic solvent to minimize waste and impurities, and employing a reprecipitation method to maintain desired molecular weight and branching.
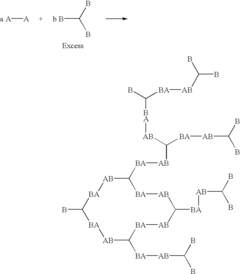
Hyperbranched polymers by multimonomer polymerization
PatentInactiveUS6812298B2
Innovation
- The process involves reacting difunctional and/or polyfunctional monomers with functional groups of one type (A) that do not react with themselves and another type (B) that reacts with A, but not with itself, to form hyperbranched copolymers, allowing for the synthesis of a wider variety of hyperbranched polymers at a lower cost by using monomers with single types of functional groups.
Safety Considerations for Perchloric Acid Use
Perchloric acid is a powerful oxidizing agent and catalyst, necessitating stringent safety measures during its use in the synthesis of hyperbranched polymers. The primary hazards associated with perchloric acid include its explosive nature, particularly when in contact with organic compounds, and its corrosive properties.
To mitigate these risks, proper handling and storage protocols must be implemented. Perchloric acid should be stored in a cool, well-ventilated area, away from combustible materials and other chemicals. Glass or PTFE containers are recommended for storage, as perchloric acid can react with many metals.
Personal protective equipment (PPE) is crucial when working with perchloric acid. This includes chemical-resistant gloves, safety goggles, and a lab coat. A face shield may be necessary for larger-scale operations. All work involving perchloric acid should be conducted in a fume hood equipped with a wash-down system to prevent the accumulation of explosive perchlorates.
Dilution of perchloric acid requires special attention. The acid should always be added to water, never the reverse, to avoid violent reactions. When preparing solutions, it is advisable to use pre-cooled water and add the acid slowly while stirring to dissipate heat.
In the context of hyperbranched polymer synthesis, additional precautions are necessary. The reaction setup should include temperature control mechanisms to prevent overheating, which could lead to rapid decomposition of the acid. Monitoring systems for detecting acid vapors are also recommended.
Emergency response procedures must be established and clearly communicated to all personnel. This includes the availability of appropriate fire extinguishing agents, as water may be ineffective against perchloric acid fires. Spill control kits specifically designed for strong oxidizers should be readily accessible.
Training is a critical component of safety management. All personnel involved in handling perchloric acid must receive comprehensive instruction on its properties, hazards, and proper handling techniques. Regular refresher courses and safety drills should be conducted to maintain awareness and preparedness.
Waste disposal presents another significant safety concern. Perchloric acid waste must be segregated from other chemical waste streams and disposed of by specialized waste management services. Any materials that have come into contact with perchloric acid, including reaction vessels and PPE, should be thoroughly decontaminated before disposal or reuse.
By adhering to these safety considerations, researchers can minimize the risks associated with perchloric acid use in hyperbranched polymer synthesis, ensuring a safer working environment while leveraging the catalytic properties of this powerful acid.
To mitigate these risks, proper handling and storage protocols must be implemented. Perchloric acid should be stored in a cool, well-ventilated area, away from combustible materials and other chemicals. Glass or PTFE containers are recommended for storage, as perchloric acid can react with many metals.
Personal protective equipment (PPE) is crucial when working with perchloric acid. This includes chemical-resistant gloves, safety goggles, and a lab coat. A face shield may be necessary for larger-scale operations. All work involving perchloric acid should be conducted in a fume hood equipped with a wash-down system to prevent the accumulation of explosive perchlorates.
Dilution of perchloric acid requires special attention. The acid should always be added to water, never the reverse, to avoid violent reactions. When preparing solutions, it is advisable to use pre-cooled water and add the acid slowly while stirring to dissipate heat.
In the context of hyperbranched polymer synthesis, additional precautions are necessary. The reaction setup should include temperature control mechanisms to prevent overheating, which could lead to rapid decomposition of the acid. Monitoring systems for detecting acid vapors are also recommended.
Emergency response procedures must be established and clearly communicated to all personnel. This includes the availability of appropriate fire extinguishing agents, as water may be ineffective against perchloric acid fires. Spill control kits specifically designed for strong oxidizers should be readily accessible.
Training is a critical component of safety management. All personnel involved in handling perchloric acid must receive comprehensive instruction on its properties, hazards, and proper handling techniques. Regular refresher courses and safety drills should be conducted to maintain awareness and preparedness.
Waste disposal presents another significant safety concern. Perchloric acid waste must be segregated from other chemical waste streams and disposed of by specialized waste management services. Any materials that have come into contact with perchloric acid, including reaction vessels and PPE, should be thoroughly decontaminated before disposal or reuse.
By adhering to these safety considerations, researchers can minimize the risks associated with perchloric acid use in hyperbranched polymer synthesis, ensuring a safer working environment while leveraging the catalytic properties of this powerful acid.
Environmental Impact of Hyperbranched Polymer Production
The production of hyperbranched polymers using perchloric acid as a catalyst has significant environmental implications that warrant careful consideration. The synthesis process, while efficient, generates various waste streams and byproducts that can impact ecosystems if not properly managed.
One of the primary environmental concerns is the potential release of perchloric acid into water systems. Perchloric acid is highly soluble in water and can persist in the environment, potentially affecting aquatic life and water quality. Proper containment and treatment of waste streams containing perchloric acid residues are crucial to mitigate these risks.
The synthesis of hyperbranched polymers often involves the use of organic solvents, which can contribute to air pollution and pose health risks if not handled correctly. Volatile organic compounds (VOCs) emitted during the production process may contribute to smog formation and have adverse effects on air quality in surrounding areas.
Energy consumption is another significant factor in the environmental impact of hyperbranched polymer production. The synthesis process typically requires elevated temperatures and prolonged reaction times, leading to substantial energy usage. This energy demand often translates to increased greenhouse gas emissions, particularly if non-renewable energy sources are utilized.
The disposal of unreacted monomers and oligomers presents additional environmental challenges. These materials may be toxic or non-biodegradable, requiring specialized treatment and disposal methods to prevent soil and groundwater contamination.
However, it is important to note that hyperbranched polymers themselves can offer environmental benefits in certain applications. Their unique structure and properties can lead to improved material efficiency, potentially reducing overall resource consumption in end-use products. Additionally, some hyperbranched polymers have shown promise in environmental remediation applications, such as water treatment and pollutant removal.
To address these environmental concerns, researchers and industry professionals are exploring greener synthesis methods for hyperbranched polymers. This includes the development of alternative catalysts that are less hazardous than perchloric acid, as well as the use of more environmentally friendly solvents and reaction conditions.
Implementing closed-loop systems and recycling processes can help minimize waste generation and resource consumption in hyperbranched polymer production. Additionally, adopting renewable energy sources for manufacturing processes can significantly reduce the carbon footprint associated with energy-intensive synthesis steps.
As regulations surrounding chemical production and environmental protection continue to evolve, manufacturers of hyperbranched polymers must prioritize sustainable practices and invest in cleaner technologies. This proactive approach not only mitigates environmental risks but also positions companies favorably in an increasingly eco-conscious market.
One of the primary environmental concerns is the potential release of perchloric acid into water systems. Perchloric acid is highly soluble in water and can persist in the environment, potentially affecting aquatic life and water quality. Proper containment and treatment of waste streams containing perchloric acid residues are crucial to mitigate these risks.
The synthesis of hyperbranched polymers often involves the use of organic solvents, which can contribute to air pollution and pose health risks if not handled correctly. Volatile organic compounds (VOCs) emitted during the production process may contribute to smog formation and have adverse effects on air quality in surrounding areas.
Energy consumption is another significant factor in the environmental impact of hyperbranched polymer production. The synthesis process typically requires elevated temperatures and prolonged reaction times, leading to substantial energy usage. This energy demand often translates to increased greenhouse gas emissions, particularly if non-renewable energy sources are utilized.
The disposal of unreacted monomers and oligomers presents additional environmental challenges. These materials may be toxic or non-biodegradable, requiring specialized treatment and disposal methods to prevent soil and groundwater contamination.
However, it is important to note that hyperbranched polymers themselves can offer environmental benefits in certain applications. Their unique structure and properties can lead to improved material efficiency, potentially reducing overall resource consumption in end-use products. Additionally, some hyperbranched polymers have shown promise in environmental remediation applications, such as water treatment and pollutant removal.
To address these environmental concerns, researchers and industry professionals are exploring greener synthesis methods for hyperbranched polymers. This includes the development of alternative catalysts that are less hazardous than perchloric acid, as well as the use of more environmentally friendly solvents and reaction conditions.
Implementing closed-loop systems and recycling processes can help minimize waste generation and resource consumption in hyperbranched polymer production. Additionally, adopting renewable energy sources for manufacturing processes can significantly reduce the carbon footprint associated with energy-intensive synthesis steps.
As regulations surrounding chemical production and environmental protection continue to evolve, manufacturers of hyperbranched polymers must prioritize sustainable practices and invest in cleaner technologies. This proactive approach not only mitigates environmental risks but also positions companies favorably in an increasingly eco-conscious market.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!