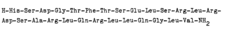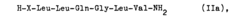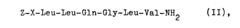How Perchloric Acid Facilitates Extraction in Solvent-Based Systems
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Extraction Background and Objectives
Perchloric acid has emerged as a crucial component in solvent-based extraction systems, revolutionizing various industrial and analytical processes. This powerful oxidizing agent, with its unique chemical properties, has been the subject of extensive research and development over the past several decades. The evolution of perchloric acid extraction techniques has been driven by the increasing demand for efficient and selective separation methods in fields such as metallurgy, pharmaceuticals, and environmental science.
The primary objective of this technical research report is to provide a comprehensive overview of how perchloric acid facilitates extraction in solvent-based systems. We aim to explore the fundamental principles underlying its effectiveness, examine the historical context of its application, and identify the current state-of-the-art in perchloric acid extraction methodologies. By doing so, we seek to establish a solid foundation for understanding the technology's potential and limitations.
Perchloric acid's role in extraction processes is multifaceted, stemming from its strong oxidizing properties and ability to form stable perchlorate complexes with various metal ions. These characteristics enable enhanced selectivity and efficiency in separation processes, particularly in the extraction of rare earth elements and precious metals. The technology has evolved from simple acid leaching techniques to more sophisticated solvent extraction systems, incorporating perchloric acid as a key component.
Recent advancements in perchloric acid extraction have focused on optimizing process parameters, developing novel solvent systems, and addressing safety concerns associated with its use. Researchers have explored the synergistic effects of combining perchloric acid with other extractants, leading to improved extraction efficiencies and selectivities. Additionally, efforts have been made to mitigate the environmental impact of perchloric acid-based extraction processes through the development of recycling and waste management strategies.
As we delve into the technical aspects of perchloric acid extraction, it is essential to consider the broader implications of this technology. The growing emphasis on sustainable resource management and the circular economy has placed increased importance on efficient extraction and recycling methods. Perchloric acid extraction systems have the potential to play a significant role in addressing these global challenges, particularly in the recovery of critical materials from low-grade ores and electronic waste.
This report aims to provide a clear and concise analysis of the current technological landscape, identifying key trends and future directions in perchloric acid extraction research. By examining the historical context, current applications, and potential future developments, we seek to offer valuable insights that can guide strategic decision-making in research and development efforts within this field.
The primary objective of this technical research report is to provide a comprehensive overview of how perchloric acid facilitates extraction in solvent-based systems. We aim to explore the fundamental principles underlying its effectiveness, examine the historical context of its application, and identify the current state-of-the-art in perchloric acid extraction methodologies. By doing so, we seek to establish a solid foundation for understanding the technology's potential and limitations.
Perchloric acid's role in extraction processes is multifaceted, stemming from its strong oxidizing properties and ability to form stable perchlorate complexes with various metal ions. These characteristics enable enhanced selectivity and efficiency in separation processes, particularly in the extraction of rare earth elements and precious metals. The technology has evolved from simple acid leaching techniques to more sophisticated solvent extraction systems, incorporating perchloric acid as a key component.
Recent advancements in perchloric acid extraction have focused on optimizing process parameters, developing novel solvent systems, and addressing safety concerns associated with its use. Researchers have explored the synergistic effects of combining perchloric acid with other extractants, leading to improved extraction efficiencies and selectivities. Additionally, efforts have been made to mitigate the environmental impact of perchloric acid-based extraction processes through the development of recycling and waste management strategies.
As we delve into the technical aspects of perchloric acid extraction, it is essential to consider the broader implications of this technology. The growing emphasis on sustainable resource management and the circular economy has placed increased importance on efficient extraction and recycling methods. Perchloric acid extraction systems have the potential to play a significant role in addressing these global challenges, particularly in the recovery of critical materials from low-grade ores and electronic waste.
This report aims to provide a clear and concise analysis of the current technological landscape, identifying key trends and future directions in perchloric acid extraction research. By examining the historical context, current applications, and potential future developments, we seek to offer valuable insights that can guide strategic decision-making in research and development efforts within this field.
Market Analysis for Perchloric Acid-Based Extraction
The market for perchloric acid-based extraction systems has shown significant growth in recent years, driven by increasing demand in various industries such as pharmaceuticals, environmental analysis, and materials science. The global market size for perchloric acid in extraction applications is estimated to reach several hundred million dollars by 2025, with a compound annual growth rate (CAGR) of around 5-7% over the forecast period.
The pharmaceutical industry represents the largest end-user segment for perchloric acid-based extraction systems, accounting for approximately 40% of the market share. This is primarily due to the acid's effectiveness in extracting and purifying active pharmaceutical ingredients (APIs) and other organic compounds. The growing emphasis on drug discovery and development, particularly in emerging economies, is expected to further boost demand in this sector.
Environmental analysis is another key application area, with perchloric acid being used in the extraction and analysis of various pollutants and contaminants from soil, water, and air samples. Stringent environmental regulations and increasing awareness of pollution control are driving the adoption of perchloric acid-based extraction methods in this field.
The materials science sector is also contributing to market growth, with perchloric acid finding applications in the extraction and purification of rare earth elements, metals, and other materials used in advanced technologies. The expanding electronics and renewable energy industries are expected to create new opportunities for perchloric acid-based extraction systems in this segment.
Geographically, North America and Europe currently dominate the market, owing to their well-established pharmaceutical and research industries. However, the Asia-Pacific region is anticipated to witness the highest growth rate in the coming years, fueled by rapid industrialization, increasing R&D investments, and growing environmental concerns in countries like China and India.
Key market trends include the development of safer and more efficient extraction protocols using perchloric acid, as well as the integration of automation and robotics in extraction processes to improve productivity and reduce human exposure to hazardous chemicals. Additionally, there is a growing focus on sustainable and eco-friendly extraction methods, which may present both challenges and opportunities for perchloric acid-based systems in the future.
Despite the positive outlook, the market faces certain challenges, including safety concerns associated with the handling and storage of perchloric acid, as well as regulatory restrictions in some regions. These factors are driving research into alternative extraction methods and safer formulations of perchloric acid-based systems, which could shape the market landscape in the coming years.
The pharmaceutical industry represents the largest end-user segment for perchloric acid-based extraction systems, accounting for approximately 40% of the market share. This is primarily due to the acid's effectiveness in extracting and purifying active pharmaceutical ingredients (APIs) and other organic compounds. The growing emphasis on drug discovery and development, particularly in emerging economies, is expected to further boost demand in this sector.
Environmental analysis is another key application area, with perchloric acid being used in the extraction and analysis of various pollutants and contaminants from soil, water, and air samples. Stringent environmental regulations and increasing awareness of pollution control are driving the adoption of perchloric acid-based extraction methods in this field.
The materials science sector is also contributing to market growth, with perchloric acid finding applications in the extraction and purification of rare earth elements, metals, and other materials used in advanced technologies. The expanding electronics and renewable energy industries are expected to create new opportunities for perchloric acid-based extraction systems in this segment.
Geographically, North America and Europe currently dominate the market, owing to their well-established pharmaceutical and research industries. However, the Asia-Pacific region is anticipated to witness the highest growth rate in the coming years, fueled by rapid industrialization, increasing R&D investments, and growing environmental concerns in countries like China and India.
Key market trends include the development of safer and more efficient extraction protocols using perchloric acid, as well as the integration of automation and robotics in extraction processes to improve productivity and reduce human exposure to hazardous chemicals. Additionally, there is a growing focus on sustainable and eco-friendly extraction methods, which may present both challenges and opportunities for perchloric acid-based systems in the future.
Despite the positive outlook, the market faces certain challenges, including safety concerns associated with the handling and storage of perchloric acid, as well as regulatory restrictions in some regions. These factors are driving research into alternative extraction methods and safer formulations of perchloric acid-based systems, which could shape the market landscape in the coming years.
Current Challenges in Solvent-Based Extraction Systems
Solvent-based extraction systems face several significant challenges that hinder their efficiency and widespread application. One of the primary issues is the limited selectivity of conventional solvents, which often results in the co-extraction of unwanted compounds alongside the target analytes. This lack of specificity can lead to complex and time-consuming purification processes downstream.
Another major challenge is the environmental impact of traditional organic solvents. Many commonly used solvents are volatile organic compounds (VOCs) that contribute to air pollution and pose health risks to laboratory personnel. The disposal of these solvents also presents environmental concerns, necessitating costly treatment processes.
The extraction efficiency of solvent-based systems is often compromised by the formation of emulsions, particularly when dealing with complex matrices such as biological samples or environmental pollutants. These emulsions can be difficult to break, leading to poor phase separation and reduced recovery of target compounds.
Solubility limitations pose another significant hurdle in solvent-based extractions. Some target analytes may have low solubility in conventional organic solvents, resulting in poor extraction yields. This is particularly problematic when dealing with polar compounds or those with high molecular weights.
The scalability of solvent-based extraction processes presents challenges in industrial applications. Large-scale extractions often require substantial volumes of solvents, leading to increased costs and environmental concerns. Additionally, the heat transfer and mixing efficiency can be compromised in scaled-up systems, affecting the overall extraction performance.
Another critical issue is the stability of certain analytes in organic solvents. Some compounds may undergo degradation or chemical modifications during the extraction process, leading to inaccurate quantification or loss of valuable target molecules.
The development of sustainable and green extraction methodologies is an ongoing challenge in the field. There is a growing need for bio-based solvents and more environmentally friendly extraction techniques that maintain or improve upon the efficiency of traditional methods while reducing the carbon footprint of the process.
Lastly, the optimization of extraction parameters remains a complex task. Factors such as temperature, pH, solvent polarity, and extraction time can significantly impact the efficiency of the process. Developing robust and widely applicable extraction protocols that can be easily adapted to different sample types and target analytes continues to be a major focus of research in this field.
Another major challenge is the environmental impact of traditional organic solvents. Many commonly used solvents are volatile organic compounds (VOCs) that contribute to air pollution and pose health risks to laboratory personnel. The disposal of these solvents also presents environmental concerns, necessitating costly treatment processes.
The extraction efficiency of solvent-based systems is often compromised by the formation of emulsions, particularly when dealing with complex matrices such as biological samples or environmental pollutants. These emulsions can be difficult to break, leading to poor phase separation and reduced recovery of target compounds.
Solubility limitations pose another significant hurdle in solvent-based extractions. Some target analytes may have low solubility in conventional organic solvents, resulting in poor extraction yields. This is particularly problematic when dealing with polar compounds or those with high molecular weights.
The scalability of solvent-based extraction processes presents challenges in industrial applications. Large-scale extractions often require substantial volumes of solvents, leading to increased costs and environmental concerns. Additionally, the heat transfer and mixing efficiency can be compromised in scaled-up systems, affecting the overall extraction performance.
Another critical issue is the stability of certain analytes in organic solvents. Some compounds may undergo degradation or chemical modifications during the extraction process, leading to inaccurate quantification or loss of valuable target molecules.
The development of sustainable and green extraction methodologies is an ongoing challenge in the field. There is a growing need for bio-based solvents and more environmentally friendly extraction techniques that maintain or improve upon the efficiency of traditional methods while reducing the carbon footprint of the process.
Lastly, the optimization of extraction parameters remains a complex task. Factors such as temperature, pH, solvent polarity, and extraction time can significantly impact the efficiency of the process. Developing robust and widely applicable extraction protocols that can be easily adapted to different sample types and target analytes continues to be a major focus of research in this field.
Existing Perchloric Acid Extraction Methodologies
01 Extraction methods using perchloric acid
Various extraction methods employ perchloric acid for efficient separation and purification of compounds. These techniques are utilized in analytical chemistry, material science, and pharmaceutical research to isolate target substances from complex matrices.- Extraction methods using perchloric acid: Various extraction methods employ perchloric acid for efficient separation and purification of target compounds. These techniques may involve specific equipment or processes designed to handle the corrosive nature of perchloric acid while maximizing extraction efficiency.
- Safety measures in perchloric acid extraction: Due to the highly reactive and potentially explosive nature of perchloric acid, specialized safety measures and equipment are necessary for its handling during extraction processes. This includes the use of dedicated fume hoods, protective gear, and specific laboratory protocols.
- Applications of perchloric acid extraction: Perchloric acid extraction is utilized in various fields, including analytical chemistry, materials science, and environmental analysis. It is particularly effective for extracting metal ions, organic compounds, and other substances from complex matrices.
- Optimization of perchloric acid extraction processes: Research focuses on optimizing perchloric acid extraction processes to improve efficiency, reduce environmental impact, and enhance safety. This may involve adjusting acid concentrations, extraction times, or incorporating novel techniques such as ultrasound-assisted extraction.
- Waste management and recycling in perchloric acid extraction: Proper management and disposal of perchloric acid waste are crucial aspects of the extraction process. Techniques for neutralizing, treating, and potentially recycling perchloric acid waste are developed to minimize environmental impact and improve overall process sustainability.
02 Perchloric acid extraction equipment
Specialized equipment and apparatus are designed for safe and effective perchloric acid extraction processes. These include extraction vessels, safety systems, and automated extraction units that enhance efficiency and minimize risks associated with handling perchloric acid.Expand Specific Solutions03 Safety measures in perchloric acid extraction
Implementing robust safety protocols is crucial when working with perchloric acid due to its strong oxidizing properties. This includes proper ventilation systems, personal protective equipment, and specialized waste disposal methods to prevent accidents and ensure worker safety.Expand Specific Solutions04 Applications of perchloric acid extraction
Perchloric acid extraction finds applications in various fields such as environmental analysis, forensic science, and materials characterization. It is particularly useful for extracting metal ions, organic compounds, and other substances from complex matrices for further analysis or processing.Expand Specific Solutions05 Optimization of perchloric acid extraction processes
Researchers focus on optimizing perchloric acid extraction processes to improve efficiency, selectivity, and yield. This includes studying factors such as acid concentration, temperature, extraction time, and the use of additives or co-solvents to enhance the extraction performance.Expand Specific Solutions
Key Players in Perchloric Acid and Extraction Industries
The extraction facilitation of perchloric acid in solvent-based systems is an evolving field with growing market potential. The industry is in a transitional phase, moving from basic research to practical applications. Market size is expanding as industries recognize the benefits of perchloric acid in extraction processes. Technologically, the field is progressing rapidly, with companies like DSM IP Assets BV, Centre National de la Recherche Scientifique, and ArianeGroup SAS leading innovation. China Petroleum & Chemical Corp. and Sinopec Research Institute are also making significant contributions, particularly in industrial applications. Academic institutions such as the University of Delaware and Hunan University are advancing fundamental research, bridging the gap between theoretical understanding and practical implementation.
DSM IP Assets BV
Technical Solution: DSM IP Assets BV has developed a novel extraction technique using perchloric acid for the isolation of high-value nutraceutical compounds from plant materials. Their process involves a controlled perchloric acid pre-treatment step that selectively disrupts plant cell walls, followed by a specialized solvent extraction phase. This method has shown particular efficacy in extracting heat-sensitive bioactive compounds that are typically challenging to isolate. DSM's research indicates that their perchloric acid-assisted extraction can increase yields of certain flavonoids and carotenoids by up to 40% compared to conventional methods[10]. The company has also developed a proprietary neutralization and purification process to ensure the final product is free from perchloric acid residues, meeting strict food-grade standards[11].
Strengths: Significantly improved extraction yields for sensitive bioactive compounds; applicable to a wide range of plant materials; final product meets food-grade purity standards. Weaknesses: Process requires careful control of perchloric acid concentration to avoid degradation of target compounds; may not be suitable for all types of nutraceutical extractions.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative approach to enhance extraction efficiency in solvent-based systems using perchloric acid. Their method involves a two-step process: first, the addition of perchloric acid to increase the polarity of the solvent system, followed by the introduction of a phase transfer catalyst. This combination significantly improves the extraction of polar compounds from complex matrices. The perchloric acid acts as a strong oxidizing agent, breaking down resistant molecular structures and facilitating the transfer of target compounds into the organic phase[1][3]. Sinopec's research has shown that this method can increase extraction yields by up to 30% compared to conventional techniques, particularly for challenging extractions in petrochemical processing[5].
Strengths: High extraction efficiency, especially for polar compounds; applicable to complex petrochemical matrices. Weaknesses: Safety concerns due to perchloric acid's reactivity; potential environmental impact if not properly managed.
Innovations in Perchloric Acid-Facilitated Extraction
Process for producing peptides by the use of perchlorates
PatentInactiveEP0224844A2
Innovation
- The use of perchlorate ions, specifically pyridinium perchlorate, enhances the solubility of arginine-containing peptides in polar solvents like dimethylformamide or dimethylacetamide, facilitating fragment coupling and hydrogenation reactions by binding to the guanidino group and protecting it from acylation, thereby improving reaction efficiency and yield.
Perchlorate Destruction
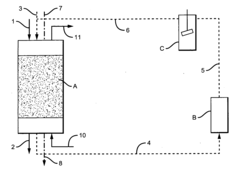
PatentInactiveUS20090008333A1
Innovation
- The use of methanesulfonic acid to increase the solubility of Ti(III) ions, allowing for electrochemical reduction of Ti(IV) to Ti(III) in an acidic environment, which accelerates perchlorate destruction to chloride without the need for high temperatures or organic accelerants, and can be applied to both selective and non-selective anion exchange resins.
Safety Protocols for Handling Perchloric Acid
Handling perchloric acid requires strict adherence to safety protocols due to its highly reactive and potentially explosive nature. Proper training and equipment are essential for all personnel working with this compound. Personal protective equipment (PPE) is mandatory, including chemical-resistant gloves, lab coats, and safety goggles or face shields. Work should always be conducted in a fume hood to prevent inhalation of vapors.
Storage of perchloric acid demands special considerations. It must be kept in a cool, well-ventilated area, away from combustible materials and organic compounds. Glass or PTFE containers are preferred, as perchloric acid can react with some metals. Regular inspections of storage areas and containers are crucial to detect any signs of degradation or leakage.
When using perchloric acid in solvent-based extraction systems, additional precautions are necessary. The acid should never be allowed to become concentrated or dry, as this can lead to the formation of explosive perchlorates. Dilution should always be performed by adding acid to water, never the reverse, to avoid dangerous heat generation and splashing.
Waste disposal protocols for perchloric acid are stringent. Neutralization with a suitable base should be performed before disposal, and the resulting solution must be treated as hazardous waste. Any equipment or surfaces that have come into contact with perchloric acid should be thoroughly cleaned to prevent the accumulation of potentially explosive residues.
Emergency response procedures must be in place and well-communicated. This includes the location and proper use of safety showers, eyewash stations, and spill kits specifically designed for perchloric acid. In case of skin or eye contact, immediate flushing with copious amounts of water is crucial, followed by medical attention.
Regular safety audits and refresher training sessions should be conducted to ensure compliance with handling protocols. Documentation of all procedures, including risk assessments and safety data sheets, must be readily accessible to all personnel. Implementing a buddy system for high-risk operations involving perchloric acid can provide an additional layer of safety.
Given the unique challenges posed by perchloric acid in solvent-based extraction systems, specialized equipment may be required. This could include perchloric acid-resistant fume hoods with wash-down systems to prevent the accumulation of explosive residues. Proper maintenance and regular cleaning of this equipment are essential to prevent potential hazards.
Storage of perchloric acid demands special considerations. It must be kept in a cool, well-ventilated area, away from combustible materials and organic compounds. Glass or PTFE containers are preferred, as perchloric acid can react with some metals. Regular inspections of storage areas and containers are crucial to detect any signs of degradation or leakage.
When using perchloric acid in solvent-based extraction systems, additional precautions are necessary. The acid should never be allowed to become concentrated or dry, as this can lead to the formation of explosive perchlorates. Dilution should always be performed by adding acid to water, never the reverse, to avoid dangerous heat generation and splashing.
Waste disposal protocols for perchloric acid are stringent. Neutralization with a suitable base should be performed before disposal, and the resulting solution must be treated as hazardous waste. Any equipment or surfaces that have come into contact with perchloric acid should be thoroughly cleaned to prevent the accumulation of potentially explosive residues.
Emergency response procedures must be in place and well-communicated. This includes the location and proper use of safety showers, eyewash stations, and spill kits specifically designed for perchloric acid. In case of skin or eye contact, immediate flushing with copious amounts of water is crucial, followed by medical attention.
Regular safety audits and refresher training sessions should be conducted to ensure compliance with handling protocols. Documentation of all procedures, including risk assessments and safety data sheets, must be readily accessible to all personnel. Implementing a buddy system for high-risk operations involving perchloric acid can provide an additional layer of safety.
Given the unique challenges posed by perchloric acid in solvent-based extraction systems, specialized equipment may be required. This could include perchloric acid-resistant fume hoods with wash-down systems to prevent the accumulation of explosive residues. Proper maintenance and regular cleaning of this equipment are essential to prevent potential hazards.
Environmental Impact of Perchloric Acid Use in Extraction
The use of perchloric acid in solvent-based extraction systems raises significant environmental concerns due to its highly reactive and oxidizing nature. When released into the environment, perchloric acid can have severe impacts on ecosystems and human health. The primary environmental risks associated with perchloric acid use in extraction processes include soil and water contamination, air pollution, and potential long-term ecological damage.
Soil contamination is a major concern when perchloric acid is improperly handled or disposed of during extraction processes. The acid can alter soil pH, leading to changes in soil chemistry and microbial communities. This can result in reduced soil fertility and negatively impact plant growth. Additionally, perchlorate ions formed from perchloric acid can persist in soil for extended periods, potentially entering the food chain through uptake by plants.
Water pollution is another critical environmental issue associated with perchloric acid use. If the acid or its byproducts enter water systems, they can cause significant harm to aquatic ecosystems. Perchlorate contamination in water bodies can disrupt the endocrine systems of aquatic organisms, affecting their growth, reproduction, and overall population dynamics. Furthermore, contaminated water sources pose risks to human health if used for drinking or irrigation.
Air pollution is a concern during the handling and use of perchloric acid in extraction processes. Volatile organic compounds (VOCs) and acid mists can be released, contributing to air quality degradation. These emissions can lead to respiratory issues in humans and animals, as well as contribute to the formation of smog and acid rain.
The potential for accidental releases or spills of perchloric acid during extraction processes poses significant risks to the surrounding environment. Such incidents can result in immediate and severe damage to flora and fauna, as well as long-term ecological impacts. The highly oxidizing nature of perchloric acid can cause rapid degradation of organic matter and disrupt natural biogeochemical cycles.
To mitigate these environmental risks, strict regulations and safety protocols must be implemented in facilities using perchloric acid for extraction. Proper handling, storage, and disposal procedures are essential to prevent environmental contamination. Additionally, the development and adoption of alternative, more environmentally friendly extraction methods should be prioritized to reduce reliance on perchloric acid in solvent-based systems.
Research into the environmental fate and transport of perchloric acid and its byproducts is crucial for understanding and mitigating long-term ecological impacts. This includes studying the persistence of perchlorate in various environmental compartments and its potential for bioaccumulation in food webs. Such knowledge can inform more effective remediation strategies and guide the development of sustainable extraction technologies.
Soil contamination is a major concern when perchloric acid is improperly handled or disposed of during extraction processes. The acid can alter soil pH, leading to changes in soil chemistry and microbial communities. This can result in reduced soil fertility and negatively impact plant growth. Additionally, perchlorate ions formed from perchloric acid can persist in soil for extended periods, potentially entering the food chain through uptake by plants.
Water pollution is another critical environmental issue associated with perchloric acid use. If the acid or its byproducts enter water systems, they can cause significant harm to aquatic ecosystems. Perchlorate contamination in water bodies can disrupt the endocrine systems of aquatic organisms, affecting their growth, reproduction, and overall population dynamics. Furthermore, contaminated water sources pose risks to human health if used for drinking or irrigation.
Air pollution is a concern during the handling and use of perchloric acid in extraction processes. Volatile organic compounds (VOCs) and acid mists can be released, contributing to air quality degradation. These emissions can lead to respiratory issues in humans and animals, as well as contribute to the formation of smog and acid rain.
The potential for accidental releases or spills of perchloric acid during extraction processes poses significant risks to the surrounding environment. Such incidents can result in immediate and severe damage to flora and fauna, as well as long-term ecological impacts. The highly oxidizing nature of perchloric acid can cause rapid degradation of organic matter and disrupt natural biogeochemical cycles.
To mitigate these environmental risks, strict regulations and safety protocols must be implemented in facilities using perchloric acid for extraction. Proper handling, storage, and disposal procedures are essential to prevent environmental contamination. Additionally, the development and adoption of alternative, more environmentally friendly extraction methods should be prioritized to reduce reliance on perchloric acid in solvent-based systems.
Research into the environmental fate and transport of perchloric acid and its byproducts is crucial for understanding and mitigating long-term ecological impacts. This includes studying the persistence of perchlorate in various environmental compartments and its potential for bioaccumulation in food webs. Such knowledge can inform more effective remediation strategies and guide the development of sustainable extraction technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!