How Perchloric Acid Facilitates the Crosslinking of Polymeric Chains
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Crosslinking Background
Perchloric acid has emerged as a significant facilitator in the crosslinking of polymeric chains, playing a crucial role in various industrial and research applications. This strong oxidizing agent, with the chemical formula HClO4, has been utilized for decades in polymer chemistry due to its unique properties and reactivity.
The history of perchloric acid in polymer crosslinking can be traced back to the mid-20th century when researchers began exploring its potential in modifying polymer structures. Initially, its use was limited due to safety concerns and the lack of understanding of its precise mechanisms. However, as analytical techniques advanced and safety protocols improved, perchloric acid gained prominence in the field of polymer science.
The fundamental principle behind perchloric acid's effectiveness in crosslinking lies in its ability to generate highly reactive species. When introduced to a polymeric system, perchloric acid can initiate the formation of free radicals or carbocations, which then trigger crosslinking reactions between polymer chains. This process results in the creation of covalent bonds between different polymer molecules, leading to a three-dimensional network structure.
One of the key advantages of perchloric acid in crosslinking is its versatility across various polymer types. It has shown efficacy in crosslinking both natural and synthetic polymers, including but not limited to polyethylene, polypropylene, polyvinyl alcohol, and cellulose derivatives. This broad applicability has made perchloric acid a valuable tool in industries ranging from textiles to advanced materials manufacturing.
The evolution of perchloric acid usage in polymer crosslinking has been marked by continuous refinement of techniques and understanding of reaction mechanisms. Early applications often involved high concentrations of perchloric acid, which posed safety risks and sometimes led to uncontrolled reactions. Over time, researchers developed methods to use lower concentrations effectively, often in combination with other catalysts or initiators, to achieve more controlled and efficient crosslinking.
Recent advancements in the field have focused on enhancing the specificity and efficiency of perchloric acid-mediated crosslinking. This includes the development of novel reaction conditions, the use of perchloric acid derivatives, and the exploration of synergistic effects with other crosslinking agents. These developments have not only improved the quality of crosslinked polymers but also expanded the range of applications where perchloric acid can be effectively employed.
The history of perchloric acid in polymer crosslinking can be traced back to the mid-20th century when researchers began exploring its potential in modifying polymer structures. Initially, its use was limited due to safety concerns and the lack of understanding of its precise mechanisms. However, as analytical techniques advanced and safety protocols improved, perchloric acid gained prominence in the field of polymer science.
The fundamental principle behind perchloric acid's effectiveness in crosslinking lies in its ability to generate highly reactive species. When introduced to a polymeric system, perchloric acid can initiate the formation of free radicals or carbocations, which then trigger crosslinking reactions between polymer chains. This process results in the creation of covalent bonds between different polymer molecules, leading to a three-dimensional network structure.
One of the key advantages of perchloric acid in crosslinking is its versatility across various polymer types. It has shown efficacy in crosslinking both natural and synthetic polymers, including but not limited to polyethylene, polypropylene, polyvinyl alcohol, and cellulose derivatives. This broad applicability has made perchloric acid a valuable tool in industries ranging from textiles to advanced materials manufacturing.
The evolution of perchloric acid usage in polymer crosslinking has been marked by continuous refinement of techniques and understanding of reaction mechanisms. Early applications often involved high concentrations of perchloric acid, which posed safety risks and sometimes led to uncontrolled reactions. Over time, researchers developed methods to use lower concentrations effectively, often in combination with other catalysts or initiators, to achieve more controlled and efficient crosslinking.
Recent advancements in the field have focused on enhancing the specificity and efficiency of perchloric acid-mediated crosslinking. This includes the development of novel reaction conditions, the use of perchloric acid derivatives, and the exploration of synergistic effects with other crosslinking agents. These developments have not only improved the quality of crosslinked polymers but also expanded the range of applications where perchloric acid can be effectively employed.
Market Analysis for Crosslinked Polymers
The market for crosslinked polymers has experienced significant growth in recent years, driven by increasing demand across various industries. These materials, characterized by their enhanced mechanical properties, thermal stability, and chemical resistance, find applications in sectors such as automotive, aerospace, electronics, and healthcare.
In the automotive industry, crosslinked polymers are extensively used in the production of high-performance tires, gaskets, and seals. The growing emphasis on fuel efficiency and vehicle lightweighting has further boosted the demand for these materials. The aerospace sector utilizes crosslinked polymers in composite materials for aircraft components, benefiting from their high strength-to-weight ratio and durability.
The electronics industry has witnessed a surge in the use of crosslinked polymers for insulation, encapsulation, and printed circuit board manufacturing. With the rapid expansion of 5G technology and the Internet of Things (IoT), the demand for reliable and high-performance electronic components has intensified, driving the market for crosslinked polymers.
In the healthcare sector, crosslinked polymers play a crucial role in medical devices, implants, and drug delivery systems. The biocompatibility and controlled degradation properties of certain crosslinked polymers make them ideal for tissue engineering and regenerative medicine applications.
The global market for crosslinked polymers is projected to continue its upward trajectory, with Asia-Pacific emerging as the fastest-growing region. This growth is attributed to rapid industrialization, increasing automotive production, and expanding electronics manufacturing in countries like China and India.
Environmental concerns and sustainability initiatives have also influenced the market dynamics. There is a growing focus on developing bio-based and recyclable crosslinked polymers to address environmental challenges associated with traditional petroleum-based materials. This trend is expected to create new opportunities for market players and drive innovation in the sector.
The competitive landscape of the crosslinked polymer market is characterized by the presence of both large multinational corporations and specialized niche players. Key market participants are investing heavily in research and development to introduce novel products and expand their application areas. Strategic collaborations and partnerships between material suppliers and end-users are becoming increasingly common to develop tailored solutions for specific industry needs.
In the automotive industry, crosslinked polymers are extensively used in the production of high-performance tires, gaskets, and seals. The growing emphasis on fuel efficiency and vehicle lightweighting has further boosted the demand for these materials. The aerospace sector utilizes crosslinked polymers in composite materials for aircraft components, benefiting from their high strength-to-weight ratio and durability.
The electronics industry has witnessed a surge in the use of crosslinked polymers for insulation, encapsulation, and printed circuit board manufacturing. With the rapid expansion of 5G technology and the Internet of Things (IoT), the demand for reliable and high-performance electronic components has intensified, driving the market for crosslinked polymers.
In the healthcare sector, crosslinked polymers play a crucial role in medical devices, implants, and drug delivery systems. The biocompatibility and controlled degradation properties of certain crosslinked polymers make them ideal for tissue engineering and regenerative medicine applications.
The global market for crosslinked polymers is projected to continue its upward trajectory, with Asia-Pacific emerging as the fastest-growing region. This growth is attributed to rapid industrialization, increasing automotive production, and expanding electronics manufacturing in countries like China and India.
Environmental concerns and sustainability initiatives have also influenced the market dynamics. There is a growing focus on developing bio-based and recyclable crosslinked polymers to address environmental challenges associated with traditional petroleum-based materials. This trend is expected to create new opportunities for market players and drive innovation in the sector.
The competitive landscape of the crosslinked polymer market is characterized by the presence of both large multinational corporations and specialized niche players. Key market participants are investing heavily in research and development to introduce novel products and expand their application areas. Strategic collaborations and partnerships between material suppliers and end-users are becoming increasingly common to develop tailored solutions for specific industry needs.
Current Challenges in Polymer Crosslinking
Polymer crosslinking, a critical process in material science, faces several significant challenges in the current technological landscape. One of the primary issues is achieving precise control over the crosslinking density and distribution. This challenge stems from the inherent complexity of polymer systems and the difficulty in predicting and manipulating molecular interactions during the crosslinking process.
Another major hurdle is the development of efficient and environmentally friendly crosslinking agents. Traditional crosslinking methods often involve the use of toxic or hazardous chemicals, which pose risks to both human health and the environment. The search for green alternatives that can provide comparable or superior performance remains an ongoing challenge in the field.
The thermal stability of crosslinked polymers presents another significant challenge. While crosslinking generally enhances the thermal properties of polymers, achieving consistent and reliable thermal stability across a wide range of temperatures and applications continues to be a complex task. This is particularly crucial in high-temperature applications where material integrity is paramount.
Compatibility issues between crosslinking agents and polymer matrices also pose substantial difficulties. Ensuring uniform dispersion and reactivity of crosslinking agents within diverse polymer systems is essential for achieving consistent material properties. However, this often requires extensive optimization and can be highly polymer-specific.
The reversibility of crosslinking is another area of ongoing research and development. While permanent crosslinks are desirable in many applications, there is a growing need for materials with reversible or dynamic crosslinks that can adapt to changing conditions or be easily recycled. Developing such systems without compromising the overall material performance remains a significant challenge.
Furthermore, the scalability of crosslinking processes from laboratory to industrial scale presents numerous technical and economic challenges. Maintaining uniform crosslinking across large volumes of material, ensuring consistent quality, and managing the associated costs are critical concerns for commercial viability.
Lastly, the characterization and quality control of crosslinked polymers continue to be challenging. Developing accurate, non-destructive methods for assessing crosslink density, distribution, and overall material properties is crucial for advancing the field and ensuring product reliability. This challenge is particularly pronounced in complex, multi-component polymer systems where traditional analytical techniques may fall short.
Another major hurdle is the development of efficient and environmentally friendly crosslinking agents. Traditional crosslinking methods often involve the use of toxic or hazardous chemicals, which pose risks to both human health and the environment. The search for green alternatives that can provide comparable or superior performance remains an ongoing challenge in the field.
The thermal stability of crosslinked polymers presents another significant challenge. While crosslinking generally enhances the thermal properties of polymers, achieving consistent and reliable thermal stability across a wide range of temperatures and applications continues to be a complex task. This is particularly crucial in high-temperature applications where material integrity is paramount.
Compatibility issues between crosslinking agents and polymer matrices also pose substantial difficulties. Ensuring uniform dispersion and reactivity of crosslinking agents within diverse polymer systems is essential for achieving consistent material properties. However, this often requires extensive optimization and can be highly polymer-specific.
The reversibility of crosslinking is another area of ongoing research and development. While permanent crosslinks are desirable in many applications, there is a growing need for materials with reversible or dynamic crosslinks that can adapt to changing conditions or be easily recycled. Developing such systems without compromising the overall material performance remains a significant challenge.
Furthermore, the scalability of crosslinking processes from laboratory to industrial scale presents numerous technical and economic challenges. Maintaining uniform crosslinking across large volumes of material, ensuring consistent quality, and managing the associated costs are critical concerns for commercial viability.
Lastly, the characterization and quality control of crosslinked polymers continue to be challenging. Developing accurate, non-destructive methods for assessing crosslink density, distribution, and overall material properties is crucial for advancing the field and ensuring product reliability. This challenge is particularly pronounced in complex, multi-component polymer systems where traditional analytical techniques may fall short.
Existing Perchloric Acid Crosslinking Methods
01 Perchloric acid as a crosslinking agent
Perchloric acid is utilized as a crosslinking agent in various applications, particularly in polymer chemistry. It can initiate and promote the formation of chemical bonds between polymer chains, resulting in improved mechanical properties and thermal stability of the final product.- Perchloric acid as a crosslinking agent: Perchloric acid is utilized as a crosslinking agent in various applications, particularly in polymer chemistry. It can initiate and promote the formation of chemical bonds between polymer chains, resulting in improved mechanical properties and thermal stability of the final product.
- Crosslinking in battery electrode materials: Perchloric acid crosslinking is employed in the production of battery electrode materials. This process enhances the structural integrity and electrochemical performance of the electrodes, leading to improved battery efficiency and longevity.
- Application in membrane technology: Perchloric acid crosslinking is used in the development of advanced membranes for various separation processes. This technique helps to create more robust and selective membranes with enhanced chemical and thermal resistance.
- Crosslinking in hydrogel formation: Perchloric acid is utilized as a crosslinking agent in the synthesis of hydrogels. This process allows for the creation of hydrogels with tailored properties, such as improved swelling behavior and mechanical strength, for applications in biomedical and environmental fields.
- Perchloric acid crosslinking in composite materials: The use of perchloric acid for crosslinking in composite materials enhances their overall performance. This technique improves the interfacial bonding between matrix and reinforcement, resulting in composites with superior mechanical properties and durability.
02 Crosslinking in battery electrode materials
Perchloric acid crosslinking is employed in the production of battery electrode materials. This process enhances the structural integrity and electrochemical performance of the electrodes, leading to improved battery efficiency and longevity.Expand Specific Solutions03 Application in membrane technology
Perchloric acid crosslinking is used in the development of advanced membrane materials. This technique helps create membranes with enhanced selectivity, permeability, and chemical resistance, suitable for various separation and filtration processes.Expand Specific Solutions04 Crosslinking in aerospace materials
The aerospace industry utilizes perchloric acid crosslinking to develop high-performance materials. This process contributes to the creation of lightweight yet strong composites and coatings that can withstand extreme conditions encountered in aerospace applications.Expand Specific Solutions05 Safety considerations in perchloric acid crosslinking
Due to the reactive nature of perchloric acid, special safety measures are necessary when using it for crosslinking processes. This includes the use of specialized equipment, proper handling procedures, and appropriate personal protective gear to mitigate potential risks associated with its use.Expand Specific Solutions
Key Players in Polymer Chemistry Industry
The competitive landscape for perchloric acid's role in polymer crosslinking is evolving, with the market in its growth phase. The global market size for this technology is expanding, driven by increasing demand in various industries such as electronics, automotive, and materials science. The technology's maturity is moderate, with ongoing research and development efforts. Key players like Nippon Shokubai, 3M Innovative Properties, and Dow Global Technologies are leading innovation in this field, leveraging their expertise in chemical engineering and materials science. Companies such as DuPont de Nemours and Eternal Materials are also making significant contributions, focusing on developing advanced polymer formulations and crosslinking techniques. The competition is intensifying as more companies recognize the potential of perchloric acid in enhancing polymer properties and performance.
Nippon Shokubai Co., Ltd.
Technical Solution: Nippon Shokubai has developed an innovative approach to polymer crosslinking using perchloric acid in combination with their proprietary initiator systems. Their method involves the controlled release of perchloric acid from a precursor compound, which gradually initiates the crosslinking process. This approach allows for a more uniform distribution of crosslinks throughout the polymer matrix. The company has also incorporated antioxidants and stabilizers to prevent degradation of the polymer during the crosslinking process[5]. Nippon Shokubai's technique has been particularly successful in producing highly crosslinked hydrogels with improved swelling properties and mechanical strength[6].
Strengths: Uniform crosslink distribution, controlled reaction kinetics, and enhanced properties of hydrogels. Weaknesses: Limited to specific polymer types and potential scalability issues for large-scale production.
3M Innovative Properties Co.
Technical Solution: 3M has developed a unique approach to polymer crosslinking using perchloric acid in conjunction with their proprietary surface modification techniques. Their method involves the creation of a perchloric acid-rich interface between polymer layers or at the surface of polymer films. This localized concentration of perchloric acid facilitates targeted crosslinking, allowing for the development of gradient materials with varying degrees of crosslinking across their thickness[7]. 3M's technique has been particularly effective in producing adhesive films with enhanced bonding strength and durability. The company has also developed specialized coating processes to protect sensitive substrates from the corrosive effects of perchloric acid during the crosslinking process[8].
Strengths: Ability to create gradient materials, improved adhesive properties, and compatibility with sensitive substrates. Weaknesses: Limited to specific geometries and potential challenges in scaling up for large-area applications.
Core Innovations in Perchloric Acid Catalysis
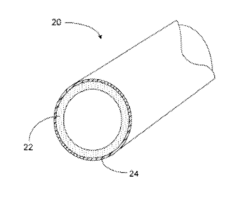
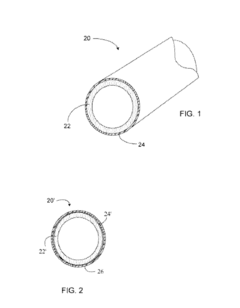
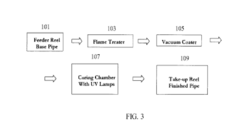
Methods for coating pipe
PatentActiveUS20150217333A1
Innovation
- A process involving radiation-curable pre-polymers is used to apply cross-linked coating layers to plastic pipes, where the surface is oxidized and then coated with a first and optionally a second layer of radiation-curable pre-polymer, which is cured using radiation to form thin, uniform, and flexible coatings with precise thickness control.
Polymeric compositions and method of making and articles thereof
PatentInactiveEP2499201A2
Innovation
- A method involving a non-self-crosslinking polymer reacted with a modifying compound containing distinct functional groups, such as aziridine amides or isocyanates, followed by an azide-alkyne cure system, allowing for controlled crosslinking under mild conditions and extended pot life.
Safety Considerations for Perchloric Acid Use
The use of perchloric acid in polymer crosslinking processes requires stringent safety measures due to its highly reactive and potentially explosive nature. Proper handling and storage of perchloric acid are crucial to prevent accidents and ensure the safety of personnel and facilities.
Perchloric acid should be stored in a cool, well-ventilated area away from combustible materials and other chemicals. It must be kept in tightly sealed containers made of compatible materials such as glass or PTFE. Regular inspections of storage areas and containers are necessary to detect any signs of leakage or degradation.
Personal protective equipment (PPE) is essential when working with perchloric acid. This includes chemical-resistant gloves, goggles, face shields, and appropriate protective clothing. Proper training on the handling and use of perchloric acid is mandatory for all personnel involved in its use.
Specialized fume hoods equipped with wash-down systems are required for work involving perchloric acid. These hoods must be regularly cleaned to prevent the accumulation of potentially explosive perchlorates. The use of wooden or other organic materials in the fume hood construction should be avoided.
Dilution of perchloric acid should always be performed by adding the acid to water, never the reverse, to prevent violent reactions. When heating perchloric acid, only use equipment specifically designed for this purpose, as standard hot plates can lead to explosions if perchloric acid residues are present.
Proper disposal of perchloric acid waste is critical. It should never be mixed with organic solvents or other incompatible materials. Specialized waste disposal procedures must be followed, often involving neutralization before disposal.
Emergency response plans must be in place, including the availability of appropriate fire extinguishing agents and spill control materials. Personnel should be trained in emergency procedures and the location of safety equipment.
Regular safety audits and risk assessments should be conducted to ensure compliance with safety protocols and to identify potential hazards. This includes monitoring the integrity of equipment and storage facilities, as well as reviewing and updating safety procedures.
In the context of polymer crosslinking, additional precautions may be necessary. The reaction environment must be carefully controlled to prevent unintended reactions between perchloric acid and polymer materials. Proper ventilation and temperature control are essential to manage the heat generated during crosslinking reactions.
By implementing these comprehensive safety measures, the risks associated with perchloric acid use in polymer crosslinking can be effectively managed, allowing for the safe exploitation of its unique properties in materials science applications.
Perchloric acid should be stored in a cool, well-ventilated area away from combustible materials and other chemicals. It must be kept in tightly sealed containers made of compatible materials such as glass or PTFE. Regular inspections of storage areas and containers are necessary to detect any signs of leakage or degradation.
Personal protective equipment (PPE) is essential when working with perchloric acid. This includes chemical-resistant gloves, goggles, face shields, and appropriate protective clothing. Proper training on the handling and use of perchloric acid is mandatory for all personnel involved in its use.
Specialized fume hoods equipped with wash-down systems are required for work involving perchloric acid. These hoods must be regularly cleaned to prevent the accumulation of potentially explosive perchlorates. The use of wooden or other organic materials in the fume hood construction should be avoided.
Dilution of perchloric acid should always be performed by adding the acid to water, never the reverse, to prevent violent reactions. When heating perchloric acid, only use equipment specifically designed for this purpose, as standard hot plates can lead to explosions if perchloric acid residues are present.
Proper disposal of perchloric acid waste is critical. It should never be mixed with organic solvents or other incompatible materials. Specialized waste disposal procedures must be followed, often involving neutralization before disposal.
Emergency response plans must be in place, including the availability of appropriate fire extinguishing agents and spill control materials. Personnel should be trained in emergency procedures and the location of safety equipment.
Regular safety audits and risk assessments should be conducted to ensure compliance with safety protocols and to identify potential hazards. This includes monitoring the integrity of equipment and storage facilities, as well as reviewing and updating safety procedures.
In the context of polymer crosslinking, additional precautions may be necessary. The reaction environment must be carefully controlled to prevent unintended reactions between perchloric acid and polymer materials. Proper ventilation and temperature control are essential to manage the heat generated during crosslinking reactions.
By implementing these comprehensive safety measures, the risks associated with perchloric acid use in polymer crosslinking can be effectively managed, allowing for the safe exploitation of its unique properties in materials science applications.
Environmental Impact of Crosslinking Processes
The environmental impact of crosslinking processes involving perchloric acid in polymer chain reactions is a critical consideration for both industrial applications and ecological sustainability. These processes, while effective in enhancing material properties, can pose significant environmental risks if not properly managed.
Perchloric acid, a strong oxidizing agent, is known for its ability to facilitate efficient crosslinking of polymeric chains. However, its use in industrial settings raises concerns about potential environmental contamination. The primary risk stems from the release of perchlorate ions into water systems. These ions are highly soluble and stable in aqueous environments, making them persistent pollutants that can travel long distances through groundwater and surface water.
The presence of perchlorates in water bodies can have detrimental effects on aquatic ecosystems. Studies have shown that perchlorate contamination can disrupt the endocrine systems of various aquatic organisms, particularly affecting thyroid function in fish and amphibians. This disruption can lead to developmental abnormalities and reproductive issues in affected species, potentially causing long-term ecological imbalances.
Furthermore, the use of perchloric acid in crosslinking processes may contribute to air pollution. Volatile organic compounds (VOCs) and other hazardous air pollutants can be released during the reaction and curing stages. These emissions not only pose health risks to workers but also contribute to the formation of ground-level ozone and smog, impacting air quality in surrounding areas.
Waste management is another crucial aspect of the environmental impact assessment. Residual perchloric acid and its byproducts require specialized disposal methods to prevent soil and water contamination. Improper disposal can lead to the accumulation of perchlorates in landfills, potentially leaching into groundwater and affecting drinking water supplies.
To mitigate these environmental risks, industries employing perchloric acid in crosslinking processes must implement stringent control measures. These include closed-loop systems to minimize emissions, advanced wastewater treatment technologies to remove perchlorate ions, and proper handling and disposal protocols for hazardous waste. Additionally, research into alternative, more environmentally friendly crosslinking agents is ongoing, aiming to reduce the reliance on perchloric acid while maintaining the desired material properties.
Regulatory bodies worldwide are increasingly focusing on the environmental impact of industrial processes involving perchloric acid. Stricter regulations and monitoring requirements are being implemented to ensure compliance with environmental standards and to protect ecosystems and human health from the potential adverse effects of perchlorate contamination.
Perchloric acid, a strong oxidizing agent, is known for its ability to facilitate efficient crosslinking of polymeric chains. However, its use in industrial settings raises concerns about potential environmental contamination. The primary risk stems from the release of perchlorate ions into water systems. These ions are highly soluble and stable in aqueous environments, making them persistent pollutants that can travel long distances through groundwater and surface water.
The presence of perchlorates in water bodies can have detrimental effects on aquatic ecosystems. Studies have shown that perchlorate contamination can disrupt the endocrine systems of various aquatic organisms, particularly affecting thyroid function in fish and amphibians. This disruption can lead to developmental abnormalities and reproductive issues in affected species, potentially causing long-term ecological imbalances.
Furthermore, the use of perchloric acid in crosslinking processes may contribute to air pollution. Volatile organic compounds (VOCs) and other hazardous air pollutants can be released during the reaction and curing stages. These emissions not only pose health risks to workers but also contribute to the formation of ground-level ozone and smog, impacting air quality in surrounding areas.
Waste management is another crucial aspect of the environmental impact assessment. Residual perchloric acid and its byproducts require specialized disposal methods to prevent soil and water contamination. Improper disposal can lead to the accumulation of perchlorates in landfills, potentially leaching into groundwater and affecting drinking water supplies.
To mitigate these environmental risks, industries employing perchloric acid in crosslinking processes must implement stringent control measures. These include closed-loop systems to minimize emissions, advanced wastewater treatment technologies to remove perchlorate ions, and proper handling and disposal protocols for hazardous waste. Additionally, research into alternative, more environmentally friendly crosslinking agents is ongoing, aiming to reduce the reliance on perchloric acid while maintaining the desired material properties.
Regulatory bodies worldwide are increasingly focusing on the environmental impact of industrial processes involving perchloric acid. Stricter regulations and monitoring requirements are being implemented to ensure compliance with environmental standards and to protect ecosystems and human health from the potential adverse effects of perchlorate contamination.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!