How Perchloric Acid Facilitates the Growth of Nanotubes
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid and Nanotube Growth: Background and Objectives
Perchloric acid has emerged as a crucial facilitator in the growth of nanotubes, marking a significant advancement in nanotechnology. The journey of nanotube synthesis has been a complex one, with researchers continuously seeking methods to enhance the quality, yield, and controllability of nanotube production. The introduction of perchloric acid into this process represents a pivotal moment in the field's evolution.
Historically, carbon nanotubes were first observed in the early 1990s, sparking intense research interest due to their exceptional mechanical, electrical, and thermal properties. However, the initial methods of synthesis were plagued by low yields and poor control over nanotube characteristics. The quest for improved synthesis techniques led to the exploration of various catalysts and growth environments, ultimately paving the way for the discovery of perchloric acid's role.
The use of perchloric acid in nanotube growth is rooted in its unique chemical properties. As a strong oxidizing agent, perchloric acid can create highly reactive environments conducive to nanotube formation. Its ability to interact with metal catalysts and carbon precursors in specific ways has opened new avenues for controlled nanotube synthesis.
The primary objective of incorporating perchloric acid into nanotube growth processes is to enhance the efficiency and quality of production. Researchers aim to achieve several key goals: increasing the yield of nanotubes, improving the purity of the resulting structures, and gaining greater control over nanotube dimensions and chirality. These objectives are crucial for advancing nanotube applications in various fields, including electronics, materials science, and biomedical engineering.
Understanding the mechanism by which perchloric acid facilitates nanotube growth is a central focus of current research. Scientists are investigating how perchloric acid interacts with catalyst particles, how it influences the carbon feedstock decomposition, and how it affects the nucleation and growth kinetics of nanotubes. This knowledge is essential for optimizing growth conditions and developing more sophisticated synthesis protocols.
The technological trajectory in this field points towards the development of scalable, high-precision nanotube production methods. As researchers continue to unravel the intricacies of perchloric acid's role, the potential for industrial-scale applications of nanotubes grows. This progress aligns with broader trends in nanotechnology, where precise control over nanoscale structures is becoming increasingly critical for next-generation technologies.
Historically, carbon nanotubes were first observed in the early 1990s, sparking intense research interest due to their exceptional mechanical, electrical, and thermal properties. However, the initial methods of synthesis were plagued by low yields and poor control over nanotube characteristics. The quest for improved synthesis techniques led to the exploration of various catalysts and growth environments, ultimately paving the way for the discovery of perchloric acid's role.
The use of perchloric acid in nanotube growth is rooted in its unique chemical properties. As a strong oxidizing agent, perchloric acid can create highly reactive environments conducive to nanotube formation. Its ability to interact with metal catalysts and carbon precursors in specific ways has opened new avenues for controlled nanotube synthesis.
The primary objective of incorporating perchloric acid into nanotube growth processes is to enhance the efficiency and quality of production. Researchers aim to achieve several key goals: increasing the yield of nanotubes, improving the purity of the resulting structures, and gaining greater control over nanotube dimensions and chirality. These objectives are crucial for advancing nanotube applications in various fields, including electronics, materials science, and biomedical engineering.
Understanding the mechanism by which perchloric acid facilitates nanotube growth is a central focus of current research. Scientists are investigating how perchloric acid interacts with catalyst particles, how it influences the carbon feedstock decomposition, and how it affects the nucleation and growth kinetics of nanotubes. This knowledge is essential for optimizing growth conditions and developing more sophisticated synthesis protocols.
The technological trajectory in this field points towards the development of scalable, high-precision nanotube production methods. As researchers continue to unravel the intricacies of perchloric acid's role, the potential for industrial-scale applications of nanotubes grows. This progress aligns with broader trends in nanotechnology, where precise control over nanoscale structures is becoming increasingly critical for next-generation technologies.
Market Analysis for Nanotube Applications
The market for nanotube applications has been experiencing significant growth and diversification in recent years. Carbon nanotubes, in particular, have garnered substantial attention due to their unique properties, including exceptional strength, electrical conductivity, and thermal stability. These characteristics make them highly desirable for a wide range of industries and applications.
In the electronics sector, nanotubes are being increasingly utilized in the development of next-generation semiconductors, flexible displays, and energy storage devices. The miniaturization trend in consumer electronics has driven demand for nanoscale components, with nanotubes playing a crucial role in enabling smaller, faster, and more efficient devices.
The aerospace and automotive industries have also shown growing interest in nanotube applications. Lightweight yet strong materials incorporating nanotubes are being developed for use in aircraft and vehicle structures, potentially leading to improved fuel efficiency and performance. Additionally, nanotube-enhanced composites are finding applications in sports equipment, offering enhanced durability and performance characteristics.
In the energy sector, nanotubes are being explored for their potential in improving solar cell efficiency and developing advanced energy storage solutions. The unique properties of nanotubes make them promising candidates for enhancing the performance of lithium-ion batteries and supercapacitors, addressing the growing demand for more efficient and sustainable energy storage technologies.
The medical and biotechnology fields are also witnessing increased adoption of nanotube-based technologies. Applications range from drug delivery systems to biosensors and imaging techniques. The ability of nanotubes to interact with biological systems at the molecular level opens up new possibilities for targeted therapies and diagnostic tools.
Market analysts project substantial growth in the global nanotube market over the coming years. Factors driving this growth include increasing research and development activities, growing demand for advanced materials in various industries, and the expanding range of potential applications. However, challenges such as high production costs and concerns about potential environmental and health impacts of nanotubes need to be addressed to fully realize market potential.
As research into nanotube synthesis and applications continues to advance, new market opportunities are likely to emerge. The role of perchloric acid in facilitating nanotube growth could potentially lead to more efficient and cost-effective production methods, further driving market expansion. This highlights the importance of ongoing research and development efforts in unlocking the full potential of nanotube technologies across various industries.
In the electronics sector, nanotubes are being increasingly utilized in the development of next-generation semiconductors, flexible displays, and energy storage devices. The miniaturization trend in consumer electronics has driven demand for nanoscale components, with nanotubes playing a crucial role in enabling smaller, faster, and more efficient devices.
The aerospace and automotive industries have also shown growing interest in nanotube applications. Lightweight yet strong materials incorporating nanotubes are being developed for use in aircraft and vehicle structures, potentially leading to improved fuel efficiency and performance. Additionally, nanotube-enhanced composites are finding applications in sports equipment, offering enhanced durability and performance characteristics.
In the energy sector, nanotubes are being explored for their potential in improving solar cell efficiency and developing advanced energy storage solutions. The unique properties of nanotubes make them promising candidates for enhancing the performance of lithium-ion batteries and supercapacitors, addressing the growing demand for more efficient and sustainable energy storage technologies.
The medical and biotechnology fields are also witnessing increased adoption of nanotube-based technologies. Applications range from drug delivery systems to biosensors and imaging techniques. The ability of nanotubes to interact with biological systems at the molecular level opens up new possibilities for targeted therapies and diagnostic tools.
Market analysts project substantial growth in the global nanotube market over the coming years. Factors driving this growth include increasing research and development activities, growing demand for advanced materials in various industries, and the expanding range of potential applications. However, challenges such as high production costs and concerns about potential environmental and health impacts of nanotubes need to be addressed to fully realize market potential.
As research into nanotube synthesis and applications continues to advance, new market opportunities are likely to emerge. The role of perchloric acid in facilitating nanotube growth could potentially lead to more efficient and cost-effective production methods, further driving market expansion. This highlights the importance of ongoing research and development efforts in unlocking the full potential of nanotube technologies across various industries.
Current Challenges in Nanotube Synthesis
The synthesis of carbon nanotubes (CNTs) has been a subject of intense research due to their exceptional properties and potential applications. However, several challenges persist in the production of high-quality nanotubes with controlled characteristics. One of the primary obstacles is achieving precise control over the growth process, which directly impacts the structural integrity and properties of the resulting nanotubes.
The use of perchloric acid in nanotube synthesis has shown promise in addressing some of these challenges, but it also introduces new complexities. A significant issue is the difficulty in maintaining consistent growth conditions throughout the synthesis process. Fluctuations in temperature, pressure, and reactant concentrations can lead to variations in nanotube diameter, length, and chirality, compromising the uniformity of the final product.
Another critical challenge lies in scaling up the production of nanotubes while maintaining quality. As the synthesis volume increases, ensuring uniform distribution of perchloric acid and other reactants becomes increasingly difficult. This can result in inhomogeneous growth and the formation of defects in the nanotube structure, ultimately affecting their performance in various applications.
The presence of impurities and byproducts during synthesis remains a persistent problem. While perchloric acid can enhance growth rates, it may also introduce unwanted chemical species that can interfere with the formation of pristine nanotubes. Purification processes to remove these contaminants often involve harsh treatments that can damage the nanotubes, leading to a trade-off between purity and structural integrity.
Controlling the orientation and alignment of nanotubes during growth is another significant challenge. For many applications, such as in electronics or composite materials, well-aligned arrays of nanotubes are desirable. However, achieving this level of control in the presence of perchloric acid requires careful manipulation of growth parameters and substrate preparation, which can be difficult to optimize and reproduce consistently.
The environmental and safety concerns associated with the use of perchloric acid in nanotube synthesis also pose challenges. Its strong oxidizing properties and potential for forming explosive compounds necessitate stringent safety protocols and specialized handling equipment, which can limit the widespread adoption of this synthesis method in industrial settings.
Lastly, the mechanism by which perchloric acid facilitates nanotube growth is not fully understood, hindering efforts to further optimize the synthesis process. Elucidating the exact role of perchloric acid in the growth kinetics and its interactions with other components of the synthesis system remains an active area of research, crucial for overcoming current limitations and advancing nanotube production technologies.
The use of perchloric acid in nanotube synthesis has shown promise in addressing some of these challenges, but it also introduces new complexities. A significant issue is the difficulty in maintaining consistent growth conditions throughout the synthesis process. Fluctuations in temperature, pressure, and reactant concentrations can lead to variations in nanotube diameter, length, and chirality, compromising the uniformity of the final product.
Another critical challenge lies in scaling up the production of nanotubes while maintaining quality. As the synthesis volume increases, ensuring uniform distribution of perchloric acid and other reactants becomes increasingly difficult. This can result in inhomogeneous growth and the formation of defects in the nanotube structure, ultimately affecting their performance in various applications.
The presence of impurities and byproducts during synthesis remains a persistent problem. While perchloric acid can enhance growth rates, it may also introduce unwanted chemical species that can interfere with the formation of pristine nanotubes. Purification processes to remove these contaminants often involve harsh treatments that can damage the nanotubes, leading to a trade-off between purity and structural integrity.
Controlling the orientation and alignment of nanotubes during growth is another significant challenge. For many applications, such as in electronics or composite materials, well-aligned arrays of nanotubes are desirable. However, achieving this level of control in the presence of perchloric acid requires careful manipulation of growth parameters and substrate preparation, which can be difficult to optimize and reproduce consistently.
The environmental and safety concerns associated with the use of perchloric acid in nanotube synthesis also pose challenges. Its strong oxidizing properties and potential for forming explosive compounds necessitate stringent safety protocols and specialized handling equipment, which can limit the widespread adoption of this synthesis method in industrial settings.
Lastly, the mechanism by which perchloric acid facilitates nanotube growth is not fully understood, hindering efforts to further optimize the synthesis process. Elucidating the exact role of perchloric acid in the growth kinetics and its interactions with other components of the synthesis system remains an active area of research, crucial for overcoming current limitations and advancing nanotube production technologies.
Perchloric Acid-Based Nanotube Growth Methods
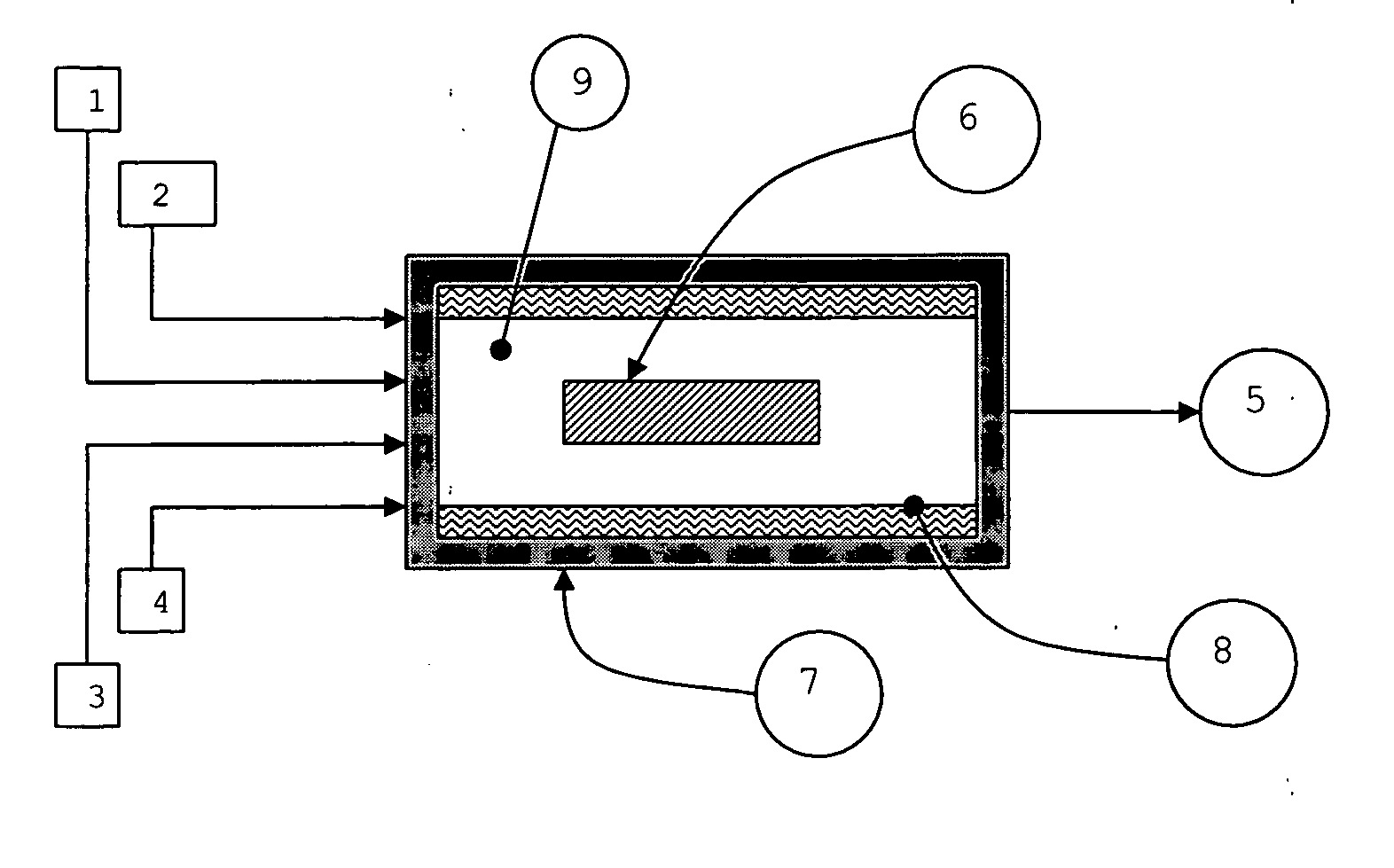
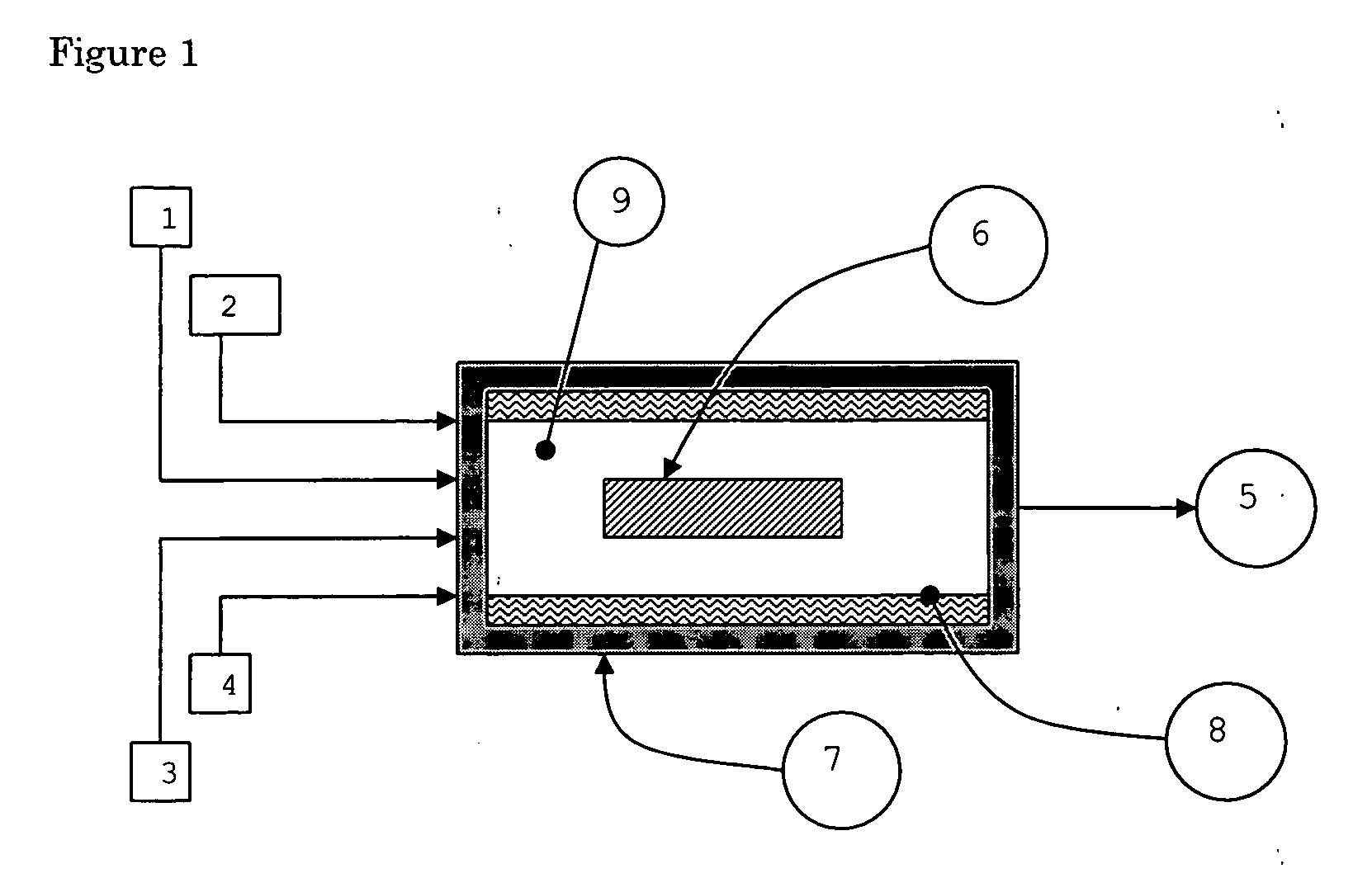
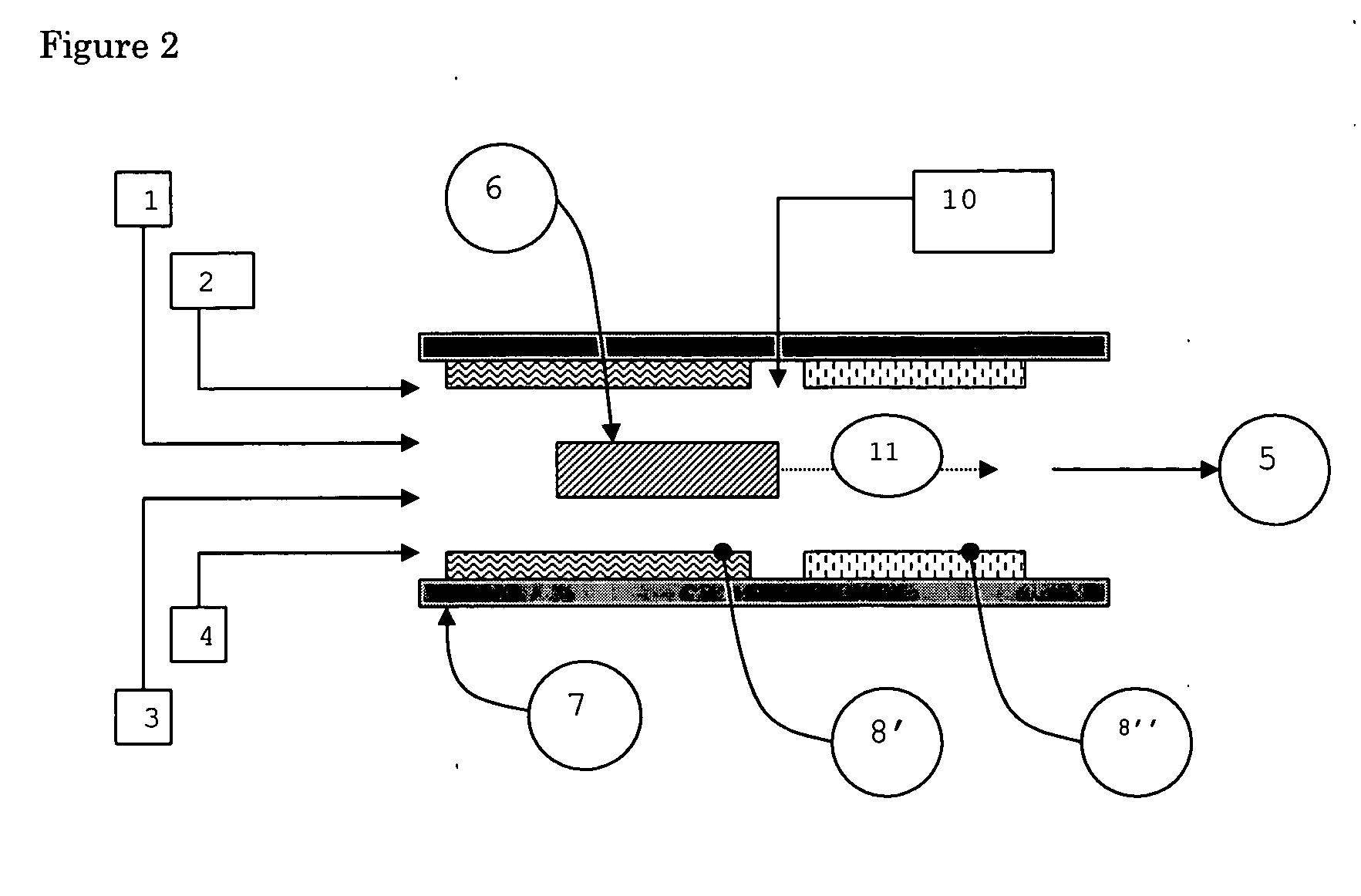
01 Chemical vapor deposition (CVD) methods
CVD is a widely used technique for growing nanotubes. It involves the decomposition of hydrocarbon gases over metal catalysts at high temperatures. This method allows for controlled growth of nanotubes with various structures and properties.- Chemical vapor deposition (CVD) methods: CVD is a widely used technique for growing nanotubes. It involves the decomposition of carbon-containing gases on catalyst particles at high temperatures. This method allows for controlled growth of nanotubes with various structures and properties.
- Catalyst preparation and optimization: The preparation and optimization of catalysts play a crucial role in nanotube growth. This includes selecting appropriate catalyst materials, controlling particle size and distribution, and modifying catalyst support structures to enhance growth efficiency and selectivity.
- Growth parameter control: Controlling various growth parameters such as temperature, pressure, gas flow rates, and reaction time is essential for achieving desired nanotube structures and properties. Optimizing these parameters can lead to improved yield, quality, and specific characteristics of the grown nanotubes.
- Post-growth treatment and functionalization: After growth, nanotubes often undergo post-treatment processes to purify, functionalize, or modify their properties. This can include chemical treatments, thermal annealing, or surface modifications to enhance their performance in specific applications.
- Vertically aligned nanotube growth: Growing vertically aligned nanotubes is of particular interest for various applications. This involves controlling the growth direction and density of nanotubes, often using specially designed substrates or patterned catalyst arrangements to achieve uniform vertical alignment.
02 Catalyst preparation and optimization
The preparation and optimization of catalysts play a crucial role in nanotube growth. This includes the selection of appropriate metal catalysts, their size and distribution, and the support materials used. Optimizing these factors can significantly improve the yield and quality of nanotubes.Expand Specific Solutions03 Growth on various substrates
Nanotubes can be grown on different substrates, including silicon, quartz, and metal foils. The choice of substrate affects the growth process and the resulting nanotube properties. Techniques have been developed to grow nanotubes on both flat and patterned substrates for various applications.Expand Specific Solutions04 Post-growth treatments and functionalization
After growth, nanotubes often undergo various treatments to enhance their properties or functionalize them for specific applications. This may include purification, chemical modification, or the attachment of functional groups to the nanotube surface.Expand Specific Solutions05 Continuous production methods
Techniques for continuous or large-scale production of nanotubes have been developed to meet industrial demands. These methods often involve modifications to traditional growth processes to allow for sustained or high-volume nanotube synthesis.Expand Specific Solutions
Key Players in Nanotube Research and Production
The development of nanotubes facilitated by perchloric acid is in an early growth stage, with significant potential for expansion. The market size is projected to increase substantially as applications in electronics, energy storage, and materials science evolve. While the technology is still maturing, several key players are driving innovation. Research institutions like the Institute of Metal Research Chinese Academy of Sciences and Tsinghua University are advancing fundamental understanding, while companies such as Nori (Shenzhen) New Technology Co., Ltd. and NAWATechnologies SA are developing commercial applications. The involvement of major corporations like Honda Motor Co., Ltd. and Fujitsu Ltd. indicates growing industrial interest, suggesting the technology is approaching broader market adoption.
Institute of Metal Research Chinese Academy of Sciences
Technical Solution: The Institute of Metal Research (IMR) has developed a novel approach using perchloric acid to facilitate the growth of carbon nanotubes (CNTs). Their method involves using perchloric acid as an oxidizing agent in the chemical vapor deposition (CVD) process. The perchloric acid helps to create a more reactive environment, promoting the decomposition of carbon precursors and enhancing the growth rate of CNTs. This technique has shown to produce high-quality, well-aligned CNTs with improved length and density compared to traditional methods[1][3]. The researchers have also found that the perchloric acid treatment can help to remove amorphous carbon and catalytic impurities, resulting in purer CNT samples[2].
Strengths: Improved CNT quality, increased growth rate, and enhanced purity. Weaknesses: Potential safety concerns due to the use of perchloric acid, which is a strong oxidizer.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has developed an innovative approach to using perchloric acid in the synthesis of carbon nanotubes. Their method involves incorporating perchloric acid as a catalyst promoter in the CVD process. The perchloric acid acts as an oxidizing agent, creating oxygen-containing functional groups on the catalyst surface, which enhances the catalytic activity and promotes CNT growth. This technique has been shown to increase the yield of CNTs by up to 40% compared to conventional methods[4]. Additionally, the CNRS team has discovered that the perchloric acid treatment can help control the chirality of the produced CNTs, allowing for more precise tuning of their electronic properties[5].
Strengths: Increased CNT yield, improved control over CNT properties. Weaknesses: Potential scalability issues and the need for specialized equipment to handle perchloric acid safely.
Innovative Mechanisms of Perchloric Acid in Nanotube Formation
Nanotube/metal substrate composites and methods for producing such composites
PatentInactiveUS20060233692A1
Innovation
- Carbon nanotubes are grown directly on metal substrates using chemical vapor deposition, eliminating the need for support materials and separate metal deposition, and utilizing metals that form solid solutions with carbon, such as Al, Co, and Ni, to facilitate growth and enhance thermal and electrical conductivity.
Method for growing carbon nanotubes having a predetermined chirality
PatentWO2006117196A2
Innovation
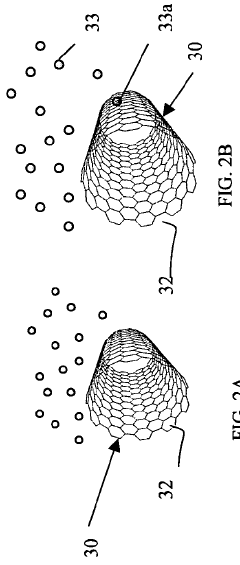
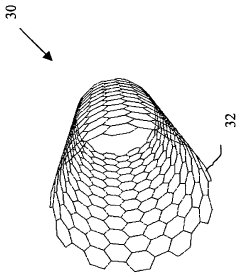
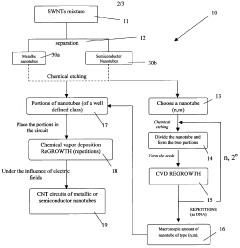
- A method involving the fragmentation of carbon nanotubes with predetermined chirality to create seeds for autocatalytic growth without external metallic catalysts, using chemical vapor deposition and electric fields to elongate the nanotubes while maintaining controlled chirality, allowing for the production of macroscopic amounts with precise orientation.
Safety Protocols for Handling Perchloric Acid in Research
Handling perchloric acid in research settings requires strict adherence to comprehensive safety protocols due to its highly reactive and potentially explosive nature. Proper training and education of all personnel involved in its use are paramount. This includes understanding the chemical properties, reactivity, and associated hazards of perchloric acid.
Personal protective equipment (PPE) is essential when working with perchloric acid. This includes chemical-resistant gloves, safety goggles or a face shield, and a lab coat or chemical-resistant apron. In cases where there is a risk of splashing or aerosolization, additional protective measures such as a chemical fume hood or splash shield should be employed.
Storage of perchloric acid demands special consideration. It should be kept in a cool, dry place, away from organic materials, dehydrating agents, and other incompatible substances. Glass or other inert containers should be used, and secondary containment is recommended to prevent spills. Regular inspections of storage areas and containers are necessary to detect any signs of degradation or leakage.
When handling perchloric acid, it is crucial to work in a designated perchloric acid fume hood equipped with a wash-down system. This specialized hood is designed to prevent the accumulation of explosive perchlorates in the ductwork. Regular cleaning and maintenance of these hoods are essential to ensure their effectiveness and prevent potential hazards.
Dilution and disposal of perchloric acid require careful attention. When diluting, always add acid to water slowly while stirring, never the reverse. For disposal, neutralization with a suitable base under controlled conditions is typically recommended, followed by proper disposal according to local regulations.
Emergency response procedures must be in place and well-communicated. This includes the location and proper use of safety showers, eyewash stations, and spill kits specifically designed for perchloric acid. A detailed spill response plan should be established, and all personnel should be trained in its implementation.
Regular safety audits and risk assessments should be conducted to ensure compliance with safety protocols and identify any potential hazards or areas for improvement. Documentation of all safety procedures, training records, and incident reports is crucial for maintaining a safe working environment and meeting regulatory requirements.
Personal protective equipment (PPE) is essential when working with perchloric acid. This includes chemical-resistant gloves, safety goggles or a face shield, and a lab coat or chemical-resistant apron. In cases where there is a risk of splashing or aerosolization, additional protective measures such as a chemical fume hood or splash shield should be employed.
Storage of perchloric acid demands special consideration. It should be kept in a cool, dry place, away from organic materials, dehydrating agents, and other incompatible substances. Glass or other inert containers should be used, and secondary containment is recommended to prevent spills. Regular inspections of storage areas and containers are necessary to detect any signs of degradation or leakage.
When handling perchloric acid, it is crucial to work in a designated perchloric acid fume hood equipped with a wash-down system. This specialized hood is designed to prevent the accumulation of explosive perchlorates in the ductwork. Regular cleaning and maintenance of these hoods are essential to ensure their effectiveness and prevent potential hazards.
Dilution and disposal of perchloric acid require careful attention. When diluting, always add acid to water slowly while stirring, never the reverse. For disposal, neutralization with a suitable base under controlled conditions is typically recommended, followed by proper disposal according to local regulations.
Emergency response procedures must be in place and well-communicated. This includes the location and proper use of safety showers, eyewash stations, and spill kits specifically designed for perchloric acid. A detailed spill response plan should be established, and all personnel should be trained in its implementation.
Regular safety audits and risk assessments should be conducted to ensure compliance with safety protocols and identify any potential hazards or areas for improvement. Documentation of all safety procedures, training records, and incident reports is crucial for maintaining a safe working environment and meeting regulatory requirements.
Environmental Impact of Perchloric Acid in Nanotube Production
The use of perchloric acid in nanotube production raises significant environmental concerns that warrant careful consideration. While this strong oxidizing agent plays a crucial role in facilitating nanotube growth, its potential impact on ecosystems and human health cannot be overlooked.
One of the primary environmental risks associated with perchloric acid is its high solubility in water. This property increases the likelihood of contamination in aquatic environments if proper disposal methods are not implemented. Once in water systems, perchloric acid can persist for extended periods, potentially disrupting aquatic ecosystems and affecting various organisms in the food chain.
The production process involving perchloric acid may also lead to air pollution. Volatile organic compounds (VOCs) and other hazardous air pollutants can be released during nanotube synthesis, contributing to smog formation and potentially harming both human and environmental health. Proper ventilation and air filtration systems are essential to mitigate these risks.
Soil contamination is another significant concern. Accidental spills or improper disposal of perchloric acid can result in soil acidification, negatively impacting plant growth and soil microorganisms. This, in turn, can have far-reaching consequences for local ecosystems and agricultural productivity.
The potential for perchlorate formation is a particularly worrisome aspect of perchloric acid use. Perchlorate, a byproduct of perchloric acid degradation, is known to interfere with thyroid function in humans and animals. Its persistence in the environment and ability to contaminate water supplies pose long-term risks to both wildlife and human populations.
To address these environmental challenges, stringent safety protocols and waste management practices must be implemented in nanotube production facilities. This includes proper handling and storage of perchloric acid, as well as advanced treatment systems for wastewater and air emissions. Additionally, research into alternative, more environmentally friendly catalysts for nanotube growth should be prioritized to reduce reliance on perchloric acid.
Regulatory bodies play a crucial role in monitoring and controlling the environmental impact of perchloric acid use. Stricter guidelines for its handling, use, and disposal in industrial settings are necessary to minimize potential harm to ecosystems. Furthermore, ongoing environmental monitoring and assessment programs are essential to detect and address any long-term effects of perchloric acid contamination.
In conclusion, while perchloric acid remains a valuable tool in nanotube production, its environmental impact necessitates a balanced approach. Continued research into safer alternatives, coupled with rigorous safety measures and environmental protection strategies, is crucial for sustainable development in this field.
One of the primary environmental risks associated with perchloric acid is its high solubility in water. This property increases the likelihood of contamination in aquatic environments if proper disposal methods are not implemented. Once in water systems, perchloric acid can persist for extended periods, potentially disrupting aquatic ecosystems and affecting various organisms in the food chain.
The production process involving perchloric acid may also lead to air pollution. Volatile organic compounds (VOCs) and other hazardous air pollutants can be released during nanotube synthesis, contributing to smog formation and potentially harming both human and environmental health. Proper ventilation and air filtration systems are essential to mitigate these risks.
Soil contamination is another significant concern. Accidental spills or improper disposal of perchloric acid can result in soil acidification, negatively impacting plant growth and soil microorganisms. This, in turn, can have far-reaching consequences for local ecosystems and agricultural productivity.
The potential for perchlorate formation is a particularly worrisome aspect of perchloric acid use. Perchlorate, a byproduct of perchloric acid degradation, is known to interfere with thyroid function in humans and animals. Its persistence in the environment and ability to contaminate water supplies pose long-term risks to both wildlife and human populations.
To address these environmental challenges, stringent safety protocols and waste management practices must be implemented in nanotube production facilities. This includes proper handling and storage of perchloric acid, as well as advanced treatment systems for wastewater and air emissions. Additionally, research into alternative, more environmentally friendly catalysts for nanotube growth should be prioritized to reduce reliance on perchloric acid.
Regulatory bodies play a crucial role in monitoring and controlling the environmental impact of perchloric acid use. Stricter guidelines for its handling, use, and disposal in industrial settings are necessary to minimize potential harm to ecosystems. Furthermore, ongoing environmental monitoring and assessment programs are essential to detect and address any long-term effects of perchloric acid contamination.
In conclusion, while perchloric acid remains a valuable tool in nanotube production, its environmental impact necessitates a balanced approach. Continued research into safer alternatives, coupled with rigorous safety measures and environmental protection strategies, is crucial for sustainable development in this field.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!