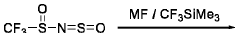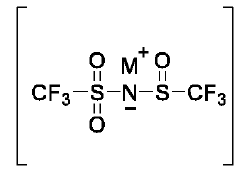How Perchloric Acid Influences Ionic Conductivity in Battery Electrolytes
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid in Electrolytes: Background and Objectives
Perchloric acid has emerged as a significant component in the development of advanced battery electrolytes, particularly in the pursuit of enhanced ionic conductivity. The evolution of battery technology has been driven by the increasing demand for high-performance energy storage solutions across various sectors, including consumer electronics, electric vehicles, and renewable energy systems. In this context, the role of electrolytes in facilitating efficient ion transport between electrodes has become a focal point of research and innovation.
The primary objective of investigating perchloric acid's influence on ionic conductivity in battery electrolytes is to unlock new possibilities for improving battery performance, specifically in terms of power output, charging speed, and overall efficiency. By understanding the mechanisms through which perchloric acid affects ion mobility and transport, researchers aim to develop electrolyte formulations that can significantly enhance the capabilities of next-generation energy storage devices.
Historically, the use of perchloric acid in electrolyte systems has been limited due to safety concerns and its highly oxidizing nature. However, recent advancements in materials science and a deeper understanding of electrochemistry have reignited interest in harnessing its unique properties. The strong dissociation of perchloric acid in solution and its ability to form stable perchlorates with various cations make it an intriguing candidate for modifying electrolyte compositions.
The technical landscape surrounding perchloric acid in battery electrolytes is characterized by a complex interplay of factors, including ion-solvent interactions, electrode-electrolyte interfaces, and the formation of solid-electrolyte interphases (SEI). Researchers are exploring how the addition of perchloric acid or its derivatives can influence these critical aspects of battery chemistry, potentially leading to breakthroughs in electrolyte design.
As the field progresses, the goals of perchloric acid research in battery electrolytes extend beyond mere improvements in ionic conductivity. Scientists and engineers are also investigating its potential to enhance other crucial battery parameters, such as thermal stability, electrochemical window, and long-term cycling performance. The ultimate aim is to develop electrolyte systems that can meet the increasingly demanding requirements of advanced energy storage applications while maintaining safety and reliability.
The primary objective of investigating perchloric acid's influence on ionic conductivity in battery electrolytes is to unlock new possibilities for improving battery performance, specifically in terms of power output, charging speed, and overall efficiency. By understanding the mechanisms through which perchloric acid affects ion mobility and transport, researchers aim to develop electrolyte formulations that can significantly enhance the capabilities of next-generation energy storage devices.
Historically, the use of perchloric acid in electrolyte systems has been limited due to safety concerns and its highly oxidizing nature. However, recent advancements in materials science and a deeper understanding of electrochemistry have reignited interest in harnessing its unique properties. The strong dissociation of perchloric acid in solution and its ability to form stable perchlorates with various cations make it an intriguing candidate for modifying electrolyte compositions.
The technical landscape surrounding perchloric acid in battery electrolytes is characterized by a complex interplay of factors, including ion-solvent interactions, electrode-electrolyte interfaces, and the formation of solid-electrolyte interphases (SEI). Researchers are exploring how the addition of perchloric acid or its derivatives can influence these critical aspects of battery chemistry, potentially leading to breakthroughs in electrolyte design.
As the field progresses, the goals of perchloric acid research in battery electrolytes extend beyond mere improvements in ionic conductivity. Scientists and engineers are also investigating its potential to enhance other crucial battery parameters, such as thermal stability, electrochemical window, and long-term cycling performance. The ultimate aim is to develop electrolyte systems that can meet the increasingly demanding requirements of advanced energy storage applications while maintaining safety and reliability.
Market Analysis for Advanced Battery Electrolytes
The market for advanced battery electrolytes is experiencing significant growth, driven by the increasing demand for high-performance energy storage solutions across various industries. The global battery electrolyte market is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) exceeding industry averages. This growth is primarily fueled by the rapid expansion of the electric vehicle (EV) sector, as well as the rising adoption of renewable energy systems and portable electronic devices.
In the EV market, the demand for advanced battery electrolytes is particularly strong. As automakers strive to improve the range, charging speed, and overall performance of electric vehicles, there is a growing need for electrolytes that can enhance ionic conductivity and stability. This has led to increased research and development efforts focused on novel electrolyte formulations, including those incorporating perchloric acid.
The renewable energy sector is another key driver for advanced battery electrolytes. With the increasing integration of intermittent renewable sources like solar and wind into power grids, there is a growing demand for large-scale energy storage solutions. Advanced electrolytes that can improve the efficiency and longevity of grid-scale batteries are highly sought after in this market segment.
Consumer electronics represent another significant market for advanced battery electrolytes. As smartphones, laptops, and wearable devices become more powerful and feature-rich, there is a constant push for batteries with higher energy density and faster charging capabilities. This has created opportunities for electrolyte innovations that can address these requirements while maintaining safety and reliability.
The Asia-Pacific region dominates the global battery electrolyte market, with China, Japan, and South Korea being the major contributors. This is largely due to the presence of key battery manufacturers and the rapid growth of the EV industry in these countries. North America and Europe are also witnessing substantial growth in demand for advanced electrolytes, driven by stringent environmental regulations and increasing investments in clean energy technologies.
Key players in the advanced battery electrolyte market include established chemical companies, specialized electrolyte manufacturers, and emerging startups focusing on innovative formulations. These companies are actively engaged in research and development activities to create electrolytes with improved ionic conductivity, thermal stability, and compatibility with high-voltage cathode materials.
The market for perchloric acid-based electrolytes, while still niche, is gaining attention due to its potential to significantly enhance ionic conductivity in certain battery systems. However, challenges related to safety, cost, and scalability need to be addressed before widespread adoption can occur. As research progresses and new applications emerge, the market for these specialized electrolytes is expected to grow, particularly in high-performance and niche battery applications.
In the EV market, the demand for advanced battery electrolytes is particularly strong. As automakers strive to improve the range, charging speed, and overall performance of electric vehicles, there is a growing need for electrolytes that can enhance ionic conductivity and stability. This has led to increased research and development efforts focused on novel electrolyte formulations, including those incorporating perchloric acid.
The renewable energy sector is another key driver for advanced battery electrolytes. With the increasing integration of intermittent renewable sources like solar and wind into power grids, there is a growing demand for large-scale energy storage solutions. Advanced electrolytes that can improve the efficiency and longevity of grid-scale batteries are highly sought after in this market segment.
Consumer electronics represent another significant market for advanced battery electrolytes. As smartphones, laptops, and wearable devices become more powerful and feature-rich, there is a constant push for batteries with higher energy density and faster charging capabilities. This has created opportunities for electrolyte innovations that can address these requirements while maintaining safety and reliability.
The Asia-Pacific region dominates the global battery electrolyte market, with China, Japan, and South Korea being the major contributors. This is largely due to the presence of key battery manufacturers and the rapid growth of the EV industry in these countries. North America and Europe are also witnessing substantial growth in demand for advanced electrolytes, driven by stringent environmental regulations and increasing investments in clean energy technologies.
Key players in the advanced battery electrolyte market include established chemical companies, specialized electrolyte manufacturers, and emerging startups focusing on innovative formulations. These companies are actively engaged in research and development activities to create electrolytes with improved ionic conductivity, thermal stability, and compatibility with high-voltage cathode materials.
The market for perchloric acid-based electrolytes, while still niche, is gaining attention due to its potential to significantly enhance ionic conductivity in certain battery systems. However, challenges related to safety, cost, and scalability need to be addressed before widespread adoption can occur. As research progresses and new applications emerge, the market for these specialized electrolytes is expected to grow, particularly in high-performance and niche battery applications.
Current Challenges in Ionic Conductivity Enhancement
Enhancing ionic conductivity in battery electrolytes remains a critical challenge in the development of high-performance energy storage systems. Despite significant advancements in electrolyte formulations, several obstacles persist in achieving optimal ionic conductivity for next-generation batteries.
One of the primary challenges is the trade-off between ionic conductivity and electrochemical stability. While higher salt concentrations can increase ionic conductivity, they often lead to increased viscosity and reduced electrochemical stability. This delicate balance necessitates careful optimization of electrolyte compositions to maintain both high conductivity and long-term stability.
The formation of a stable solid electrolyte interphase (SEI) layer is another crucial factor affecting ionic conductivity. An ideal SEI should allow efficient ion transport while preventing continuous electrolyte decomposition. However, achieving this balance remains challenging, particularly in high-voltage battery systems where the SEI is prone to degradation.
Temperature dependence of ionic conductivity poses another significant hurdle. Many electrolytes exhibit reduced conductivity at low temperatures, limiting battery performance in cold environments. Conversely, high temperatures can accelerate electrolyte decomposition and compromise safety. Developing electrolytes with consistent conductivity across a wide temperature range is essential for improving battery reliability and safety.
The limited understanding of ion transport mechanisms in complex electrolyte systems hinders the rational design of high-conductivity electrolytes. The interplay between different ionic species, solvent molecules, and additives creates a complex environment that is challenging to model and predict accurately. This knowledge gap impedes the development of tailored electrolyte solutions for specific battery chemistries.
Scaling up the production of high-performance electrolytes presents additional challenges. Many promising electrolyte formulations that demonstrate excellent conductivity in laboratory settings face difficulties in large-scale manufacturing due to cost, stability, or safety concerns. Bridging this gap between research and commercial viability is crucial for the widespread adoption of advanced electrolytes.
The influence of electrolyte additives, such as perchloric acid, on ionic conductivity introduces both opportunities and challenges. While additives can enhance conductivity through various mechanisms, their long-term effects on battery performance and safety require extensive investigation. Balancing the benefits of conductivity enhancement with potential drawbacks, such as increased corrosion or gas generation, remains a significant challenge in electrolyte design.
One of the primary challenges is the trade-off between ionic conductivity and electrochemical stability. While higher salt concentrations can increase ionic conductivity, they often lead to increased viscosity and reduced electrochemical stability. This delicate balance necessitates careful optimization of electrolyte compositions to maintain both high conductivity and long-term stability.
The formation of a stable solid electrolyte interphase (SEI) layer is another crucial factor affecting ionic conductivity. An ideal SEI should allow efficient ion transport while preventing continuous electrolyte decomposition. However, achieving this balance remains challenging, particularly in high-voltage battery systems where the SEI is prone to degradation.
Temperature dependence of ionic conductivity poses another significant hurdle. Many electrolytes exhibit reduced conductivity at low temperatures, limiting battery performance in cold environments. Conversely, high temperatures can accelerate electrolyte decomposition and compromise safety. Developing electrolytes with consistent conductivity across a wide temperature range is essential for improving battery reliability and safety.
The limited understanding of ion transport mechanisms in complex electrolyte systems hinders the rational design of high-conductivity electrolytes. The interplay between different ionic species, solvent molecules, and additives creates a complex environment that is challenging to model and predict accurately. This knowledge gap impedes the development of tailored electrolyte solutions for specific battery chemistries.
Scaling up the production of high-performance electrolytes presents additional challenges. Many promising electrolyte formulations that demonstrate excellent conductivity in laboratory settings face difficulties in large-scale manufacturing due to cost, stability, or safety concerns. Bridging this gap between research and commercial viability is crucial for the widespread adoption of advanced electrolytes.
The influence of electrolyte additives, such as perchloric acid, on ionic conductivity introduces both opportunities and challenges. While additives can enhance conductivity through various mechanisms, their long-term effects on battery performance and safety require extensive investigation. Balancing the benefits of conductivity enhancement with potential drawbacks, such as increased corrosion or gas generation, remains a significant challenge in electrolyte design.
Existing Solutions for Improving Ionic Conductivity
01 Ionic liquid-based electrolytes
Ionic liquids are used as electrolytes in batteries to enhance ionic conductivity. These materials have low volatility, high thermal stability, and excellent electrochemical properties. They can be tailored to optimize conductivity and improve overall battery performance.- Ionic liquid electrolytes: Ionic liquids are used as electrolytes in batteries to enhance ionic conductivity. These materials have low volatility, high thermal stability, and excellent electrochemical properties. They can improve the overall performance and safety of batteries by providing better ion transport and reducing the risk of electrolyte leakage.
- Polymer-based electrolytes: Polymer-based electrolytes are developed to improve ionic conductivity in batteries. These materials combine the mechanical stability of polymers with the ion-conducting properties of electrolytes. They can be designed as solid or gel-like structures, offering advantages such as improved safety, flexibility, and compatibility with various electrode materials.
- Composite electrolytes: Composite electrolytes are created by combining different materials to enhance ionic conductivity. These may include mixtures of organic and inorganic components, ceramic particles in polymer matrices, or hybrid structures. Composite electrolytes can offer improved mechanical properties, higher ionic conductivity, and better interfacial stability with electrodes.
- Additives for enhancing ionic conductivity: Various additives are incorporated into battery electrolytes to improve ionic conductivity. These may include salts, solvents, or nanoparticles that can enhance ion transport, modify the electrolyte structure, or create additional pathways for ion movement. Careful selection and optimization of additives can significantly improve battery performance and cycling stability.
- Novel electrolyte structures and designs: Innovative electrolyte structures and designs are explored to enhance ionic conductivity in batteries. These may include three-dimensional networks, hierarchical porous structures, or engineered interfaces between electrolytes and electrodes. Such designs aim to optimize ion transport pathways, reduce interfacial resistance, and improve overall battery performance.
02 Polymer-based solid electrolytes
Polymer-based solid electrolytes are developed to improve safety and stability in batteries. These materials offer advantages such as flexibility, ease of processing, and compatibility with electrode materials. Research focuses on enhancing their ionic conductivity through various polymer compositions and additives.Expand Specific Solutions03 Composite electrolytes
Composite electrolytes combine different materials to achieve improved ionic conductivity and mechanical properties. These may include polymer-ceramic composites or gel polymer electrolytes. The synergistic effects of the components can lead to enhanced overall performance in battery applications.Expand Specific Solutions04 Additives for enhancing ionic conductivity
Various additives are incorporated into electrolytes to boost ionic conductivity. These may include salts, plasticizers, or nanoparticles. The additives can modify the structure of the electrolyte, create additional conduction pathways, or improve ion dissociation, resulting in higher overall conductivity.Expand Specific Solutions05 Novel electrolyte structures and designs
Innovative electrolyte structures and designs are explored to enhance ionic conductivity. These may include hierarchical or porous structures, layered electrolytes, or novel material combinations. Such designs aim to optimize ion transport pathways and improve overall battery performance.Expand Specific Solutions
Key Players in Battery Electrolyte Industry
The development of perchloric acid's influence on ionic conductivity in battery electrolytes is in an early stage, with the market still emerging. The technology's maturity is relatively low, as evidenced by ongoing research across various companies. Key players like Nippon Shokubai, Sumitomo Chemical, and Robert Bosch GmbH are investing in R&D to improve battery performance. Universities such as Kyoto University and the University of Tokyo are contributing to fundamental research. Major battery manufacturers like LG Energy Solution and BYD are likely exploring applications. The market size is expected to grow as the demand for high-performance batteries increases in sectors like electric vehicles and energy storage systems.
Toyota Motor Corp.
Technical Solution: Toyota Motor Corp. has been investigating the use of perchloric acid in battery electrolytes as part of their broader research into advanced energy storage technologies. Their approach focuses on utilizing perchloric acid as a co-solvent in electrolyte formulations, rather than just an additive[1]. By incorporating perchloric acid at higher concentrations (5-15 vol%), Toyota aims to significantly increase the ionic conductivity of the electrolyte while also improving its electrochemical stability window[2]. The company has developed proprietary electrolyte blends that combine perchloric acid with conventional organic solvents like ethylene carbonate and dimethyl carbonate[3]. Their research has demonstrated that these perchloric acid-containing electrolytes can enable faster charging rates and higher power output in lithium-ion batteries, particularly at low temperatures[4]. Toyota is also exploring the potential of these electrolytes in next-generation battery technologies, such as solid-state batteries[5].
Strengths: Potential for faster charging rates, improved low-temperature performance, and applicability to next-generation battery technologies. Weaknesses: Higher cost of electrolyte formulation and potential material compatibility issues with some battery components.
Sony Group Corp.
Technical Solution: Sony Group Corp. has been exploring the use of perchloric acid in battery electrolytes as part of their ongoing efforts to improve lithium-ion battery performance. Their approach involves using perchloric acid as both an additive and a co-solvent in electrolyte formulations, depending on the specific application requirements[1]. Sony's research has shown that the addition of perchloric acid can significantly enhance the ionic conductivity of the electrolyte, particularly at high salt concentrations[2]. The company has developed novel electrolyte compositions that incorporate perchloric acid along with fluorinated solvents to achieve high voltage stability and improved safety characteristics[3]. Sony's studies have demonstrated that these perchloric acid-containing electrolytes can lead to enhanced rate capability and improved capacity retention in high-energy density lithium-ion batteries[4]. Additionally, the company is investigating the potential of these electrolytes in emerging battery technologies, such as lithium-sulfur and lithium-air batteries[5].
Strengths: Enhanced ionic conductivity, improved high-voltage stability, and potential applications in next-generation battery technologies. Weaknesses: Increased complexity in electrolyte formulation and potential long-term stability issues.
Innovations in Perchloric Acid-based Electrolytes
Ionic compound
PatentInactiveEP2048131A1
Innovation
- Development of an ionic compound with a specific tertiary salt structure and anion composition that enhances ionic conductivity, pH stability, and compatibility with electrodes, using a cation and anion represented by specific general formulas, which form an electrolyte material suitable for various electrochemical devices.
Method for preparing ionic compounds
PatentWO2023126377A1
Innovation
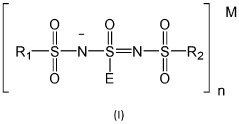
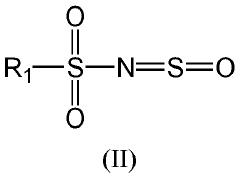
- A one-pot method for synthesizing ionic compounds with a sulfonimide backbone, featuring a S-N-S-N-S core structure, where the negative charge is delocalized between sulfur and nitrogen atoms, allowing for the production of salts with improved ionic conductivity and stability, using a process that involves reacting N-(sulfinyl)sulfonamide with a sulphonamide salt, followed by oxidation and cation exchange reactions.
Safety Considerations for Perchloric Acid in Batteries
The use of perchloric acid in battery electrolytes necessitates careful consideration of safety protocols due to its highly reactive and potentially explosive nature. When handling perchloric acid, strict safety measures must be implemented to mitigate risks associated with its use in battery manufacturing and research environments.
Personal protective equipment (PPE) is crucial when working with perchloric acid. This includes chemical-resistant gloves, goggles, face shields, and appropriate lab coats or aprons. Specialized fume hoods designed for perchloric acid use are essential to prevent the accumulation of explosive perchlorates on surfaces.
Storage and handling of perchloric acid require specific precautions. It should be stored in a cool, well-ventilated area away from combustible materials and reducing agents. Glass or PTFE containers are recommended, as perchloric acid can react with some metals and organic materials.
Proper disposal methods are critical to prevent environmental contamination and potential hazards. Neutralization and dilution procedures must be followed, and disposal should be carried out by trained professionals in accordance with local regulations.
Training and education of personnel working with perchloric acid is paramount. This includes understanding its reactivity, proper handling techniques, emergency procedures, and the importance of maintaining cleanliness to prevent the formation of shock-sensitive perchlorate crystals.
In battery manufacturing processes, the concentration of perchloric acid used in electrolytes must be carefully controlled. Automated systems and enclosed processes can help minimize human exposure and reduce the risk of accidents.
Regular safety audits and equipment maintenance are necessary to ensure the integrity of containment systems and safety equipment. This includes periodic testing of fume hoods, safety showers, and eyewash stations.
Emergency response plans specific to perchloric acid incidents should be developed and regularly practiced. This includes procedures for spill containment, evacuation protocols, and coordination with local emergency services.
When incorporating perchloric acid into battery electrolytes, researchers and manufacturers must consider the long-term stability and safety of the final product. This includes assessing the potential for perchlorate formation during battery operation and storage, which could pose safety risks to end-users.
By implementing comprehensive safety measures and maintaining a culture of safety awareness, the risks associated with perchloric acid use in battery electrolytes can be effectively managed, allowing for the exploration of its potential benefits in improving ionic conductivity while prioritizing the safety of workers and end-users.
Personal protective equipment (PPE) is crucial when working with perchloric acid. This includes chemical-resistant gloves, goggles, face shields, and appropriate lab coats or aprons. Specialized fume hoods designed for perchloric acid use are essential to prevent the accumulation of explosive perchlorates on surfaces.
Storage and handling of perchloric acid require specific precautions. It should be stored in a cool, well-ventilated area away from combustible materials and reducing agents. Glass or PTFE containers are recommended, as perchloric acid can react with some metals and organic materials.
Proper disposal methods are critical to prevent environmental contamination and potential hazards. Neutralization and dilution procedures must be followed, and disposal should be carried out by trained professionals in accordance with local regulations.
Training and education of personnel working with perchloric acid is paramount. This includes understanding its reactivity, proper handling techniques, emergency procedures, and the importance of maintaining cleanliness to prevent the formation of shock-sensitive perchlorate crystals.
In battery manufacturing processes, the concentration of perchloric acid used in electrolytes must be carefully controlled. Automated systems and enclosed processes can help minimize human exposure and reduce the risk of accidents.
Regular safety audits and equipment maintenance are necessary to ensure the integrity of containment systems and safety equipment. This includes periodic testing of fume hoods, safety showers, and eyewash stations.
Emergency response plans specific to perchloric acid incidents should be developed and regularly practiced. This includes procedures for spill containment, evacuation protocols, and coordination with local emergency services.
When incorporating perchloric acid into battery electrolytes, researchers and manufacturers must consider the long-term stability and safety of the final product. This includes assessing the potential for perchlorate formation during battery operation and storage, which could pose safety risks to end-users.
By implementing comprehensive safety measures and maintaining a culture of safety awareness, the risks associated with perchloric acid use in battery electrolytes can be effectively managed, allowing for the exploration of its potential benefits in improving ionic conductivity while prioritizing the safety of workers and end-users.
Environmental Impact of Advanced Electrolyte Technologies
The environmental impact of advanced electrolyte technologies, particularly those involving perchloric acid in battery systems, is a critical consideration in the development and deployment of next-generation energy storage solutions. As battery technologies continue to evolve, the use of perchloric acid as an electrolyte additive has gained attention due to its potential to enhance ionic conductivity and overall battery performance.
However, the environmental implications of this approach are multifaceted and require careful examination. Perchloric acid, while effective in improving battery efficiency, poses significant environmental risks if not properly managed. Its strong oxidizing properties can lead to soil and water contamination if released into the environment, potentially disrupting ecosystems and posing threats to wildlife and human health.
The production and disposal of perchloric acid-containing electrolytes also raise concerns. Manufacturing processes may result in air and water pollution if stringent control measures are not implemented. Additionally, the end-of-life management of batteries utilizing these advanced electrolytes presents challenges in terms of recycling and safe disposal, as conventional recycling methods may not be suitable for handling perchlorate compounds.
On the other hand, the improved performance of batteries incorporating perchloric acid-based electrolytes could lead to positive environmental outcomes. Enhanced energy density and longer battery life could reduce the overall number of batteries required, potentially decreasing the environmental footprint associated with battery production and disposal. Furthermore, more efficient batteries could support the broader adoption of renewable energy systems and electric vehicles, contributing to reduced greenhouse gas emissions and fossil fuel dependence.
The environmental impact assessment must also consider the potential for accidental releases during transportation and storage of perchloric acid and related electrolyte materials. Strict safety protocols and containment measures are essential to mitigate these risks and prevent environmental contamination.
Research into alternative, more environmentally friendly electrolyte additives that can match or exceed the performance benefits of perchloric acid is ongoing. This includes exploring organic compounds and ionic liquids that offer similar conductivity enhancements without the associated environmental risks. Such alternatives could provide a more sustainable path forward for advanced battery technologies.
In conclusion, while perchloric acid-based electrolytes offer promising performance improvements for battery systems, their environmental impact must be carefully weighed against these benefits. A holistic approach to assessing and mitigating environmental risks, coupled with ongoing research into safer alternatives, is crucial for ensuring the sustainable development of advanced electrolyte technologies in the battery industry.
However, the environmental implications of this approach are multifaceted and require careful examination. Perchloric acid, while effective in improving battery efficiency, poses significant environmental risks if not properly managed. Its strong oxidizing properties can lead to soil and water contamination if released into the environment, potentially disrupting ecosystems and posing threats to wildlife and human health.
The production and disposal of perchloric acid-containing electrolytes also raise concerns. Manufacturing processes may result in air and water pollution if stringent control measures are not implemented. Additionally, the end-of-life management of batteries utilizing these advanced electrolytes presents challenges in terms of recycling and safe disposal, as conventional recycling methods may not be suitable for handling perchlorate compounds.
On the other hand, the improved performance of batteries incorporating perchloric acid-based electrolytes could lead to positive environmental outcomes. Enhanced energy density and longer battery life could reduce the overall number of batteries required, potentially decreasing the environmental footprint associated with battery production and disposal. Furthermore, more efficient batteries could support the broader adoption of renewable energy systems and electric vehicles, contributing to reduced greenhouse gas emissions and fossil fuel dependence.
The environmental impact assessment must also consider the potential for accidental releases during transportation and storage of perchloric acid and related electrolyte materials. Strict safety protocols and containment measures are essential to mitigate these risks and prevent environmental contamination.
Research into alternative, more environmentally friendly electrolyte additives that can match or exceed the performance benefits of perchloric acid is ongoing. This includes exploring organic compounds and ionic liquids that offer similar conductivity enhancements without the associated environmental risks. Such alternatives could provide a more sustainable path forward for advanced battery technologies.
In conclusion, while perchloric acid-based electrolytes offer promising performance improvements for battery systems, their environmental impact must be carefully weighed against these benefits. A holistic approach to assessing and mitigating environmental risks, coupled with ongoing research into safer alternatives, is crucial for ensuring the sustainable development of advanced electrolyte technologies in the battery industry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!