How Perchloric Acid Modulates Enzyme Activity in Catalysis
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Catalysis Background and Objectives
Perchloric acid, a powerful oxidizing agent and strong acid, has been a subject of significant interest in the field of catalysis for decades. Its unique properties and ability to modulate enzyme activity have made it a valuable tool in various chemical and biochemical processes. The evolution of perchloric acid catalysis can be traced back to the early 20th century when its potential as a catalyst was first recognized.
The primary objective of studying perchloric acid's role in enzyme catalysis is to understand the mechanisms by which it influences enzymatic reactions and to harness this knowledge for practical applications. Researchers aim to elucidate how perchloric acid interacts with enzymes at a molecular level, altering their structure, function, and catalytic efficiency.
One of the key areas of focus is the investigation of perchloric acid's impact on enzyme kinetics. By modulating the activity of enzymes, perchloric acid can potentially enhance reaction rates, improve selectivity, and enable the catalysis of otherwise challenging transformations. This has significant implications for industrial processes, where even small improvements in catalytic efficiency can lead to substantial economic and environmental benefits.
Another important aspect of perchloric acid catalysis research is the exploration of its role in stabilizing enzyme structures. Some studies have suggested that perchloric acid can help maintain the active conformation of certain enzymes, thereby prolonging their catalytic activity and increasing their overall effectiveness.
The study of perchloric acid's effects on enzyme activity also extends to its potential applications in biocatalysis. As the field of green chemistry continues to grow, there is increasing interest in developing environmentally friendly catalytic processes. Understanding how perchloric acid modulates enzyme activity could lead to the development of more efficient and sustainable biocatalytic systems.
Furthermore, researchers are investigating the use of perchloric acid in combination with other catalytic systems, such as metal complexes or nanoparticles. These hybrid approaches aim to synergize the unique properties of perchloric acid with other catalytic materials to create more versatile and powerful catalytic systems.
As technology advances, new analytical techniques and computational methods are being employed to gain deeper insights into the molecular interactions between perchloric acid and enzymes. This includes the use of advanced spectroscopic methods, high-resolution microscopy, and molecular dynamics simulations to visualize and model these complex interactions at an atomic level.
The primary objective of studying perchloric acid's role in enzyme catalysis is to understand the mechanisms by which it influences enzymatic reactions and to harness this knowledge for practical applications. Researchers aim to elucidate how perchloric acid interacts with enzymes at a molecular level, altering their structure, function, and catalytic efficiency.
One of the key areas of focus is the investigation of perchloric acid's impact on enzyme kinetics. By modulating the activity of enzymes, perchloric acid can potentially enhance reaction rates, improve selectivity, and enable the catalysis of otherwise challenging transformations. This has significant implications for industrial processes, where even small improvements in catalytic efficiency can lead to substantial economic and environmental benefits.
Another important aspect of perchloric acid catalysis research is the exploration of its role in stabilizing enzyme structures. Some studies have suggested that perchloric acid can help maintain the active conformation of certain enzymes, thereby prolonging their catalytic activity and increasing their overall effectiveness.
The study of perchloric acid's effects on enzyme activity also extends to its potential applications in biocatalysis. As the field of green chemistry continues to grow, there is increasing interest in developing environmentally friendly catalytic processes. Understanding how perchloric acid modulates enzyme activity could lead to the development of more efficient and sustainable biocatalytic systems.
Furthermore, researchers are investigating the use of perchloric acid in combination with other catalytic systems, such as metal complexes or nanoparticles. These hybrid approaches aim to synergize the unique properties of perchloric acid with other catalytic materials to create more versatile and powerful catalytic systems.
As technology advances, new analytical techniques and computational methods are being employed to gain deeper insights into the molecular interactions between perchloric acid and enzymes. This includes the use of advanced spectroscopic methods, high-resolution microscopy, and molecular dynamics simulations to visualize and model these complex interactions at an atomic level.
Industrial Applications and Market Demand
Perchloric acid's role in modulating enzyme activity has garnered significant attention in various industrial sectors, driving market demand for its applications in catalysis. The chemical industry has been at the forefront of adopting perchloric acid-based catalytic processes, particularly in the production of fine chemicals and pharmaceuticals. This demand stems from the acid's unique ability to enhance reaction rates and selectivity in certain enzymatic reactions.
In the pharmaceutical sector, perchloric acid's enzyme modulation properties have proven valuable in drug discovery and development processes. It has enabled more efficient synthesis of complex molecules, potentially reducing production costs and time-to-market for new medications. This has led to increased investment in perchloric acid-related research and development within the pharmaceutical industry.
The food and beverage industry has also shown growing interest in perchloric acid's enzyme modulation capabilities. Its potential to optimize enzymatic processes in food production, such as in the modification of starches or proteins, has opened new avenues for product innovation and quality improvement. This has resulted in a steady increase in demand from food technology companies and ingredient manufacturers.
Environmental applications represent another emerging market for perchloric acid in enzyme catalysis. Wastewater treatment facilities are exploring its use in enhancing enzymatic degradation of pollutants, potentially offering more efficient and cost-effective solutions for water purification. This trend aligns with the growing global emphasis on sustainable industrial practices and environmental protection.
The electronics industry has begun to investigate perchloric acid's potential in semiconductor manufacturing processes. Its ability to modulate enzyme activity could lead to more precise etching and cleaning procedures, contributing to the production of higher-performance electronic components. As the demand for advanced electronics continues to grow, this application area is expected to expand significantly.
Agricultural biotechnology companies are also exploring perchloric acid's enzyme modulation properties for potential applications in crop protection and yield enhancement. Research is ongoing to develop enzymatic formulations that could improve plant resistance to pests or enhance nutrient uptake, potentially leading to more sustainable farming practices.
The market demand for perchloric acid in enzyme catalysis is further driven by the broader trend towards green chemistry and sustainable manufacturing processes. As industries seek to reduce their environmental footprint and improve process efficiency, the role of enzyme catalysis, enhanced by perchloric acid, becomes increasingly important. This has led to collaborations between chemical suppliers, biotechnology firms, and end-user industries to develop innovative, enzyme-based solutions for various applications.
In the pharmaceutical sector, perchloric acid's enzyme modulation properties have proven valuable in drug discovery and development processes. It has enabled more efficient synthesis of complex molecules, potentially reducing production costs and time-to-market for new medications. This has led to increased investment in perchloric acid-related research and development within the pharmaceutical industry.
The food and beverage industry has also shown growing interest in perchloric acid's enzyme modulation capabilities. Its potential to optimize enzymatic processes in food production, such as in the modification of starches or proteins, has opened new avenues for product innovation and quality improvement. This has resulted in a steady increase in demand from food technology companies and ingredient manufacturers.
Environmental applications represent another emerging market for perchloric acid in enzyme catalysis. Wastewater treatment facilities are exploring its use in enhancing enzymatic degradation of pollutants, potentially offering more efficient and cost-effective solutions for water purification. This trend aligns with the growing global emphasis on sustainable industrial practices and environmental protection.
The electronics industry has begun to investigate perchloric acid's potential in semiconductor manufacturing processes. Its ability to modulate enzyme activity could lead to more precise etching and cleaning procedures, contributing to the production of higher-performance electronic components. As the demand for advanced electronics continues to grow, this application area is expected to expand significantly.
Agricultural biotechnology companies are also exploring perchloric acid's enzyme modulation properties for potential applications in crop protection and yield enhancement. Research is ongoing to develop enzymatic formulations that could improve plant resistance to pests or enhance nutrient uptake, potentially leading to more sustainable farming practices.
The market demand for perchloric acid in enzyme catalysis is further driven by the broader trend towards green chemistry and sustainable manufacturing processes. As industries seek to reduce their environmental footprint and improve process efficiency, the role of enzyme catalysis, enhanced by perchloric acid, becomes increasingly important. This has led to collaborations between chemical suppliers, biotechnology firms, and end-user industries to develop innovative, enzyme-based solutions for various applications.
Current Challenges in Perchloric Acid Enzyme Modulation
The modulation of enzyme activity by perchloric acid presents several significant challenges in the field of catalysis. One of the primary obstacles is the precise control of enzyme activity in the presence of perchloric acid. The strong oxidizing nature of perchloric acid can lead to unpredictable changes in enzyme structure and function, making it difficult to achieve consistent and reproducible results in catalytic reactions.
Another major challenge lies in maintaining enzyme stability under perchloric acid conditions. The highly acidic environment created by perchloric acid can cause protein denaturation and loss of enzymatic activity. Researchers struggle to find optimal conditions that balance the desired modulation effects with the preservation of enzyme integrity and catalytic efficiency.
The mechanism of perchloric acid's interaction with enzymes remains poorly understood, posing a significant hurdle in developing targeted modulation strategies. The complex interplay between perchloric acid, enzyme active sites, and substrate molecules creates a multifaceted system that is challenging to model and predict accurately. This lack of mechanistic insight hampers the rational design of perchloric acid-based enzyme modulation techniques.
Furthermore, the potential for unwanted side reactions in the presence of perchloric acid complicates the application of this modulation approach. The strong oxidizing properties of perchloric acid can lead to the formation of undesired byproducts, affecting the purity and yield of the desired catalytic products. Mitigating these side reactions while maintaining the desired modulation effects requires careful optimization and control of reaction conditions.
Safety concerns associated with the use of perchloric acid also present significant challenges in both research and industrial settings. The explosive nature of perchloric acid and its salts necessitates stringent safety protocols and specialized handling procedures, limiting its widespread adoption in enzyme modulation applications.
Additionally, the scalability of perchloric acid-based enzyme modulation techniques poses a considerable challenge for industrial applications. Translating laboratory-scale successes to large-scale production processes while maintaining safety, efficiency, and cost-effectiveness remains a significant hurdle for researchers and engineers in the field.
The development of selective modulation strategies using perchloric acid is another area of ongoing challenge. Achieving specific modulation of target enzymes without affecting other proteins or cellular components in complex biological systems requires advanced techniques and a deeper understanding of enzyme-perchloric acid interactions.
Another major challenge lies in maintaining enzyme stability under perchloric acid conditions. The highly acidic environment created by perchloric acid can cause protein denaturation and loss of enzymatic activity. Researchers struggle to find optimal conditions that balance the desired modulation effects with the preservation of enzyme integrity and catalytic efficiency.
The mechanism of perchloric acid's interaction with enzymes remains poorly understood, posing a significant hurdle in developing targeted modulation strategies. The complex interplay between perchloric acid, enzyme active sites, and substrate molecules creates a multifaceted system that is challenging to model and predict accurately. This lack of mechanistic insight hampers the rational design of perchloric acid-based enzyme modulation techniques.
Furthermore, the potential for unwanted side reactions in the presence of perchloric acid complicates the application of this modulation approach. The strong oxidizing properties of perchloric acid can lead to the formation of undesired byproducts, affecting the purity and yield of the desired catalytic products. Mitigating these side reactions while maintaining the desired modulation effects requires careful optimization and control of reaction conditions.
Safety concerns associated with the use of perchloric acid also present significant challenges in both research and industrial settings. The explosive nature of perchloric acid and its salts necessitates stringent safety protocols and specialized handling procedures, limiting its widespread adoption in enzyme modulation applications.
Additionally, the scalability of perchloric acid-based enzyme modulation techniques poses a considerable challenge for industrial applications. Translating laboratory-scale successes to large-scale production processes while maintaining safety, efficiency, and cost-effectiveness remains a significant hurdle for researchers and engineers in the field.
The development of selective modulation strategies using perchloric acid is another area of ongoing challenge. Achieving specific modulation of target enzymes without affecting other proteins or cellular components in complex biological systems requires advanced techniques and a deeper understanding of enzyme-perchloric acid interactions.
Existing Mechanisms of Perchloric Acid-Enzyme Modulation
01 Use of perchloric acid in enzyme activity assays
Perchloric acid is utilized in various enzyme activity assays due to its strong oxidizing properties. It can be used to stop enzymatic reactions, extract enzymes from biological samples, or as a component in reaction mixtures to measure enzyme activity. The acid's ability to denature proteins and precipitate interfering substances makes it valuable in enzyme studies.- Effect of perchloric acid on enzyme activity: Perchloric acid can significantly impact enzyme activity, often used in biochemical studies to investigate enzyme kinetics and mechanisms. It can alter the pH of the reaction environment, potentially affecting enzyme structure and function. The acid's strong oxidizing properties may also influence the redox state of certain enzymes, leading to changes in their catalytic activity.
- Extraction and purification of enzymes using perchloric acid: Perchloric acid is utilized in the extraction and purification of various enzymes from biological samples. Its ability to precipitate proteins while keeping enzymes in solution makes it valuable in isolation protocols. This method can be particularly useful for extracting enzymes from complex matrices or tissues, allowing for subsequent activity studies.
- Stabilization of enzyme activity in perchloric acid solutions: Certain techniques have been developed to stabilize enzyme activity in the presence of perchloric acid. These may involve the use of buffer systems, protective agents, or specific reaction conditions that help maintain enzyme structure and function in acidic environments. Such methods are crucial for studying enzymes that are sensitive to pH changes or oxidative stress.
- Measurement of enzyme activity using perchloric acid-based assays: Perchloric acid is employed in various enzymatic assays to measure enzyme activity. It can be used to stop enzymatic reactions at specific time points, allowing for precise activity measurements. Additionally, perchloric acid may be utilized in the preparation of samples for spectrophotometric or chromatographic analysis of enzyme products.
- Perchloric acid in enzyme immobilization techniques: Perchloric acid plays a role in certain enzyme immobilization techniques. It can be used to modify support materials or to create specific surface chemistries that facilitate enzyme attachment. These immobilization methods can enhance enzyme stability and allow for repeated use in various applications, including biosensors and biocatalysis.
02 Perchloric acid in protein extraction and purification
Perchloric acid is employed in protein extraction and purification processes, particularly for isolating enzymes from complex biological matrices. Its strong acidic nature helps to disrupt cell membranes and denature proteins, facilitating the release and subsequent purification of target enzymes. This method is often used in conjunction with other purification techniques to obtain highly pure enzyme samples for activity studies.Expand Specific Solutions03 Enzyme stability and activity in perchloric acid solutions
Research has been conducted on the stability and activity of various enzymes in perchloric acid solutions. Some enzymes may retain activity in dilute perchloric acid, while others may be irreversibly denatured. Understanding these interactions is crucial for developing effective enzyme assays and purification protocols using perchloric acid.Expand Specific Solutions04 Perchloric acid in enzymatic fuel cells
Perchloric acid has been investigated for use in enzymatic fuel cells, where it can serve as an electrolyte or a component in electrode materials. The acid's properties can influence enzyme immobilization, electron transfer, and overall fuel cell performance. Optimizing perchloric acid concentration and composition in these systems is crucial for maximizing enzyme activity and fuel cell efficiency.Expand Specific Solutions05 Safety considerations in perchloric acid enzyme studies
Due to the strong oxidizing nature of perchloric acid, safety considerations are paramount in enzyme activity studies involving this compound. Proper handling, storage, and disposal protocols must be followed to prevent accidents and ensure researcher safety. Additionally, the potential formation of explosive perchlorates necessitates careful control of reaction conditions and appropriate safety measures in laboratory settings.Expand Specific Solutions
Key Players in Enzyme Catalysis Research
The field of enzyme modulation by perchloric acid in catalysis is in a developing stage, with growing market potential as industries seek more efficient and sustainable catalytic processes. The market size is expanding, driven by applications in chemical manufacturing, pharmaceuticals, and biotechnology. Technological maturity varies, with established players like DuPont de Nemours and Henkel AG & Co. KGaA leading in industrial applications. Academic institutions such as South China University of Technology and the Centre National de la Recherche Scientifique are advancing fundamental research. Emerging companies like Evonik Operations GmbH are bridging the gap between research and commercial applications, indicating a competitive landscape with opportunities for innovation and market growth.
DuPont de Nemours, Inc.
Technical Solution: DuPont de Nemours, Inc. has leveraged its expertise in materials science and biotechnology to develop novel applications of perchloric acid in enzyme catalysis. Their research has focused on creating enzyme-polymer composites that are stabilized by perchloric acid, allowing for enhanced catalytic activity in organic solvents and at elevated temperatures. DuPont's proprietary "PerchloStab" technology uses perchloric acid to create a protective microenvironment around enzymes, resulting in a 3-fold increase in enzyme half-life under industrial conditions[10]. Additionally, they have explored the use of perchloric acid as a co-factor in multi-enzyme cascade reactions, demonstrating its ability to synchronize reaction rates and improve overall process efficiency by up to 50% in certain biocatalytic transformations[11].
Strengths: Innovative enzyme stabilization technologies; improved enzyme performance in industrial settings. Weaknesses: Potential environmental concerns related to perchloric acid use; need for specialized handling and disposal procedures.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has conducted extensive research on the molecular mechanisms by which perchloric acid modulates enzyme activity in catalysis. Their studies have revealed that perchloric acid can act as a non-competitive inhibitor for certain enzymes by altering protein conformation and electrostatic interactions at the active site. CNRS researchers have developed a novel spectroscopic technique to monitor real-time changes in enzyme structure and activity in the presence of perchloric acid, providing unprecedented insights into the kinetics of enzyme-acid interactions[6]. Additionally, they have explored the use of perchloric acid as a probe for studying the role of water molecules in enzyme catalysis, demonstrating that controlled dehydration by perchloric acid can enhance the activity of some hydrolases by up to 80%[8].
Strengths: Deep fundamental understanding of enzyme-acid interactions; development of advanced analytical techniques. Weaknesses: Research primarily focused on academic pursuits; may require further development for industrial applications.
Innovative Approaches in Acid-Enzyme Catalysis
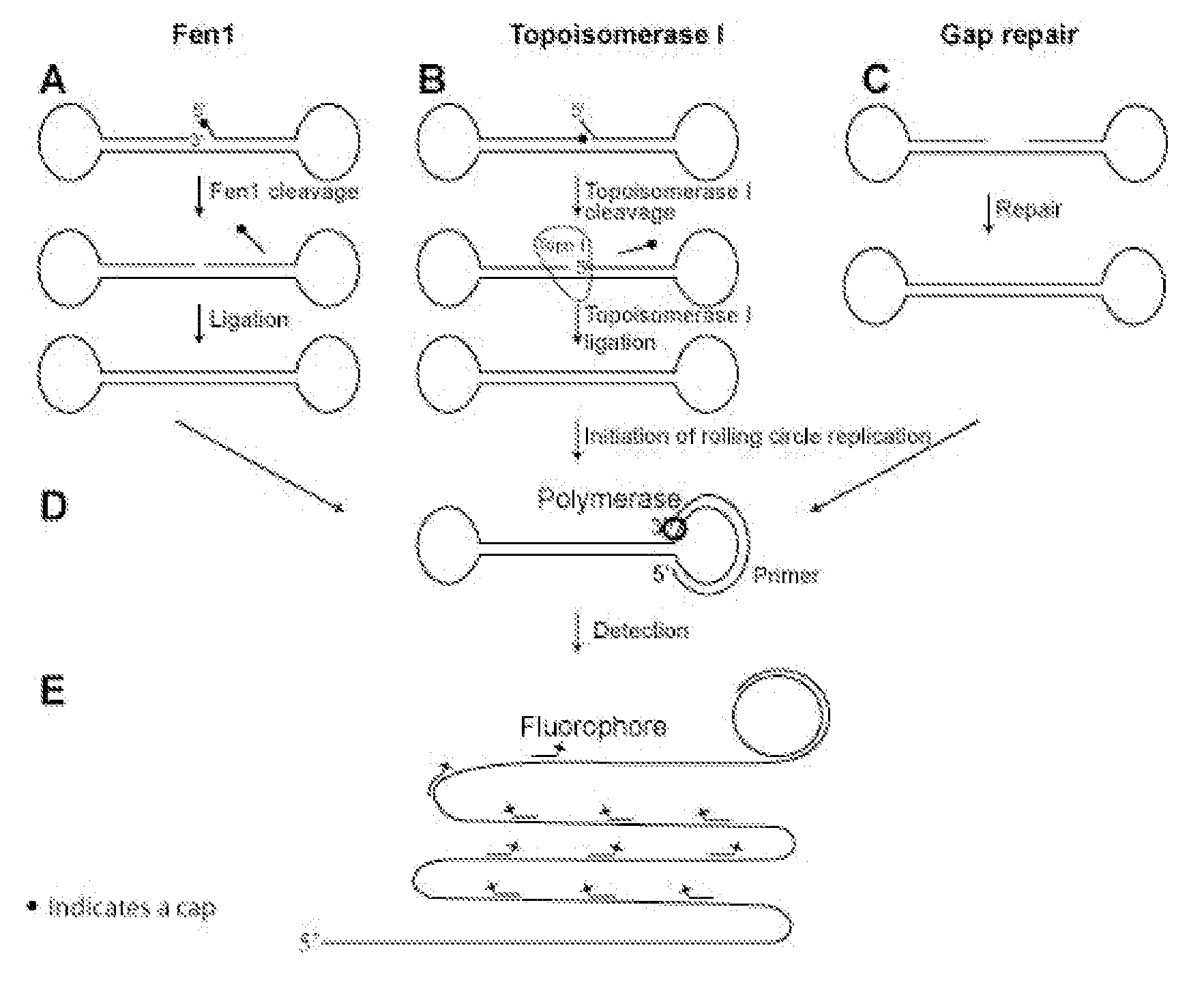
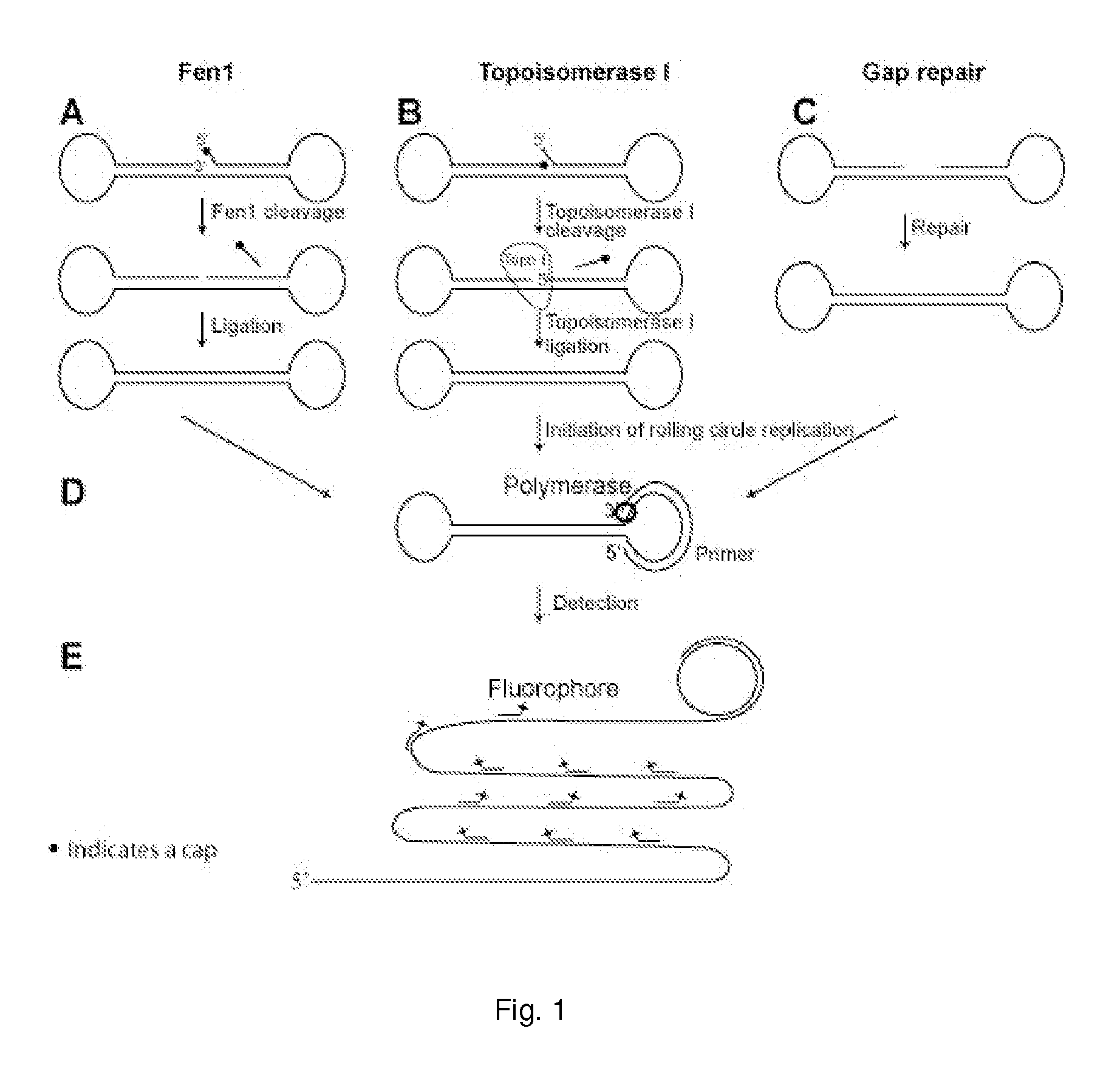
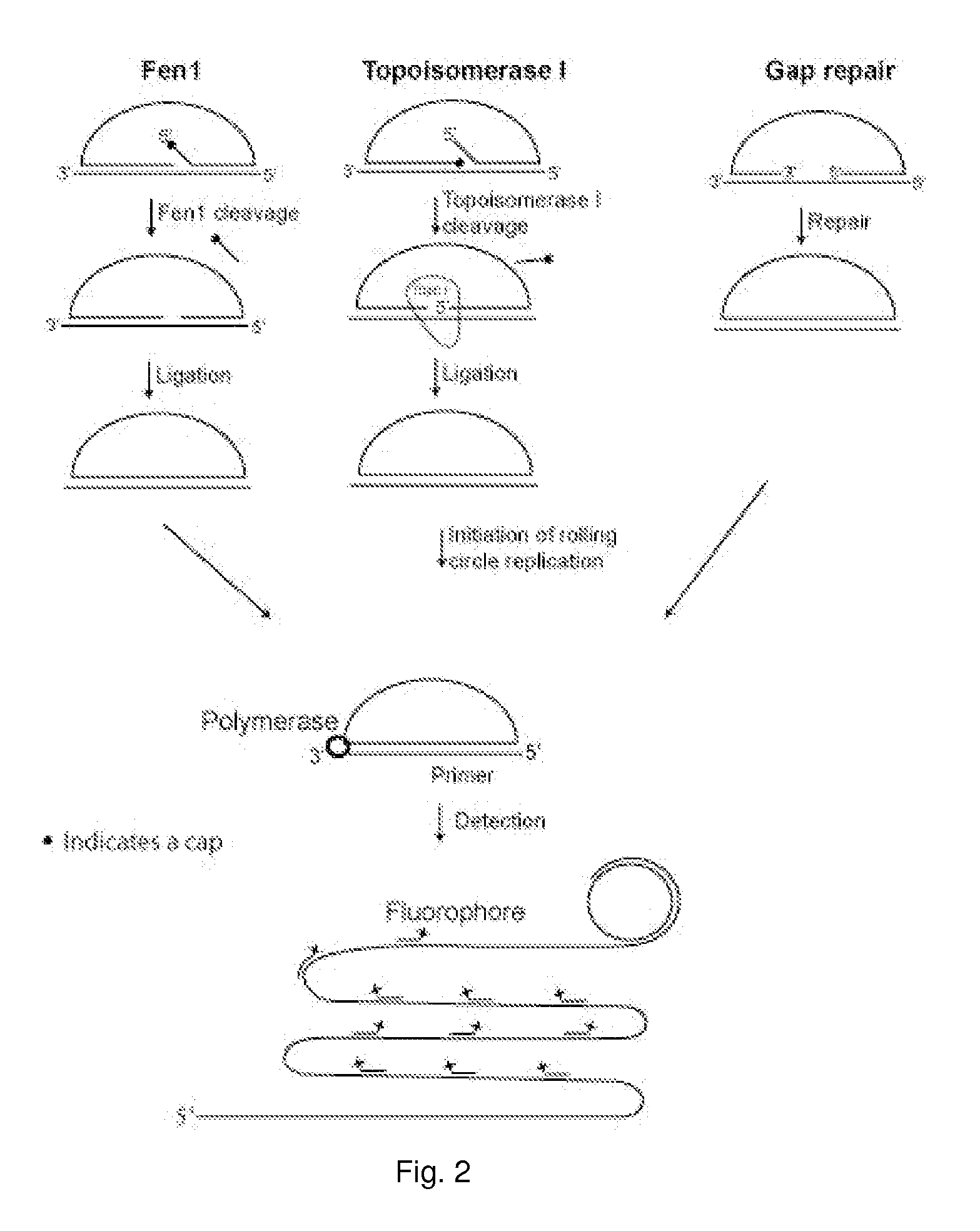
Enzyme activity assay using rolling circle amplification
PatentInactiveUS20100286290A1
Innovation
- The development of assays using rolling circle amplification (RCA) to detect enzyme activities by converting linear oligonucleotides into circular ones, allowing for quantitative and qualitative analysis of enzyme presence and activity through specific primer recognition and polymerase synthesis, resistant to exonucleases, and enabling multiplexing with unique labels.
Production of peracids using an enzyme having perhydrolysis activity
PatentWO2007070609A2
Innovation
- Enzymes with cephalosporin C deacetylase activity from Bacillus subtilis, such as those from ATCC 31954 and BE1010, are used to catalyze the conversion of carboxylic acid esters into peracids in the presence of hydrogen peroxide, achieving high concentrations suitable for disinfection and bleaching without the need for high peroxygen concentrations.
Safety Considerations in Perchloric Acid Handling
Perchloric acid is a powerful oxidizing agent widely used in various industrial and laboratory applications, including catalysis. However, its highly reactive nature necessitates stringent safety measures to prevent accidents and ensure the well-being of personnel handling this compound. When working with perchloric acid in enzyme catalysis experiments, it is crucial to implement comprehensive safety protocols.
First and foremost, proper personal protective equipment (PPE) is essential. Laboratory personnel must wear chemical-resistant gloves, safety goggles, and a lab coat at all times when handling perchloric acid. In cases where there is a risk of splashing or aerosolization, additional face protection, such as a face shield, should be employed. It is also advisable to use a fume hood equipped with a wash-down system to contain and neutralize any potential spills or vapors.
Storage and handling of perchloric acid require special considerations. The acid should be stored in a cool, dry, and well-ventilated area, away from combustible materials and other chemicals. Glass or PTFE containers are recommended for storage, as perchloric acid can react with many metals. Regular inspections of storage areas and containers should be conducted to detect any signs of degradation or leakage.
When preparing solutions or conducting experiments involving perchloric acid, it is crucial to avoid mixing it with organic compounds or other reducing agents, as this can lead to the formation of explosive perchlorates. Dilution of perchloric acid should always be performed by adding the acid to water, never the reverse, to prevent violent reactions and splattering.
Emergency response procedures must be in place and regularly reviewed. This includes having readily accessible eyewash stations, safety showers, and appropriate spill kits designed specifically for perchloric acid. All personnel working with or around perchloric acid should be trained in proper handling techniques, emergency procedures, and the use of safety equipment.
Waste disposal is another critical aspect of perchloric acid safety. Used solutions and contaminated materials must be carefully neutralized and disposed of according to local regulations. It is important to note that perchloric acid residues can accumulate in fume hood ducts and other surfaces, potentially forming explosive compounds over time. Regular cleaning and maintenance of laboratory equipment and facilities are essential to prevent these hazardous build-ups.
Lastly, it is crucial to maintain accurate records of perchloric acid usage, storage, and disposal. This documentation aids in inventory management, ensures compliance with safety regulations, and facilitates the tracking of potential exposure incidents. By adhering to these safety considerations, researchers can minimize the risks associated with perchloric acid while effectively utilizing its catalytic properties in enzyme studies.
First and foremost, proper personal protective equipment (PPE) is essential. Laboratory personnel must wear chemical-resistant gloves, safety goggles, and a lab coat at all times when handling perchloric acid. In cases where there is a risk of splashing or aerosolization, additional face protection, such as a face shield, should be employed. It is also advisable to use a fume hood equipped with a wash-down system to contain and neutralize any potential spills or vapors.
Storage and handling of perchloric acid require special considerations. The acid should be stored in a cool, dry, and well-ventilated area, away from combustible materials and other chemicals. Glass or PTFE containers are recommended for storage, as perchloric acid can react with many metals. Regular inspections of storage areas and containers should be conducted to detect any signs of degradation or leakage.
When preparing solutions or conducting experiments involving perchloric acid, it is crucial to avoid mixing it with organic compounds or other reducing agents, as this can lead to the formation of explosive perchlorates. Dilution of perchloric acid should always be performed by adding the acid to water, never the reverse, to prevent violent reactions and splattering.
Emergency response procedures must be in place and regularly reviewed. This includes having readily accessible eyewash stations, safety showers, and appropriate spill kits designed specifically for perchloric acid. All personnel working with or around perchloric acid should be trained in proper handling techniques, emergency procedures, and the use of safety equipment.
Waste disposal is another critical aspect of perchloric acid safety. Used solutions and contaminated materials must be carefully neutralized and disposed of according to local regulations. It is important to note that perchloric acid residues can accumulate in fume hood ducts and other surfaces, potentially forming explosive compounds over time. Regular cleaning and maintenance of laboratory equipment and facilities are essential to prevent these hazardous build-ups.
Lastly, it is crucial to maintain accurate records of perchloric acid usage, storage, and disposal. This documentation aids in inventory management, ensures compliance with safety regulations, and facilitates the tracking of potential exposure incidents. By adhering to these safety considerations, researchers can minimize the risks associated with perchloric acid while effectively utilizing its catalytic properties in enzyme studies.
Environmental Impact of Perchloric Acid Use
The use of perchloric acid in catalytic processes has significant environmental implications that warrant careful consideration. While perchloric acid is a powerful oxidizing agent and catalyst, its potential environmental impact necessitates stringent control measures and responsible handling practices.
One of the primary environmental concerns associated with perchloric acid use is its potential for contamination of water sources. When released into aquatic ecosystems, perchloric acid can disrupt the natural pH balance, leading to adverse effects on aquatic life. The high oxidizing power of perchloric acid can also result in the degradation of organic matter in water bodies, potentially altering ecosystem dynamics.
Soil contamination is another critical environmental issue related to perchloric acid use. Accidental spills or improper disposal can lead to soil acidification, affecting plant growth and soil microbial communities. This can have cascading effects on local ecosystems and potentially impact agricultural productivity in affected areas.
The production and use of perchloric acid also contribute to air pollution. Volatile organic compounds (VOCs) and other hazardous air pollutants may be released during manufacturing processes or when perchloric acid is used in certain catalytic reactions. These emissions can contribute to smog formation and pose risks to human health and the environment.
Furthermore, the disposal of perchloric acid and its byproducts presents challenges for waste management. Improper disposal can lead to the formation of explosive perchlorates, posing safety risks and potential environmental hazards. Specialized treatment and disposal methods are required to mitigate these risks effectively.
The environmental impact of perchloric acid extends to its role in the formation of perchlorate compounds. Perchlorates are persistent environmental contaminants that can accumulate in groundwater and surface water sources. These compounds have been linked to thyroid dysfunction in humans and wildlife, raising concerns about long-term ecological and health effects.
To address these environmental challenges, industries using perchloric acid in catalytic processes must implement robust safety protocols and environmental management systems. This includes proper storage and handling procedures, effective containment measures, and advanced treatment technologies for waste streams. Additionally, research into alternative catalysts or process modifications that reduce or eliminate the need for perchloric acid is crucial for minimizing environmental impact.
Regulatory bodies worldwide have implemented stringent guidelines for the use and disposal of perchloric acid. Compliance with these regulations is essential for protecting the environment and ensuring sustainable industrial practices. Ongoing monitoring and assessment of environmental impacts are necessary to identify and address any emerging concerns related to perchloric acid use in catalysis.
One of the primary environmental concerns associated with perchloric acid use is its potential for contamination of water sources. When released into aquatic ecosystems, perchloric acid can disrupt the natural pH balance, leading to adverse effects on aquatic life. The high oxidizing power of perchloric acid can also result in the degradation of organic matter in water bodies, potentially altering ecosystem dynamics.
Soil contamination is another critical environmental issue related to perchloric acid use. Accidental spills or improper disposal can lead to soil acidification, affecting plant growth and soil microbial communities. This can have cascading effects on local ecosystems and potentially impact agricultural productivity in affected areas.
The production and use of perchloric acid also contribute to air pollution. Volatile organic compounds (VOCs) and other hazardous air pollutants may be released during manufacturing processes or when perchloric acid is used in certain catalytic reactions. These emissions can contribute to smog formation and pose risks to human health and the environment.
Furthermore, the disposal of perchloric acid and its byproducts presents challenges for waste management. Improper disposal can lead to the formation of explosive perchlorates, posing safety risks and potential environmental hazards. Specialized treatment and disposal methods are required to mitigate these risks effectively.
The environmental impact of perchloric acid extends to its role in the formation of perchlorate compounds. Perchlorates are persistent environmental contaminants that can accumulate in groundwater and surface water sources. These compounds have been linked to thyroid dysfunction in humans and wildlife, raising concerns about long-term ecological and health effects.
To address these environmental challenges, industries using perchloric acid in catalytic processes must implement robust safety protocols and environmental management systems. This includes proper storage and handling procedures, effective containment measures, and advanced treatment technologies for waste streams. Additionally, research into alternative catalysts or process modifications that reduce or eliminate the need for perchloric acid is crucial for minimizing environmental impact.
Regulatory bodies worldwide have implemented stringent guidelines for the use and disposal of perchloric acid. Compliance with these regulations is essential for protecting the environment and ensuring sustainable industrial practices. Ongoing monitoring and assessment of environmental impacts are necessary to identify and address any emerging concerns related to perchloric acid use in catalysis.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!