How Polysilane Reinforces Safe Design in Biomedical Devices?
JUL 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Polysilane in Biomedical Devices: Background and Objectives
Polysilane, a class of silicon-based polymers, has emerged as a promising material in the field of biomedical devices due to its unique properties and potential applications. The evolution of polysilane technology can be traced back to the 1980s when researchers first synthesized these materials. Since then, significant advancements have been made in understanding their structure, properties, and potential uses.
The primary objective of incorporating polysilane in biomedical devices is to enhance safety and performance. Polysilanes offer a combination of properties that make them particularly suitable for this purpose. These include biocompatibility, controllable degradation rates, and the ability to be functionalized with various bioactive molecules. Such characteristics allow for the development of devices that can interact more effectively with biological systems while minimizing adverse reactions.
One of the key trends in polysilane technology is the development of hybrid materials that combine polysilanes with other polymers or inorganic components. This approach aims to create materials with tailored properties for specific biomedical applications. For instance, polysilane-based nanocomposites have shown promise in drug delivery systems and tissue engineering scaffolds.
The safe design of biomedical devices is a critical concern in the healthcare industry. Polysilanes contribute to this goal by offering improved mechanical properties, enhanced biocompatibility, and the potential for controlled degradation. These features can lead to devices that are less likely to cause complications or adverse reactions in patients.
Recent research has focused on exploring the potential of polysilanes in various biomedical applications. These include drug delivery systems, biosensors, tissue engineering scaffolds, and implantable devices. The versatility of polysilanes allows for their use in a wide range of medical technologies, from diagnostic tools to therapeutic interventions.
As the field progresses, researchers are working towards overcoming challenges such as optimizing synthesis methods, improving long-term stability, and enhancing the processability of polysilane-based materials. The goal is to develop polysilanes that can be easily integrated into existing manufacturing processes for biomedical devices while maintaining their beneficial properties.
The future of polysilane technology in biomedical devices looks promising, with ongoing research aimed at expanding its applications and improving its performance. As our understanding of these materials grows, so does the potential for creating safer, more effective medical devices that can significantly impact patient care and outcomes.
The primary objective of incorporating polysilane in biomedical devices is to enhance safety and performance. Polysilanes offer a combination of properties that make them particularly suitable for this purpose. These include biocompatibility, controllable degradation rates, and the ability to be functionalized with various bioactive molecules. Such characteristics allow for the development of devices that can interact more effectively with biological systems while minimizing adverse reactions.
One of the key trends in polysilane technology is the development of hybrid materials that combine polysilanes with other polymers or inorganic components. This approach aims to create materials with tailored properties for specific biomedical applications. For instance, polysilane-based nanocomposites have shown promise in drug delivery systems and tissue engineering scaffolds.
The safe design of biomedical devices is a critical concern in the healthcare industry. Polysilanes contribute to this goal by offering improved mechanical properties, enhanced biocompatibility, and the potential for controlled degradation. These features can lead to devices that are less likely to cause complications or adverse reactions in patients.
Recent research has focused on exploring the potential of polysilanes in various biomedical applications. These include drug delivery systems, biosensors, tissue engineering scaffolds, and implantable devices. The versatility of polysilanes allows for their use in a wide range of medical technologies, from diagnostic tools to therapeutic interventions.
As the field progresses, researchers are working towards overcoming challenges such as optimizing synthesis methods, improving long-term stability, and enhancing the processability of polysilane-based materials. The goal is to develop polysilanes that can be easily integrated into existing manufacturing processes for biomedical devices while maintaining their beneficial properties.
The future of polysilane technology in biomedical devices looks promising, with ongoing research aimed at expanding its applications and improving its performance. As our understanding of these materials grows, so does the potential for creating safer, more effective medical devices that can significantly impact patient care and outcomes.
Market Demand for Enhanced Biomedical Device Safety
The demand for enhanced safety in biomedical devices has been steadily increasing, driven by a combination of regulatory pressures, technological advancements, and growing patient awareness. This market trend is particularly evident in the field of implantable devices, where the long-term interaction between the device and the human body necessitates stringent safety measures.
Polysilane, a novel material with unique properties, has emerged as a promising solution to address these safety concerns. Its potential to reinforce the safe design of biomedical devices has attracted significant attention from both manufacturers and healthcare providers. The market for polysilane-enhanced biomedical devices is expected to grow substantially in the coming years, as the material's benefits become more widely recognized and validated through clinical studies.
One of the key drivers of this market demand is the increasing prevalence of chronic diseases that require long-term implantable devices. As the global population ages, the number of patients requiring such devices is projected to rise, creating a larger market for safer, more reliable biomedical solutions. Polysilane's ability to improve device biocompatibility and reduce the risk of adverse reactions addresses a critical need in this expanding market segment.
Furthermore, the healthcare industry's shift towards value-based care models has intensified the focus on patient outcomes and device longevity. Biomedical devices that incorporate polysilane have the potential to offer improved durability and reduced complication rates, aligning with these new healthcare priorities. This alignment is likely to drive adoption among healthcare providers seeking to optimize patient care while managing costs effectively.
The regulatory landscape also plays a crucial role in shaping market demand for enhanced biomedical device safety. Stricter regulations and more rigorous approval processes have compelled manufacturers to invest in advanced materials and technologies that can meet or exceed safety standards. Polysilane's potential to enhance device safety profiles positions it as an attractive option for manufacturers looking to navigate this complex regulatory environment.
Additionally, the growing trend of personalized medicine has created a demand for biomedical devices that can be tailored to individual patient needs. Polysilane's versatility and adaptability make it well-suited for customized applications, potentially opening up new market opportunities in precision medicine and patient-specific treatments.
As awareness of polysilane's benefits spreads throughout the medical community, it is anticipated that demand will further increase. Healthcare professionals and patients alike are becoming more informed about the materials used in medical devices, leading to a preference for solutions that offer enhanced safety features. This growing awareness is expected to drive market growth and innovation in polysilane-reinforced biomedical devices across various medical specialties.
Polysilane, a novel material with unique properties, has emerged as a promising solution to address these safety concerns. Its potential to reinforce the safe design of biomedical devices has attracted significant attention from both manufacturers and healthcare providers. The market for polysilane-enhanced biomedical devices is expected to grow substantially in the coming years, as the material's benefits become more widely recognized and validated through clinical studies.
One of the key drivers of this market demand is the increasing prevalence of chronic diseases that require long-term implantable devices. As the global population ages, the number of patients requiring such devices is projected to rise, creating a larger market for safer, more reliable biomedical solutions. Polysilane's ability to improve device biocompatibility and reduce the risk of adverse reactions addresses a critical need in this expanding market segment.
Furthermore, the healthcare industry's shift towards value-based care models has intensified the focus on patient outcomes and device longevity. Biomedical devices that incorporate polysilane have the potential to offer improved durability and reduced complication rates, aligning with these new healthcare priorities. This alignment is likely to drive adoption among healthcare providers seeking to optimize patient care while managing costs effectively.
The regulatory landscape also plays a crucial role in shaping market demand for enhanced biomedical device safety. Stricter regulations and more rigorous approval processes have compelled manufacturers to invest in advanced materials and technologies that can meet or exceed safety standards. Polysilane's potential to enhance device safety profiles positions it as an attractive option for manufacturers looking to navigate this complex regulatory environment.
Additionally, the growing trend of personalized medicine has created a demand for biomedical devices that can be tailored to individual patient needs. Polysilane's versatility and adaptability make it well-suited for customized applications, potentially opening up new market opportunities in precision medicine and patient-specific treatments.
As awareness of polysilane's benefits spreads throughout the medical community, it is anticipated that demand will further increase. Healthcare professionals and patients alike are becoming more informed about the materials used in medical devices, leading to a preference for solutions that offer enhanced safety features. This growing awareness is expected to drive market growth and innovation in polysilane-reinforced biomedical devices across various medical specialties.
Current Challenges in Biomedical Device Safety
The field of biomedical devices is rapidly evolving, yet it faces significant challenges in ensuring safety and reliability. One of the primary concerns is the biocompatibility of materials used in these devices. As devices become more complex and are designed for long-term implantation, the risk of adverse reactions and immune responses increases. This necessitates the development of advanced materials that can withstand the harsh biological environment while remaining inert to the body's defense mechanisms.
Another critical challenge is the durability and longevity of biomedical devices. Many implantable devices are expected to function for years or even decades, requiring materials that can resist degradation, maintain structural integrity, and continue to perform their intended functions over extended periods. This is particularly challenging in dynamic environments such as the cardiovascular system or joints, where devices are subjected to constant mechanical stress and chemical exposure.
The miniaturization of biomedical devices presents another set of safety concerns. As devices become smaller and more intricate, ensuring their structural integrity and preventing component failure becomes increasingly difficult. This is especially crucial for devices like neural implants or drug delivery systems, where even minor malfunctions can have severe consequences for patient health.
Infection control remains a persistent challenge in biomedical device safety. Despite advances in sterilization techniques and antimicrobial coatings, device-associated infections continue to be a significant risk, particularly for implantable devices. The development of materials and surface treatments that can effectively resist bacterial colonization without compromising device functionality is an ongoing area of research.
Furthermore, the integration of electronic components in biomedical devices introduces additional safety considerations. Ensuring the proper insulation and shielding of electronic elements is crucial to prevent electromagnetic interference and protect surrounding tissues from potential thermal or electrical damage. This challenge is compounded by the need for these devices to operate in the presence of strong magnetic fields, such as during MRI scans.
Lastly, the regulatory landscape for biomedical devices is becoming increasingly complex. Stringent safety standards and approval processes, while necessary, can slow down innovation and increase development costs. Balancing the need for thorough safety testing with the rapid pace of technological advancement remains a significant challenge for the industry.
Another critical challenge is the durability and longevity of biomedical devices. Many implantable devices are expected to function for years or even decades, requiring materials that can resist degradation, maintain structural integrity, and continue to perform their intended functions over extended periods. This is particularly challenging in dynamic environments such as the cardiovascular system or joints, where devices are subjected to constant mechanical stress and chemical exposure.
The miniaturization of biomedical devices presents another set of safety concerns. As devices become smaller and more intricate, ensuring their structural integrity and preventing component failure becomes increasingly difficult. This is especially crucial for devices like neural implants or drug delivery systems, where even minor malfunctions can have severe consequences for patient health.
Infection control remains a persistent challenge in biomedical device safety. Despite advances in sterilization techniques and antimicrobial coatings, device-associated infections continue to be a significant risk, particularly for implantable devices. The development of materials and surface treatments that can effectively resist bacterial colonization without compromising device functionality is an ongoing area of research.
Furthermore, the integration of electronic components in biomedical devices introduces additional safety considerations. Ensuring the proper insulation and shielding of electronic elements is crucial to prevent electromagnetic interference and protect surrounding tissues from potential thermal or electrical damage. This challenge is compounded by the need for these devices to operate in the presence of strong magnetic fields, such as during MRI scans.
Lastly, the regulatory landscape for biomedical devices is becoming increasingly complex. Stringent safety standards and approval processes, while necessary, can slow down innovation and increase development costs. Balancing the need for thorough safety testing with the rapid pace of technological advancement remains a significant challenge for the industry.
Existing Polysilane Applications in Biomedical Devices
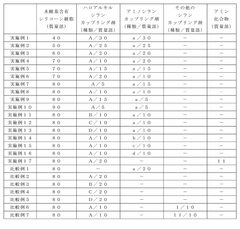
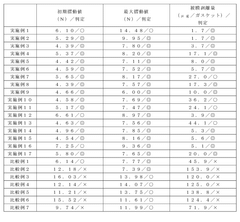
01 Synthesis and structure of polysilanes
Polysilanes are synthesized through various methods, including Wurtz coupling and catalytic dehydrogenation. The structure of polysilanes can be tailored to achieve specific properties, such as improved thermal stability and optical characteristics. Controlling the molecular weight and side-chain substituents is crucial for optimizing the performance and safety of polysilane materials.- Synthesis and structure of polysilanes: Polysilanes are synthesized through various methods, including reductive coupling of dichlorosilanes. The structure of polysilanes can be tailored to achieve specific properties, such as improved thermal stability and optical characteristics. Controlling the molecular weight and distribution is crucial for optimizing performance in different applications.
- Safety considerations in polysilane production: Safe design of polysilane production processes involves careful handling of reactive precursors and intermediates. Implementing proper containment systems, inert atmospheres, and temperature control measures are essential to prevent undesired reactions or decomposition. Additionally, using less hazardous solvents and reagents can enhance overall process safety.
- Polysilane applications in electronics and photonics: Polysilanes find applications in electronic and photonic devices due to their unique optical and electrical properties. They can be used as photoresists, semiconducting materials, and in optoelectronic components. Safe design considerations for these applications include optimizing film formation, enhancing stability, and minimizing potential environmental impacts.
- Environmental and health safety of polysilanes: Ensuring the environmental and health safety of polysilanes involves studying their degradation pathways, potential toxicity, and long-term stability. Developing eco-friendly synthesis routes and exploring biodegradable variants can contribute to safer polysilane designs. Proper disposal and recycling methods should also be considered to minimize environmental impact.
- Functionalization and modification of polysilanes: Safe design of functionalized polysilanes involves careful selection of side groups and modification techniques. These modifications can enhance stability, solubility, and compatibility with other materials. Controlled functionalization can also improve the safety profile of polysilanes by reducing reactivity or introducing desired properties for specific applications.
02 Safety considerations in polysilane production
Safe design of polysilanes involves careful control of reaction conditions and handling procedures. Proper ventilation and protective equipment are essential during synthesis and processing. Minimizing exposure to potentially harmful byproducts and ensuring the purity of the final product are key aspects of safe polysilane production.Expand Specific Solutions03 Applications and coating techniques
Polysilanes find applications in various fields, including electronics, optics, and protective coatings. Safe design considerations for polysilane coatings include proper adhesion to substrates, resistance to environmental factors, and minimizing the release of volatile compounds. Techniques such as spin-coating and vapor deposition are used to apply polysilane films safely and effectively.Expand Specific Solutions04 Modification and functionalization of polysilanes
Enhancing the safety and performance of polysilanes often involves modification and functionalization. This can include the incorporation of specific functional groups, crosslinking agents, or the creation of copolymers. These modifications can improve thermal stability, mechanical properties, and reduce potential health and environmental risks associated with the material.Expand Specific Solutions05 Environmental and disposal considerations
Safe design of polysilanes extends to their entire lifecycle, including disposal and potential environmental impact. Developing biodegradable or easily recyclable polysilanes is an important area of research. Proper disposal methods and potential for recovery of silicon-based materials from used polysilanes are considered to minimize environmental risks and promote sustainability.Expand Specific Solutions
Key Players in Polysilane-Based Biomedical Devices
The polysilane reinforcement in biomedical devices is an emerging field within the broader medical technology sector. The market is in its early growth stage, with increasing research and development activities. While the exact market size is not yet substantial, it shows promising potential due to the growing demand for advanced biomedical materials. Technologically, polysilane reinforcement is still evolving, with companies like Wacker Chemie AG, Covestro Deutschland AG, and Henkel AG & Co. KGaA leading research efforts. These firms are exploring innovative applications in biomedical devices, leveraging polysilane's unique properties to enhance safety and performance. As the technology matures, we can expect increased adoption across various biomedical applications, potentially reshaping the landscape of medical device manufacturing.
Wacker Chemie AG
Technical Solution: Wacker Chemie AG has developed innovative polysilane-based materials for biomedical devices. Their approach involves incorporating polysilane into silicone elastomers, creating a hybrid material with enhanced mechanical properties and biocompatibility[1]. This composite material exhibits improved tensile strength and elongation at break, making it suitable for applications such as implantable medical devices and drug delivery systems[2]. Wacker's polysilane-reinforced silicones also demonstrate excellent resistance to sterilization processes, maintaining their structural integrity and performance after multiple sterilization cycles[3].
Strengths: Enhanced mechanical properties, improved biocompatibility, and resistance to sterilization. Weaknesses: Potential higher production costs and limited long-term clinical data on the performance of polysilane-reinforced devices.
3M Innovative Properties Co.
Technical Solution: 3M has developed a polysilane-based coating technology for biomedical devices, focusing on improving surface properties and reducing bacterial adhesion[4]. Their approach involves creating a thin, uniform layer of polysilane on device surfaces, which can be further functionalized with antimicrobial agents or bioactive molecules[5]. This coating technology has shown promising results in reducing infection rates in implantable devices and improving the overall safety profile of medical equipment[6]. 3M's polysilane coatings also demonstrate excellent durability and resistance to wear, ensuring long-term effectiveness in various biomedical applications[7].
Strengths: Reduced bacterial adhesion, improved surface properties, and versatility in functionalization. Weaknesses: Potential challenges in scaling up the coating process for large-scale production and ensuring uniform coverage on complex device geometries.
Core Innovations in Polysilane for Device Safety
Medical instrument, treatment solution, and method for manufacturing medical instrument
PatentWO2024204412A1
Innovation
- A medical device with a sliding portion coated using a composition containing a silicone resin with hydroxyl groups, a silane coupling agent, and haloalkylsilane, which forms a durable and lubricious film that bonds well to various materials, including polymers and metals.
Organic silicon compound and silane coupling agent containing same
PatentWO2013038901A1
Innovation
- Development of betaine-type or sulfobetaine-type organosilicon compounds with stable positive and negative charges, formulated into a silane coupling agent for surface treatment of inorganic substances and resins, preventing adhesion without the need for polymers like MPC or CMB polymers.
Regulatory Framework for Polysilane in Medical Devices
The regulatory framework for polysilane in medical devices is a critical aspect of ensuring the safe and effective use of this material in biomedical applications. As polysilane continues to gain traction in the medical device industry, regulatory bodies worldwide have been developing and refining guidelines to govern its use.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating medical devices containing polysilane. The FDA's Center for Devices and Radiological Health (CDRH) oversees the premarket approval process, which includes rigorous testing and evaluation of devices incorporating polysilane. Manufacturers must demonstrate compliance with Good Manufacturing Practices (GMP) and provide substantial evidence of safety and efficacy through clinical trials and laboratory studies.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which set stringent requirements for medical devices, including those utilizing polysilane. These regulations emphasize risk management, post-market surveillance, and clinical evaluation throughout the product lifecycle. Manufacturers must obtain CE marking to indicate compliance with EU health, safety, and environmental protection standards.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates medical devices containing polysilane. The PMDA requires manufacturers to submit detailed technical documentation and clinical data to obtain approval for marketing these devices in Japan. The agency also conducts regular inspections to ensure ongoing compliance with safety and quality standards.
International standards, such as ISO 13485 for quality management systems in medical devices, provide a framework for manufacturers to ensure consistent quality and safety in the production of polysilane-based devices. Adherence to these standards is often a prerequisite for regulatory approval in many countries.
Regulatory bodies are increasingly focusing on the long-term safety and biocompatibility of polysilane in medical devices. This includes evaluating potential degradation products, interactions with biological systems, and any potential toxicity concerns. Manufacturers are required to conduct extensive biocompatibility testing in accordance with ISO 10993 standards to assess the material's safety profile.
As the use of polysilane in medical devices evolves, regulatory frameworks are adapting to address emerging challenges. This includes considerations for nanotechnology applications, combination products, and personalized medicine. Regulatory agencies are also emphasizing the importance of post-market surveillance and real-world evidence to monitor the long-term performance and safety of polysilane-based devices.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating medical devices containing polysilane. The FDA's Center for Devices and Radiological Health (CDRH) oversees the premarket approval process, which includes rigorous testing and evaluation of devices incorporating polysilane. Manufacturers must demonstrate compliance with Good Manufacturing Practices (GMP) and provide substantial evidence of safety and efficacy through clinical trials and laboratory studies.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which set stringent requirements for medical devices, including those utilizing polysilane. These regulations emphasize risk management, post-market surveillance, and clinical evaluation throughout the product lifecycle. Manufacturers must obtain CE marking to indicate compliance with EU health, safety, and environmental protection standards.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates medical devices containing polysilane. The PMDA requires manufacturers to submit detailed technical documentation and clinical data to obtain approval for marketing these devices in Japan. The agency also conducts regular inspections to ensure ongoing compliance with safety and quality standards.
International standards, such as ISO 13485 for quality management systems in medical devices, provide a framework for manufacturers to ensure consistent quality and safety in the production of polysilane-based devices. Adherence to these standards is often a prerequisite for regulatory approval in many countries.
Regulatory bodies are increasingly focusing on the long-term safety and biocompatibility of polysilane in medical devices. This includes evaluating potential degradation products, interactions with biological systems, and any potential toxicity concerns. Manufacturers are required to conduct extensive biocompatibility testing in accordance with ISO 10993 standards to assess the material's safety profile.
As the use of polysilane in medical devices evolves, regulatory frameworks are adapting to address emerging challenges. This includes considerations for nanotechnology applications, combination products, and personalized medicine. Regulatory agencies are also emphasizing the importance of post-market surveillance and real-world evidence to monitor the long-term performance and safety of polysilane-based devices.
Biocompatibility and Long-term Safety Considerations
Biocompatibility and long-term safety are critical considerations in the development and application of polysilane-reinforced biomedical devices. The integration of polysilane into these devices offers potential improvements in structural integrity and functionality, but it also necessitates a thorough evaluation of its interaction with biological systems over extended periods.
Polysilanes, as silicon-based polymers, exhibit unique properties that contribute to their biocompatibility. Their chemical structure, consisting of a silicon backbone with organic side groups, allows for tailored interactions with biological tissues. This adaptability enables the design of polysilane-reinforced devices that can mimic natural tissue properties, potentially reducing the risk of adverse reactions and improving overall biocompatibility.
Long-term safety assessments of polysilane-reinforced biomedical devices focus on several key aspects. Firstly, the stability of the polysilane structure within the physiological environment is crucial. Studies have shown that certain polysilane compositions demonstrate excellent resistance to degradation, maintaining their structural integrity over extended periods. This stability is essential for ensuring consistent device performance and minimizing the release of potentially harmful degradation products.
The potential for polysilane to elicit immune responses or inflammation is another critical area of investigation. Research indicates that carefully engineered polysilane surfaces can exhibit low immunogenicity, reducing the risk of chronic inflammation or rejection. This characteristic is particularly valuable for implantable devices, where long-term tissue integration is desired.
Toxicity evaluations of polysilane-reinforced devices have yielded promising results. In vitro and in vivo studies have demonstrated low cytotoxicity and minimal systemic effects, supporting the safety profile of these materials. However, ongoing research continues to explore potential long-term effects, particularly in relation to specific organ systems and diverse patient populations.
The incorporation of polysilane into biomedical devices also presents opportunities for enhancing long-term safety through innovative design features. For instance, the ability to functionalize polysilane surfaces allows for the integration of bioactive molecules that can promote tissue healing, reduce infection risks, or modulate local biological responses. These capabilities contribute to improved long-term outcomes and reduced complications associated with device implantation.
As the field advances, researchers are developing sophisticated in vitro models and long-term in vivo studies to comprehensively assess the biocompatibility and safety of polysilane-reinforced devices. These efforts aim to provide a more nuanced understanding of tissue-material interactions over extended timeframes, ultimately informing the design of safer and more effective biomedical devices.
Polysilanes, as silicon-based polymers, exhibit unique properties that contribute to their biocompatibility. Their chemical structure, consisting of a silicon backbone with organic side groups, allows for tailored interactions with biological tissues. This adaptability enables the design of polysilane-reinforced devices that can mimic natural tissue properties, potentially reducing the risk of adverse reactions and improving overall biocompatibility.
Long-term safety assessments of polysilane-reinforced biomedical devices focus on several key aspects. Firstly, the stability of the polysilane structure within the physiological environment is crucial. Studies have shown that certain polysilane compositions demonstrate excellent resistance to degradation, maintaining their structural integrity over extended periods. This stability is essential for ensuring consistent device performance and minimizing the release of potentially harmful degradation products.
The potential for polysilane to elicit immune responses or inflammation is another critical area of investigation. Research indicates that carefully engineered polysilane surfaces can exhibit low immunogenicity, reducing the risk of chronic inflammation or rejection. This characteristic is particularly valuable for implantable devices, where long-term tissue integration is desired.
Toxicity evaluations of polysilane-reinforced devices have yielded promising results. In vitro and in vivo studies have demonstrated low cytotoxicity and minimal systemic effects, supporting the safety profile of these materials. However, ongoing research continues to explore potential long-term effects, particularly in relation to specific organ systems and diverse patient populations.
The incorporation of polysilane into biomedical devices also presents opportunities for enhancing long-term safety through innovative design features. For instance, the ability to functionalize polysilane surfaces allows for the integration of bioactive molecules that can promote tissue healing, reduce infection risks, or modulate local biological responses. These capabilities contribute to improved long-term outcomes and reduced complications associated with device implantation.
As the field advances, researchers are developing sophisticated in vitro models and long-term in vivo studies to comprehensively assess the biocompatibility and safety of polysilane-reinforced devices. These efforts aim to provide a more nuanced understanding of tissue-material interactions over extended timeframes, ultimately informing the design of safer and more effective biomedical devices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!