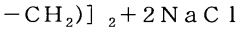Novel Synthesis Methods for Polysilane Compounds
JUL 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Polysilane Synthesis Evolution and Objectives
Polysilane compounds have been a subject of interest in materials science and polymer chemistry for several decades. The evolution of polysilane synthesis methods has been driven by the unique properties and potential applications of these silicon-based polymers. Initially discovered in the 1920s, polysilanes were first synthesized through the Wurtz coupling reaction of dichlorosilanes. This method, while groundbreaking, had limitations in terms of molecular weight control and structural diversity.
The 1980s saw a significant leap in polysilane synthesis with the development of anionic polymerization techniques. This approach allowed for better control over molecular weight and enabled the creation of more complex architectures. Concurrently, researchers explored catalytic dehydrogenative coupling of primary silanes, opening new avenues for polysilane synthesis with different substituents.
As the field progressed, the focus shifted towards developing methods that could yield high molecular weight polysilanes with controlled structures. The 1990s and early 2000s witnessed the emergence of transition metal-catalyzed polymerization techniques, which offered improved control over the polymerization process and the ability to incorporate various functional groups into the polymer backbone.
Recent years have seen a surge in interest in novel synthesis methods for polysilanes, driven by the demand for materials with tailored properties for specific applications. One of the key objectives in this field is to develop sustainable and environmentally friendly synthesis routes. This includes exploring green chemistry approaches, such as using non-toxic catalysts and solvents, as well as investigating bio-inspired synthesis methods.
Another important goal is to achieve precise control over the polymer structure, including molecular weight, polydispersity, and tacticity. This level of control is crucial for optimizing the optical, electronic, and mechanical properties of polysilanes for various applications, including photoresists, precursors for silicon carbide ceramics, and optoelectronic devices.
Researchers are also focusing on expanding the range of monomers that can be incorporated into polysilane structures. This includes the development of methods for synthesizing block copolymers, graft copolymers, and hyperbranched polysilanes. Such structural diversity is essential for tailoring the properties of polysilanes to meet specific application requirements.
Furthermore, there is a growing interest in developing scalable and cost-effective synthesis methods that can facilitate the industrial production of polysilanes. This involves optimizing reaction conditions, exploring continuous flow synthesis techniques, and investigating alternative energy sources for polymerization reactions.
The 1980s saw a significant leap in polysilane synthesis with the development of anionic polymerization techniques. This approach allowed for better control over molecular weight and enabled the creation of more complex architectures. Concurrently, researchers explored catalytic dehydrogenative coupling of primary silanes, opening new avenues for polysilane synthesis with different substituents.
As the field progressed, the focus shifted towards developing methods that could yield high molecular weight polysilanes with controlled structures. The 1990s and early 2000s witnessed the emergence of transition metal-catalyzed polymerization techniques, which offered improved control over the polymerization process and the ability to incorporate various functional groups into the polymer backbone.
Recent years have seen a surge in interest in novel synthesis methods for polysilanes, driven by the demand for materials with tailored properties for specific applications. One of the key objectives in this field is to develop sustainable and environmentally friendly synthesis routes. This includes exploring green chemistry approaches, such as using non-toxic catalysts and solvents, as well as investigating bio-inspired synthesis methods.
Another important goal is to achieve precise control over the polymer structure, including molecular weight, polydispersity, and tacticity. This level of control is crucial for optimizing the optical, electronic, and mechanical properties of polysilanes for various applications, including photoresists, precursors for silicon carbide ceramics, and optoelectronic devices.
Researchers are also focusing on expanding the range of monomers that can be incorporated into polysilane structures. This includes the development of methods for synthesizing block copolymers, graft copolymers, and hyperbranched polysilanes. Such structural diversity is essential for tailoring the properties of polysilanes to meet specific application requirements.
Furthermore, there is a growing interest in developing scalable and cost-effective synthesis methods that can facilitate the industrial production of polysilanes. This involves optimizing reaction conditions, exploring continuous flow synthesis techniques, and investigating alternative energy sources for polymerization reactions.
Industrial Applications and Market Demand
Polysilane compounds have garnered significant attention in various industrial sectors due to their unique properties and versatile applications. The market demand for these materials has been steadily growing, driven by advancements in electronics, photonics, and materials science. In the semiconductor industry, polysilanes are increasingly utilized as precursors for silicon carbide production, which is essential for high-power electronic devices and LED manufacturing. This application alone has created a substantial market, with the global silicon carbide market projected to expand at a compound annual growth rate of over 15% in the coming years.
The automotive sector has also shown keen interest in polysilane-based materials, particularly for their potential in improving the performance and durability of coatings and composites. As the automotive industry shifts towards electric vehicles and lightweight materials, the demand for advanced polymers like polysilanes is expected to rise. Additionally, the aerospace industry has begun exploring polysilanes for their thermal stability and resistance to extreme conditions, opening up new avenues for high-performance materials in aircraft and spacecraft components.
In the field of optoelectronics, polysilanes have demonstrated promising properties for use in photovoltaic cells and organic light-emitting diodes (OLEDs). The global OLED market is experiencing rapid growth, with estimates suggesting it will reach tens of billions of dollars by 2025. This growth presents a significant opportunity for novel polysilane materials that can enhance the efficiency and longevity of OLED devices.
The healthcare and biomedical sectors are emerging as potential growth areas for polysilane applications. Research into biocompatible polysilane-based materials for drug delivery systems and tissue engineering scaffolds has shown promising results. As the global healthcare market continues to expand, driven by aging populations and increased healthcare spending, the demand for advanced biomaterials is expected to create new opportunities for polysilane compounds.
Environmental concerns and the push for sustainable technologies have also influenced the market demand for polysilanes. Their potential use in water treatment processes, particularly in the removal of heavy metals and organic pollutants, has attracted attention from environmental agencies and industrial wastewater treatment facilities. This application aligns with the growing global focus on water scarcity and pollution control, potentially opening up a significant market for polysilane-based purification technologies.
As novel synthesis methods for polysilane compounds continue to evolve, they are likely to unlock new applications and markets. The ability to tailor the properties of polysilanes through innovative synthesis techniques could lead to breakthroughs in fields such as energy storage, flexible electronics, and advanced coatings. This versatility, combined with the growing demand across multiple industries, suggests a robust and expanding market for polysilane compounds in the foreseeable future.
The automotive sector has also shown keen interest in polysilane-based materials, particularly for their potential in improving the performance and durability of coatings and composites. As the automotive industry shifts towards electric vehicles and lightweight materials, the demand for advanced polymers like polysilanes is expected to rise. Additionally, the aerospace industry has begun exploring polysilanes for their thermal stability and resistance to extreme conditions, opening up new avenues for high-performance materials in aircraft and spacecraft components.
In the field of optoelectronics, polysilanes have demonstrated promising properties for use in photovoltaic cells and organic light-emitting diodes (OLEDs). The global OLED market is experiencing rapid growth, with estimates suggesting it will reach tens of billions of dollars by 2025. This growth presents a significant opportunity for novel polysilane materials that can enhance the efficiency and longevity of OLED devices.
The healthcare and biomedical sectors are emerging as potential growth areas for polysilane applications. Research into biocompatible polysilane-based materials for drug delivery systems and tissue engineering scaffolds has shown promising results. As the global healthcare market continues to expand, driven by aging populations and increased healthcare spending, the demand for advanced biomaterials is expected to create new opportunities for polysilane compounds.
Environmental concerns and the push for sustainable technologies have also influenced the market demand for polysilanes. Their potential use in water treatment processes, particularly in the removal of heavy metals and organic pollutants, has attracted attention from environmental agencies and industrial wastewater treatment facilities. This application aligns with the growing global focus on water scarcity and pollution control, potentially opening up a significant market for polysilane-based purification technologies.
As novel synthesis methods for polysilane compounds continue to evolve, they are likely to unlock new applications and markets. The ability to tailor the properties of polysilanes through innovative synthesis techniques could lead to breakthroughs in fields such as energy storage, flexible electronics, and advanced coatings. This versatility, combined with the growing demand across multiple industries, suggests a robust and expanding market for polysilane compounds in the foreseeable future.
Current Challenges in Polysilane Synthesis
Polysilane synthesis faces several significant challenges that hinder the widespread application and commercialization of these unique silicon-based polymers. One of the primary obstacles is the limited availability of efficient and scalable synthesis methods. Traditional approaches, such as Wurtz coupling reactions, often result in low molecular weight products and suffer from poor control over polymer structure and molecular weight distribution.
The synthesis of high molecular weight polysilanes remains a considerable challenge. Current methods struggle to produce polymers with sufficiently long chain lengths, which are crucial for many potential applications. This limitation is partly due to the inherent reactivity of silicon-silicon bonds and the tendency for chain termination during polymerization processes.
Another significant hurdle is the control of polymer architecture and composition. Achieving precise control over the sequence and distribution of different silicon units along the polymer backbone is challenging, limiting the ability to tailor polysilane properties for specific applications. This lack of control also hampers the development of block copolymers and other advanced polysilane structures.
The sensitivity of polysilanes to air and moisture presents additional challenges in both synthesis and handling. Many polysilane compounds are prone to oxidation and hydrolysis, necessitating stringent reaction conditions and careful post-synthesis processing. This sensitivity not only complicates the synthesis process but also limits the long-term stability of polysilane materials.
Purification and characterization of polysilanes pose further challenges. The presence of cyclic oligomers and other by-products in reaction mixtures often complicates isolation of the desired linear polymers. Additionally, the characterization of polysilane structures, particularly for high molecular weight species, can be challenging due to limitations in conventional polymer analysis techniques.
The development of environmentally friendly and sustainable synthesis routes for polysilanes is an ongoing challenge. Many current methods rely on hazardous reagents or generate significant waste, which is not aligned with the principles of green chemistry. Finding alternative, more sustainable synthesis pathways is crucial for the broader adoption of polysilane technologies.
Lastly, the cost-effectiveness of polysilane synthesis remains a significant barrier to commercial applications. Current methods often involve expensive starting materials and complex, multi-step processes, making large-scale production economically challenging. Overcoming these economic hurdles is essential for realizing the full potential of polysilanes in various industrial and technological applications.
The synthesis of high molecular weight polysilanes remains a considerable challenge. Current methods struggle to produce polymers with sufficiently long chain lengths, which are crucial for many potential applications. This limitation is partly due to the inherent reactivity of silicon-silicon bonds and the tendency for chain termination during polymerization processes.
Another significant hurdle is the control of polymer architecture and composition. Achieving precise control over the sequence and distribution of different silicon units along the polymer backbone is challenging, limiting the ability to tailor polysilane properties for specific applications. This lack of control also hampers the development of block copolymers and other advanced polysilane structures.
The sensitivity of polysilanes to air and moisture presents additional challenges in both synthesis and handling. Many polysilane compounds are prone to oxidation and hydrolysis, necessitating stringent reaction conditions and careful post-synthesis processing. This sensitivity not only complicates the synthesis process but also limits the long-term stability of polysilane materials.
Purification and characterization of polysilanes pose further challenges. The presence of cyclic oligomers and other by-products in reaction mixtures often complicates isolation of the desired linear polymers. Additionally, the characterization of polysilane structures, particularly for high molecular weight species, can be challenging due to limitations in conventional polymer analysis techniques.
The development of environmentally friendly and sustainable synthesis routes for polysilanes is an ongoing challenge. Many current methods rely on hazardous reagents or generate significant waste, which is not aligned with the principles of green chemistry. Finding alternative, more sustainable synthesis pathways is crucial for the broader adoption of polysilane technologies.
Lastly, the cost-effectiveness of polysilane synthesis remains a significant barrier to commercial applications. Current methods often involve expensive starting materials and complex, multi-step processes, making large-scale production economically challenging. Overcoming these economic hurdles is essential for realizing the full potential of polysilanes in various industrial and technological applications.
State-of-the-Art Polysilane Synthesis Techniques
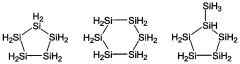
01 Synthesis and structure of polysilane compounds
Polysilane compounds are synthesized through various methods, including catalytic dehydrogenative coupling of hydrosilanes. These compounds have unique structural properties, often featuring a silicon backbone with organic substituents. The synthesis and structure of polysilanes are crucial for their applications in various fields.- Synthesis and structure of polysilane compounds: Polysilane compounds are synthesized through various methods, including catalytic dehydrocoupling of hydrosilanes and reductive coupling of dichlorosilanes. These compounds have unique structures with silicon-silicon backbones, which contribute to their electronic and optical properties.
- Applications in electronic and optical materials: Polysilane compounds are utilized in electronic and optical materials due to their unique electronic properties. They can be used in photoresists, semiconductors, and as precursors for silicon carbide materials. Their applications extend to areas such as photovoltaics and light-emitting devices.
- Polysilane-based coatings and films: Polysilane compounds are employed in the development of coatings and films with specific properties. These materials can be used for protective coatings, barrier films, and in the production of thin-film transistors. The polysilane-based coatings often exhibit improved thermal stability and adhesion properties.
- Functionalization and modification of polysilanes: Polysilane compounds can be functionalized or modified to enhance their properties or introduce new functionalities. This includes the incorporation of various organic groups, crosslinking agents, or other elements to tailor the polysilanes for specific applications or improve their performance in existing uses.
- Polysilane-based composites and hybrid materials: Polysilane compounds are used in the development of composite and hybrid materials. These materials combine the unique properties of polysilanes with other organic or inorganic components, resulting in materials with enhanced thermal, mechanical, or optical properties for various applications.
02 Applications of polysilane compounds in electronics
Polysilane compounds find applications in electronic devices due to their unique electronic properties. They can be used as semiconductors, photoconductors, and in the fabrication of electronic components. These materials exhibit interesting optical and electrical characteristics, making them suitable for various electronic applications.Expand Specific Solutions03 Polysilane compounds as precursors for ceramic materials
Polysilane compounds serve as precursors for the production of silicon-based ceramic materials. When subjected to high temperatures, these compounds can be converted into silicon carbide or other silicon-containing ceramics. This property makes them valuable in the field of advanced materials and high-temperature applications.Expand Specific Solutions04 Modification and functionalization of polysilane compounds
Polysilane compounds can be modified and functionalized to enhance their properties or introduce new functionalities. This includes the incorporation of various organic groups, crosslinking, or the addition of other elements to the silicon backbone. Such modifications allow for the tailoring of polysilanes for specific applications.Expand Specific Solutions05 Polysilane compounds in coating and film applications
Polysilane compounds are utilized in coating and film applications due to their unique properties. They can form thin films with interesting optical and electronic characteristics, making them suitable for use in various coating technologies. These compounds can also be incorporated into composite materials to enhance their properties.Expand Specific Solutions
Key Players in Polysilane Research and Production
The development of novel synthesis methods for polysilane compounds is in a nascent stage, with significant potential for growth. The market size is relatively small but expanding, driven by increasing applications in electronics, photonics, and materials science. The technology's maturity is still evolving, with key players like JSR Corp., Wacker Chemie AG, and Tokyo Ohka Kogyo Co., Ltd. leading research efforts. These companies are investing in R&D to improve synthesis efficiency and expand polysilane applications. Academic institutions such as the National University of Defense Technology and Hangzhou Normal University are also contributing to fundamental research, indicating a collaborative industry-academia approach to advancing this technology.
JSR Corp.
Technical Solution: JSR Corp. has pioneered a novel synthesis method for polysilane compounds focusing on photosensitive applications. Their approach utilizes a combination of anionic polymerization and post-polymerization modification to create well-defined polysilane structures[4]. The company has developed a unique initiator system that allows for precise control over the molecular weight and polydispersity of the resulting polysilanes[5]. JSR's method also incorporates the use of specially designed end-capping agents to introduce specific functionalities at the chain ends, enhancing the materials' compatibility with various substrates and improving their performance in photoresist applications[6]. Additionally, JSR has implemented a controlled degradation process to fine-tune the photosensitivity of their polysilane products[7].
Strengths: Excellent control over molecular structure, tailored photosensitivity, high performance in photoresist applications. Weaknesses: Potentially complex synthesis process, limited to specific application areas.
Wacker Chemie AG
Technical Solution: Wacker Chemie AG has developed a novel synthesis method for polysilane compounds using their proprietary catalytic dehydrocoupling process. This method involves the use of transition metal catalysts to promote Si-Si bond formation from silane precursors. The process allows for controlled molecular weight distribution and enables the production of high-purity polysilanes with tailored properties[1]. Wacker's approach also incorporates the use of advanced purification techniques to remove catalyst residues and achieve ultra-high purity levels suitable for electronic applications[2]. The company has further optimized their synthesis to allow for the incorporation of functional groups along the polysilane backbone, enhancing the versatility of the resulting materials[3].
Strengths: High purity products, controlled molecular weight, versatile functionalization. Weaknesses: Potentially high production costs, limited scalability for some specialized polysilanes.
Breakthrough Patents in Novel Polysilane Synthesis
Polysilane manufacturing method
PatentWO2010005107A1
Innovation
- A method involving specific silane compounds, such as cyclic and cage silane compounds, is used to produce polysilane under more reasonable conditions, utilizing reactions that do not require large-scale apparatus and can be performed at room temperature, with preferred silane compounds like cyclopentasilane and cyclohexasilane, and specific reaction conditions to achieve high molecular weight polysilane.
Method for the synthesis of a chlorine-free, pre-ceramic polymer for the production of ceramic molded bodies
PatentWO2014090934A8
Innovation
- A method involving the disproportionation of methylchlorodisilane mixtures to oligosilanes, followed by chlorine substitution with primary amines, and crosslinking using chain formers to produce chlorine-free, infusible polysilanes, which can be processed into high-quality green and ceramic fibers without the need for thermal post-treatment.
Environmental Impact of Polysilane Production
The production of polysilane compounds, while offering significant technological advancements, raises important environmental concerns that must be addressed. Traditional synthesis methods often involve energy-intensive processes and the use of hazardous chemicals, leading to potential environmental risks. The environmental impact of polysilane production can be categorized into several key areas.
Firstly, the energy consumption associated with polysilane synthesis is a major concern. High-temperature reactions and prolonged processing times contribute to increased carbon emissions and overall energy footprint. This aspect of production aligns with broader issues of climate change and the need for more sustainable industrial practices.
Chemical waste generation is another significant environmental challenge. Many synthesis routes produce by-products and unreacted materials that require proper disposal. These waste streams may contain toxic or persistent compounds, posing risks to soil and water ecosystems if not managed correctly. The development of more efficient synthesis methods that minimize waste production is crucial for reducing this environmental burden.
Air quality is also affected by polysilane production. Volatile organic compounds (VOCs) and other airborne pollutants can be released during synthesis and processing stages. These emissions may contribute to local air pollution and potentially impact human health in surrounding communities. Implementing robust air filtration and containment systems is essential to mitigate these risks.
Water usage and contamination present additional environmental concerns. Some synthesis methods require substantial amounts of water for cooling or as reaction media. Ensuring proper treatment and recycling of process water is vital to conserve this valuable resource and prevent the release of contaminated effluents into natural water bodies.
The raw materials used in polysilane production also have upstream environmental implications. The extraction and processing of silicon and other precursor materials can lead to habitat disruption, energy consumption, and emissions associated with mining and refining activities. Sourcing more sustainable raw materials and optimizing resource efficiency in the supply chain are important considerations for reducing the overall environmental footprint of polysilane production.
As the demand for polysilane compounds grows, particularly in emerging technologies like photovoltaics and microelectronics, addressing these environmental challenges becomes increasingly critical. Research into novel synthesis methods for polysilanes must prioritize not only improved material properties and production efficiency but also reduced environmental impact. This includes exploring green chemistry principles, developing closed-loop production systems, and investigating alternative precursors and reaction pathways that minimize resource consumption and waste generation.
Firstly, the energy consumption associated with polysilane synthesis is a major concern. High-temperature reactions and prolonged processing times contribute to increased carbon emissions and overall energy footprint. This aspect of production aligns with broader issues of climate change and the need for more sustainable industrial practices.
Chemical waste generation is another significant environmental challenge. Many synthesis routes produce by-products and unreacted materials that require proper disposal. These waste streams may contain toxic or persistent compounds, posing risks to soil and water ecosystems if not managed correctly. The development of more efficient synthesis methods that minimize waste production is crucial for reducing this environmental burden.
Air quality is also affected by polysilane production. Volatile organic compounds (VOCs) and other airborne pollutants can be released during synthesis and processing stages. These emissions may contribute to local air pollution and potentially impact human health in surrounding communities. Implementing robust air filtration and containment systems is essential to mitigate these risks.
Water usage and contamination present additional environmental concerns. Some synthesis methods require substantial amounts of water for cooling or as reaction media. Ensuring proper treatment and recycling of process water is vital to conserve this valuable resource and prevent the release of contaminated effluents into natural water bodies.
The raw materials used in polysilane production also have upstream environmental implications. The extraction and processing of silicon and other precursor materials can lead to habitat disruption, energy consumption, and emissions associated with mining and refining activities. Sourcing more sustainable raw materials and optimizing resource efficiency in the supply chain are important considerations for reducing the overall environmental footprint of polysilane production.
As the demand for polysilane compounds grows, particularly in emerging technologies like photovoltaics and microelectronics, addressing these environmental challenges becomes increasingly critical. Research into novel synthesis methods for polysilanes must prioritize not only improved material properties and production efficiency but also reduced environmental impact. This includes exploring green chemistry principles, developing closed-loop production systems, and investigating alternative precursors and reaction pathways that minimize resource consumption and waste generation.
Safety Considerations in Polysilane Synthesis
Safety considerations are paramount in the synthesis of polysilane compounds due to their unique chemical properties and potential hazards. The high reactivity of silicon-silicon bonds and the presence of organic substituents necessitate stringent safety protocols throughout the synthesis process. One primary concern is the pyrophoric nature of many polysilane precursors, particularly those containing alkyl or hydrogen substituents. These compounds can spontaneously ignite upon exposure to air, requiring careful handling under inert atmospheres such as argon or nitrogen.
The use of organometallic reagents, often employed in polysilane synthesis, presents additional safety challenges. Compounds like alkyllithium or Grignard reagents are highly reactive and moisture-sensitive, demanding rigorous exclusion of water and oxygen. Proper personal protective equipment (PPE), including fire-resistant lab coats, goggles, and gloves, is essential when working with these materials. Furthermore, the potential for exothermic reactions during synthesis necessitates careful temperature control and monitoring to prevent runaway reactions.
Solvent selection is another critical safety aspect in polysilane synthesis. Many common organic solvents used in these reactions, such as tetrahydrofuran (THF) or diethyl ether, are highly flammable and can form explosive peroxides over time. Regular testing for peroxide formation and proper storage of these solvents is crucial to minimize risks. Additionally, the use of cryogenic temperatures in some synthetic routes requires special precautions to prevent cold burns and asphyxiation hazards from evaporating liquid nitrogen or dry ice.
The potential for silicon dust formation during handling of solid polysilane products or intermediates poses respiratory risks. Fine silicon particles can be pyrophoric and may cause lung irritation or more severe health effects if inhaled. Adequate ventilation, dust control measures, and appropriate respiratory protection are essential in mitigating these risks. Moreover, the disposal of polysilane waste requires careful consideration due to its potential reactivity and environmental impact.
In light of these safety considerations, comprehensive risk assessments should be conducted prior to undertaking polysilane synthesis. This includes evaluating the specific hazards associated with each reagent and reaction step, implementing appropriate engineering controls, and developing detailed standard operating procedures (SOPs). Regular safety training for personnel involved in polysilane research and production is crucial to ensure awareness of potential hazards and proper emergency response protocols. By prioritizing safety in polysilane synthesis, researchers can minimize risks while advancing this important field of silicon chemistry.
The use of organometallic reagents, often employed in polysilane synthesis, presents additional safety challenges. Compounds like alkyllithium or Grignard reagents are highly reactive and moisture-sensitive, demanding rigorous exclusion of water and oxygen. Proper personal protective equipment (PPE), including fire-resistant lab coats, goggles, and gloves, is essential when working with these materials. Furthermore, the potential for exothermic reactions during synthesis necessitates careful temperature control and monitoring to prevent runaway reactions.
Solvent selection is another critical safety aspect in polysilane synthesis. Many common organic solvents used in these reactions, such as tetrahydrofuran (THF) or diethyl ether, are highly flammable and can form explosive peroxides over time. Regular testing for peroxide formation and proper storage of these solvents is crucial to minimize risks. Additionally, the use of cryogenic temperatures in some synthetic routes requires special precautions to prevent cold burns and asphyxiation hazards from evaporating liquid nitrogen or dry ice.
The potential for silicon dust formation during handling of solid polysilane products or intermediates poses respiratory risks. Fine silicon particles can be pyrophoric and may cause lung irritation or more severe health effects if inhaled. Adequate ventilation, dust control measures, and appropriate respiratory protection are essential in mitigating these risks. Moreover, the disposal of polysilane waste requires careful consideration due to its potential reactivity and environmental impact.
In light of these safety considerations, comprehensive risk assessments should be conducted prior to undertaking polysilane synthesis. This includes evaluating the specific hazards associated with each reagent and reaction step, implementing appropriate engineering controls, and developing detailed standard operating procedures (SOPs). Regular safety training for personnel involved in polysilane research and production is crucial to ensure awareness of potential hazards and proper emergency response protocols. By prioritizing safety in polysilane synthesis, researchers can minimize risks while advancing this important field of silicon chemistry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!