How Sodium Alginate Aids in Oral Drug Delivery Systems?
JUL 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Alginate in Drug Delivery: Background and Objectives
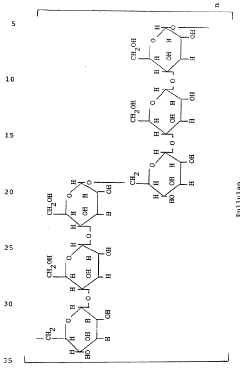
Sodium alginate has emerged as a pivotal component in oral drug delivery systems, revolutionizing the pharmaceutical industry's approach to medication administration. This natural polysaccharide, derived from brown seaweed, has garnered significant attention due to its unique properties that enhance drug delivery efficacy and patient compliance.
The journey of sodium alginate in drug delivery can be traced back to the mid-20th century when researchers began exploring its potential in pharmaceutical applications. Initially utilized for its gelling properties, sodium alginate's role in drug delivery has evolved substantially over the decades. The primary objective of incorporating sodium alginate into oral drug delivery systems is to overcome the limitations associated with conventional dosage forms, such as poor bioavailability, rapid drug degradation, and undesirable side effects.
As the field of pharmacology progressed, the demand for more sophisticated drug delivery mechanisms grew. Sodium alginate's versatility in forming hydrogels, its biocompatibility, and its ability to respond to environmental stimuli made it an ideal candidate for addressing these challenges. The technology aims to achieve controlled release of drugs, protect sensitive compounds from harsh gastrointestinal conditions, and improve the overall therapeutic efficacy of various medications.
The development of sodium alginate-based drug delivery systems aligns with the broader trend in personalized medicine, where tailored drug release profiles can be designed to meet individual patient needs. This approach has the potential to revolutionize treatment strategies for chronic diseases, enhancing patient outcomes and quality of life.
Recent advancements in nanotechnology and polymer science have further expanded the possibilities for sodium alginate in drug delivery. Researchers are now exploring its use in combination with other materials to create hybrid systems that offer unprecedented control over drug release kinetics and targeting capabilities.
The objectives of current research in this field are multifaceted. Scientists aim to optimize the formulation of sodium alginate-based delivery systems to enhance their stability, increase drug loading capacity, and improve their ability to target specific sites within the body. Additionally, there is a focus on developing smart delivery systems that can respond to physiological cues, enabling on-demand drug release.
As we look to the future, the potential applications of sodium alginate in oral drug delivery continue to expand. From improving the bioavailability of poorly soluble drugs to enabling the delivery of large biomolecules like proteins and peptides, the versatility of this natural polymer promises to address some of the most pressing challenges in modern pharmacotherapy.
The journey of sodium alginate in drug delivery can be traced back to the mid-20th century when researchers began exploring its potential in pharmaceutical applications. Initially utilized for its gelling properties, sodium alginate's role in drug delivery has evolved substantially over the decades. The primary objective of incorporating sodium alginate into oral drug delivery systems is to overcome the limitations associated with conventional dosage forms, such as poor bioavailability, rapid drug degradation, and undesirable side effects.
As the field of pharmacology progressed, the demand for more sophisticated drug delivery mechanisms grew. Sodium alginate's versatility in forming hydrogels, its biocompatibility, and its ability to respond to environmental stimuli made it an ideal candidate for addressing these challenges. The technology aims to achieve controlled release of drugs, protect sensitive compounds from harsh gastrointestinal conditions, and improve the overall therapeutic efficacy of various medications.
The development of sodium alginate-based drug delivery systems aligns with the broader trend in personalized medicine, where tailored drug release profiles can be designed to meet individual patient needs. This approach has the potential to revolutionize treatment strategies for chronic diseases, enhancing patient outcomes and quality of life.
Recent advancements in nanotechnology and polymer science have further expanded the possibilities for sodium alginate in drug delivery. Researchers are now exploring its use in combination with other materials to create hybrid systems that offer unprecedented control over drug release kinetics and targeting capabilities.
The objectives of current research in this field are multifaceted. Scientists aim to optimize the formulation of sodium alginate-based delivery systems to enhance their stability, increase drug loading capacity, and improve their ability to target specific sites within the body. Additionally, there is a focus on developing smart delivery systems that can respond to physiological cues, enabling on-demand drug release.
As we look to the future, the potential applications of sodium alginate in oral drug delivery continue to expand. From improving the bioavailability of poorly soluble drugs to enabling the delivery of large biomolecules like proteins and peptides, the versatility of this natural polymer promises to address some of the most pressing challenges in modern pharmacotherapy.
Market Analysis for Alginate-Based Oral Drug Delivery
The global market for alginate-based oral drug delivery systems has been experiencing significant growth in recent years, driven by the increasing demand for more effective and patient-friendly drug administration methods. Sodium alginate, a natural polysaccharide derived from brown seaweed, has emerged as a versatile and promising excipient in oral drug delivery formulations.
The market for alginate-based oral drug delivery systems is primarily fueled by the rising prevalence of chronic diseases, the growing geriatric population, and the need for controlled-release medications. These factors have led to an increased focus on developing innovative drug delivery technologies that can enhance bioavailability, improve patient compliance, and reduce side effects.
In terms of market segmentation, alginate-based oral drug delivery systems find applications across various therapeutic areas, including gastrointestinal disorders, cardiovascular diseases, diabetes, and cancer. The gastroenterology segment currently holds the largest market share due to the excellent mucoadhesive properties of sodium alginate, which make it particularly suitable for targeted drug delivery in the gastrointestinal tract.
Geographically, North America and Europe dominate the market for alginate-based oral drug delivery systems, owing to the presence of well-established pharmaceutical industries, high healthcare expenditure, and favorable regulatory environments. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in healthcare infrastructure, rising disposable incomes, and growing awareness about advanced drug delivery technologies.
The market landscape is characterized by intense competition among key players, including pharmaceutical companies, contract development and manufacturing organizations (CDMOs), and specialty chemical manufacturers. These companies are actively engaged in research and development activities to enhance the properties of alginate-based formulations and expand their applications in oral drug delivery.
Looking ahead, the market for alginate-based oral drug delivery systems is projected to continue its upward trajectory. Factors such as the increasing adoption of personalized medicine, the growing focus on patient-centric drug development, and the rising demand for sustained-release formulations are expected to drive market growth. Additionally, the ongoing research into novel alginate derivatives and hybrid systems combining alginate with other polymers is likely to open up new opportunities for market expansion and product innovation in the oral drug delivery space.
The market for alginate-based oral drug delivery systems is primarily fueled by the rising prevalence of chronic diseases, the growing geriatric population, and the need for controlled-release medications. These factors have led to an increased focus on developing innovative drug delivery technologies that can enhance bioavailability, improve patient compliance, and reduce side effects.
In terms of market segmentation, alginate-based oral drug delivery systems find applications across various therapeutic areas, including gastrointestinal disorders, cardiovascular diseases, diabetes, and cancer. The gastroenterology segment currently holds the largest market share due to the excellent mucoadhesive properties of sodium alginate, which make it particularly suitable for targeted drug delivery in the gastrointestinal tract.
Geographically, North America and Europe dominate the market for alginate-based oral drug delivery systems, owing to the presence of well-established pharmaceutical industries, high healthcare expenditure, and favorable regulatory environments. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in healthcare infrastructure, rising disposable incomes, and growing awareness about advanced drug delivery technologies.
The market landscape is characterized by intense competition among key players, including pharmaceutical companies, contract development and manufacturing organizations (CDMOs), and specialty chemical manufacturers. These companies are actively engaged in research and development activities to enhance the properties of alginate-based formulations and expand their applications in oral drug delivery.
Looking ahead, the market for alginate-based oral drug delivery systems is projected to continue its upward trajectory. Factors such as the increasing adoption of personalized medicine, the growing focus on patient-centric drug development, and the rising demand for sustained-release formulations are expected to drive market growth. Additionally, the ongoing research into novel alginate derivatives and hybrid systems combining alginate with other polymers is likely to open up new opportunities for market expansion and product innovation in the oral drug delivery space.
Current Challenges in Sodium Alginate Drug Delivery Systems
Despite the promising potential of sodium alginate in oral drug delivery systems, several challenges persist that hinder its widespread adoption and optimal performance. One of the primary issues is the variability in the composition and quality of sodium alginate derived from different sources. This inconsistency can lead to unpredictable drug release profiles and compromised therapeutic efficacy.
Another significant challenge is the pH-dependent behavior of sodium alginate. While its ability to form gels in acidic environments is advantageous for gastric protection, it can also result in premature drug release or incomplete absorption in the intestinal tract. This pH sensitivity necessitates careful formulation design to ensure targeted and controlled drug delivery throughout the gastrointestinal system.
The mechanical strength and stability of sodium alginate matrices pose additional concerns. In some cases, the gel network formed by sodium alginate may not be robust enough to withstand the harsh conditions of the gastrointestinal tract, leading to premature erosion or disintegration of the drug delivery system. This can result in uncontrolled drug release and reduced bioavailability.
Furthermore, the high water solubility of sodium alginate can sometimes lead to rapid dissolution and drug release, particularly for highly water-soluble drugs. This characteristic can limit its effectiveness in achieving sustained release formulations, necessitating the development of modified alginate systems or combination with other polymers to overcome this limitation.
The interaction between sodium alginate and certain drugs or excipients can also present challenges. Some ionic drugs may form complexes with alginate, altering their release kinetics or bioavailability. Additionally, the presence of multivalent cations in the gastrointestinal environment can lead to uncontrolled cross-linking of alginate chains, potentially affecting drug release patterns.
Scalability and reproducibility in manufacturing processes remain ongoing concerns. The properties of sodium alginate-based formulations can be highly sensitive to processing parameters, making it challenging to maintain consistent quality across different batches or scale up production for commercial manufacturing.
Lastly, regulatory considerations and the need for extensive in vivo studies to establish the safety and efficacy of sodium alginate-based drug delivery systems pose significant hurdles. The complex nature of these formulations often requires comprehensive characterization and validation, which can be time-consuming and resource-intensive.
Another significant challenge is the pH-dependent behavior of sodium alginate. While its ability to form gels in acidic environments is advantageous for gastric protection, it can also result in premature drug release or incomplete absorption in the intestinal tract. This pH sensitivity necessitates careful formulation design to ensure targeted and controlled drug delivery throughout the gastrointestinal system.
The mechanical strength and stability of sodium alginate matrices pose additional concerns. In some cases, the gel network formed by sodium alginate may not be robust enough to withstand the harsh conditions of the gastrointestinal tract, leading to premature erosion or disintegration of the drug delivery system. This can result in uncontrolled drug release and reduced bioavailability.
Furthermore, the high water solubility of sodium alginate can sometimes lead to rapid dissolution and drug release, particularly for highly water-soluble drugs. This characteristic can limit its effectiveness in achieving sustained release formulations, necessitating the development of modified alginate systems or combination with other polymers to overcome this limitation.
The interaction between sodium alginate and certain drugs or excipients can also present challenges. Some ionic drugs may form complexes with alginate, altering their release kinetics or bioavailability. Additionally, the presence of multivalent cations in the gastrointestinal environment can lead to uncontrolled cross-linking of alginate chains, potentially affecting drug release patterns.
Scalability and reproducibility in manufacturing processes remain ongoing concerns. The properties of sodium alginate-based formulations can be highly sensitive to processing parameters, making it challenging to maintain consistent quality across different batches or scale up production for commercial manufacturing.
Lastly, regulatory considerations and the need for extensive in vivo studies to establish the safety and efficacy of sodium alginate-based drug delivery systems pose significant hurdles. The complex nature of these formulations often requires comprehensive characterization and validation, which can be time-consuming and resource-intensive.
Existing Sodium Alginate Drug Delivery Mechanisms
01 Sodium alginate-based drug delivery systems
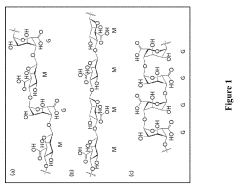
Sodium alginate is widely used in drug delivery systems due to its biocompatibility and ability to form hydrogels. These systems can be designed to control drug release rates and improve bioavailability. Various formulations incorporate sodium alginate as a matrix for encapsulating drugs, providing sustained release and enhanced therapeutic efficacy.- Sodium alginate-based drug delivery systems: Sodium alginate is widely used in drug delivery systems due to its biocompatibility and ability to form hydrogels. It can be used to encapsulate various drugs, providing controlled release and improved bioavailability. These systems can be formulated as beads, microspheres, or nanoparticles, offering versatility in drug delivery applications.
- Combination with other polymers for enhanced drug delivery: Sodium alginate is often combined with other polymers to create composite drug delivery systems with improved properties. These combinations can enhance drug loading capacity, control release kinetics, and provide additional functionalities such as mucoadhesion or pH-responsiveness. Common polymers used in combination with sodium alginate include chitosan, pectin, and synthetic polymers.
- Sodium alginate in targeted drug delivery: Sodium alginate-based systems can be modified for targeted drug delivery to specific organs or tissues. This can be achieved through surface modification, incorporation of targeting ligands, or by exploiting the inherent properties of alginate gels. Targeted delivery can improve therapeutic efficacy and reduce side effects by concentrating the drug at the desired site of action.
- Stimuli-responsive sodium alginate drug delivery systems: Sodium alginate can be used to create stimuli-responsive drug delivery systems that release their payload in response to specific environmental triggers. These triggers can include pH changes, temperature variations, or the presence of certain enzymes. Such systems allow for precise control over drug release, improving therapeutic outcomes and patient compliance.
- Sodium alginate in oral drug delivery: Sodium alginate is particularly useful in oral drug delivery applications due to its ability to form protective gels in the acidic environment of the stomach. This property can be exploited to develop gastroretentive dosage forms, enhance the stability of acid-labile drugs, and achieve controlled release in the gastrointestinal tract. Oral formulations based on sodium alginate can improve the bioavailability of poorly soluble drugs and reduce dosing frequency.
02 Nanoparticle formulations with sodium alginate
Sodium alginate is utilized in the preparation of nanoparticles for drug delivery. These nanoparticles can improve drug solubility, stability, and targeted delivery. The use of sodium alginate in nanoparticle formulations allows for better control over particle size and drug release kinetics, enhancing therapeutic outcomes.Expand Specific Solutions03 Sodium alginate in combination with other polymers
Combining sodium alginate with other polymers can create advanced drug delivery systems with improved properties. These composite materials can offer better mechanical strength, mucoadhesion, or pH-responsiveness. Such combinations allow for tailored drug release profiles and enhanced drug delivery efficiency.Expand Specific Solutions04 Sodium alginate in oral drug delivery
Sodium alginate is extensively used in oral drug delivery formulations. It can form protective barriers in the stomach, allowing for targeted intestinal drug release. This property is particularly useful for drugs that are sensitive to gastric conditions or require site-specific delivery in the gastrointestinal tract.Expand Specific Solutions05 Crosslinking of sodium alginate for controlled release
Crosslinking of sodium alginate is employed to create hydrogels with enhanced drug retention and controlled release properties. Various crosslinking agents and methods are used to modify the physical and chemical properties of alginate matrices, allowing for fine-tuning of drug release rates and durations.Expand Specific Solutions
Key Players in Alginate-Based Pharmaceutical Industry
The oral drug delivery market utilizing sodium alginate is in a growth phase, driven by increasing demand for innovative drug formulations. The market size is expanding, with a projected CAGR of 7-8% over the next five years. Technologically, sodium alginate-based systems are moderately mature, with ongoing research focused on enhancing bioavailability and controlled release properties. Key players like LTS LOHMANN Therapie-Systeme AG, Hanmi Pharmaceutical Co., Ltd., and Novo Nordisk A/S are investing in R&D to develop advanced formulations. Universities such as Zhejiang University and Soochow University are contributing to fundamental research, while companies like NorInvent AB and Oralance Pharma SA are specializing in novel oral delivery technologies, indicating a competitive and diverse landscape.
LTS LOHMANN Therapie-Systeme AG
Technical Solution: LTS LOHMANN Therapie-Systeme AG has developed innovative oral drug delivery systems utilizing sodium alginate. Their approach involves creating a multi-layered film technology that incorporates sodium alginate as a key component. This technology allows for controlled release of active pharmaceutical ingredients (APIs) in the gastrointestinal tract. The sodium alginate layer forms a gel-like barrier when in contact with gastric fluids, providing protection to the drug and enabling sustained release[1]. LTS has also combined this technology with other polymers to create hybrid systems that can target specific areas of the GI tract, enhancing bioavailability and reducing side effects[3].
Strengths: Precise control over drug release kinetics, improved patient compliance due to reduced dosing frequency. Weaknesses: Potential variability in gel formation depending on individual gastric conditions, may require additional excipients for optimal performance.
Novo Nordisk A/S
Technical Solution: Novo Nordisk A/S has developed a novel oral delivery system for peptides and proteins, particularly focusing on insulin delivery, using sodium alginate as a key component. Their approach involves encapsulating the drug molecules within a sodium alginate matrix, which is then coated with a pH-responsive polymer. This dual-layer system protects the drug from degradation in the stomach and allows for targeted release in the small intestine[2]. The sodium alginate core provides a hydrophilic environment that helps maintain the stability and activity of the peptide drugs. Additionally, Novo Nordisk has incorporated permeation enhancers into the alginate matrix to improve the absorption of large molecules across the intestinal epithelium[4].
Strengths: Enables oral delivery of traditionally injectable drugs, potentially improving patient adherence and quality of life. Weaknesses: Complexity of the system may lead to higher manufacturing costs, potential variability in absorption rates between patients.
Innovative Alginate Formulations for Enhanced Drug Release
Calcium Alginate Dosage Formulations, and Methods of Making and Using Thereof
PatentActiveUS20190321298A1
Innovation
- The development of a method to create larger, uniform calcium alginate tablets using a mesh basket process, incorporating stearic acid and ethyl cellulose, and applying an electric current to control drug release, allowing for adjustable release profiles and improved drug encapsulation.
Oral drug delivery systems
PatentInactiveCA1313620C
Innovation
- A bioadhesive oral ointment formulation combining hydrophilic polymers, pullulan as a disintegrating agent, and an ointment base, which absorbs water to adhere to oral tissues, maintaining a uniform distribution of medicaments at the treatment site for prolonged periods without causing irritation.
Regulatory Framework for Alginate-Based Pharmaceuticals
The regulatory framework for alginate-based pharmaceuticals is a critical aspect of their development and commercialization. In the United States, the Food and Drug Administration (FDA) oversees the approval process for these drug delivery systems. Sodium alginate, as a key component in oral drug delivery, falls under the purview of both the Center for Drug Evaluation and Research (CDER) and the Center for Food Safety and Applied Nutrition (CFSAN).
The FDA classifies sodium alginate as Generally Recognized as Safe (GRAS) for food applications, but its use in pharmaceuticals requires additional scrutiny. Manufacturers must demonstrate the safety and efficacy of alginate-based drug delivery systems through rigorous clinical trials and comprehensive documentation. This includes providing data on the sourcing, purity, and consistency of the sodium alginate used in the formulation.
In the European Union, the European Medicines Agency (EMA) regulates alginate-based pharmaceuticals. The EMA's guidelines on excipients require manufacturers to provide detailed information on the quality and safety of sodium alginate when used in oral drug delivery systems. This includes specifications on impurities, microbial limits, and potential interactions with active pharmaceutical ingredients.
Globally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides guidelines that influence the regulatory approach to alginate-based pharmaceuticals. These guidelines address quality control, stability testing, and good manufacturing practices, which are essential for ensuring the consistency and reliability of alginate-based drug delivery systems.
Regulatory bodies also focus on the potential environmental impact of alginate production and disposal. Manufacturers must adhere to sustainability guidelines and provide environmental risk assessments as part of their regulatory submissions. This includes evaluating the sourcing of algae and the potential for marine ecosystem disruption.
As research in alginate-based drug delivery systems advances, regulatory frameworks continue to evolve. Agencies are developing new guidance documents to address emerging technologies, such as 3D-printed alginate scaffolds for controlled drug release. These regulations aim to balance innovation with patient safety, ensuring that novel alginate-based pharmaceuticals meet stringent quality and efficacy standards.
Compliance with these regulatory requirements is essential for companies developing alginate-based oral drug delivery systems. Failure to meet these standards can result in delays in product approval, market withdrawal, or legal consequences. Therefore, pharmaceutical companies must invest significant resources in regulatory affairs to navigate the complex landscape of alginate-based drug delivery regulations.
The FDA classifies sodium alginate as Generally Recognized as Safe (GRAS) for food applications, but its use in pharmaceuticals requires additional scrutiny. Manufacturers must demonstrate the safety and efficacy of alginate-based drug delivery systems through rigorous clinical trials and comprehensive documentation. This includes providing data on the sourcing, purity, and consistency of the sodium alginate used in the formulation.
In the European Union, the European Medicines Agency (EMA) regulates alginate-based pharmaceuticals. The EMA's guidelines on excipients require manufacturers to provide detailed information on the quality and safety of sodium alginate when used in oral drug delivery systems. This includes specifications on impurities, microbial limits, and potential interactions with active pharmaceutical ingredients.
Globally, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides guidelines that influence the regulatory approach to alginate-based pharmaceuticals. These guidelines address quality control, stability testing, and good manufacturing practices, which are essential for ensuring the consistency and reliability of alginate-based drug delivery systems.
Regulatory bodies also focus on the potential environmental impact of alginate production and disposal. Manufacturers must adhere to sustainability guidelines and provide environmental risk assessments as part of their regulatory submissions. This includes evaluating the sourcing of algae and the potential for marine ecosystem disruption.
As research in alginate-based drug delivery systems advances, regulatory frameworks continue to evolve. Agencies are developing new guidance documents to address emerging technologies, such as 3D-printed alginate scaffolds for controlled drug release. These regulations aim to balance innovation with patient safety, ensuring that novel alginate-based pharmaceuticals meet stringent quality and efficacy standards.
Compliance with these regulatory requirements is essential for companies developing alginate-based oral drug delivery systems. Failure to meet these standards can result in delays in product approval, market withdrawal, or legal consequences. Therefore, pharmaceutical companies must invest significant resources in regulatory affairs to navigate the complex landscape of alginate-based drug delivery regulations.
Biocompatibility and Safety Considerations of Sodium Alginate
Sodium alginate has gained significant attention in oral drug delivery systems due to its biocompatibility and safety profile. As a natural polysaccharide derived from brown seaweed, sodium alginate exhibits low toxicity and minimal immunogenicity, making it an attractive option for pharmaceutical applications. The biocompatibility of sodium alginate is attributed to its structural similarity to extracellular matrices, allowing for seamless integration with biological systems.
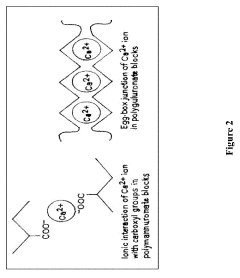
In oral drug delivery, sodium alginate's safety is further enhanced by its ability to form hydrogels in the presence of divalent cations, such as calcium ions found in the gastrointestinal tract. This gelation process creates a protective barrier around encapsulated drugs, shielding them from harsh gastric conditions and promoting controlled release. The resulting hydrogel matrix also demonstrates mucoadhesive properties, prolonging the residence time of drugs in the gastrointestinal tract and improving bioavailability.
Extensive in vitro and in vivo studies have consistently demonstrated the non-toxic nature of sodium alginate. Cytotoxicity assays on various cell lines have shown minimal adverse effects, even at high concentrations. Moreover, animal studies have reported no significant systemic toxicity or local irritation following oral administration of sodium alginate-based formulations. These findings support the safety profile of sodium alginate for long-term use in oral drug delivery systems.
The biodegradability of sodium alginate contributes to its safety profile, as it can be broken down by enzymes in the gastrointestinal tract. This property ensures that the polymer does not accumulate in the body, reducing the risk of long-term adverse effects. Additionally, the degradation products of sodium alginate are readily excreted, further minimizing potential toxicity concerns.
Regulatory agencies, including the FDA and EMA, have recognized the safety of sodium alginate for use in pharmaceutical and food applications. It is classified as Generally Recognized as Safe (GRAS) by the FDA, reflecting its long history of safe use. This regulatory acceptance facilitates the development and approval of sodium alginate-based oral drug delivery systems, streamlining the path to market for innovative formulations.
While sodium alginate demonstrates an excellent safety profile, considerations must be made for potential allergic reactions in individuals with sensitivities to algae or seaweed-derived products. However, such cases are rare, and proper screening can mitigate this risk. Furthermore, the interaction of sodium alginate with certain drugs or nutrients should be evaluated to ensure optimal therapeutic outcomes and avoid any unintended effects on drug absorption or bioavailability.
In conclusion, the biocompatibility and safety of sodium alginate make it an ideal candidate for oral drug delivery systems. Its natural origin, low toxicity, and biodegradability, combined with regulatory acceptance, position sodium alginate as a versatile and reliable excipient for developing advanced oral drug formulations. Ongoing research continues to explore novel applications and further validate the long-term safety of sodium alginate in diverse pharmaceutical contexts.
In oral drug delivery, sodium alginate's safety is further enhanced by its ability to form hydrogels in the presence of divalent cations, such as calcium ions found in the gastrointestinal tract. This gelation process creates a protective barrier around encapsulated drugs, shielding them from harsh gastric conditions and promoting controlled release. The resulting hydrogel matrix also demonstrates mucoadhesive properties, prolonging the residence time of drugs in the gastrointestinal tract and improving bioavailability.
Extensive in vitro and in vivo studies have consistently demonstrated the non-toxic nature of sodium alginate. Cytotoxicity assays on various cell lines have shown minimal adverse effects, even at high concentrations. Moreover, animal studies have reported no significant systemic toxicity or local irritation following oral administration of sodium alginate-based formulations. These findings support the safety profile of sodium alginate for long-term use in oral drug delivery systems.
The biodegradability of sodium alginate contributes to its safety profile, as it can be broken down by enzymes in the gastrointestinal tract. This property ensures that the polymer does not accumulate in the body, reducing the risk of long-term adverse effects. Additionally, the degradation products of sodium alginate are readily excreted, further minimizing potential toxicity concerns.
Regulatory agencies, including the FDA and EMA, have recognized the safety of sodium alginate for use in pharmaceutical and food applications. It is classified as Generally Recognized as Safe (GRAS) by the FDA, reflecting its long history of safe use. This regulatory acceptance facilitates the development and approval of sodium alginate-based oral drug delivery systems, streamlining the path to market for innovative formulations.
While sodium alginate demonstrates an excellent safety profile, considerations must be made for potential allergic reactions in individuals with sensitivities to algae or seaweed-derived products. However, such cases are rare, and proper screening can mitigate this risk. Furthermore, the interaction of sodium alginate with certain drugs or nutrients should be evaluated to ensure optimal therapeutic outcomes and avoid any unintended effects on drug absorption or bioavailability.
In conclusion, the biocompatibility and safety of sodium alginate make it an ideal candidate for oral drug delivery systems. Its natural origin, low toxicity, and biodegradability, combined with regulatory acceptance, position sodium alginate as a versatile and reliable excipient for developing advanced oral drug formulations. Ongoing research continues to explore novel applications and further validate the long-term safety of sodium alginate in diverse pharmaceutical contexts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!