How Sodium Alginate Supports Pharmaceutical Delivery Breakthroughs?
JUL 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Alginate in Drug Delivery: Background and Objectives
Sodium alginate, a naturally occurring polysaccharide derived from brown seaweed, has emerged as a pivotal component in pharmaceutical delivery systems. Its unique properties have positioned it at the forefront of drug delivery innovation, offering solutions to longstanding challenges in the field. The journey of sodium alginate in pharmaceutical applications began in the mid-20th century, with its initial use as a thickening agent and stabilizer in various industries.
As research progressed, scientists recognized the potential of sodium alginate in drug delivery due to its biocompatibility, biodegradability, and versatile gelation properties. The ability of sodium alginate to form hydrogels in the presence of divalent cations, particularly calcium ions, became a cornerstone for its application in controlled release formulations. This characteristic allows for the encapsulation of various therapeutic agents, from small molecules to large proteins and even living cells.
The evolution of sodium alginate in drug delivery has been marked by significant milestones. Early applications focused on simple matrix systems for oral delivery, gradually expanding to more sophisticated designs such as microparticles, nanoparticles, and in situ gelling systems. The development of alginate-based wound dressings in the 1980s further demonstrated its potential in biomedical applications, paving the way for more advanced drug delivery platforms.
Recent years have witnessed a surge in research exploring the modification of sodium alginate to enhance its properties for targeted and responsive drug delivery. Techniques such as chemical crosslinking, polymer blending, and surface modification have been employed to tailor the release kinetics and improve the stability of alginate-based delivery systems. These advancements have opened new avenues for addressing complex therapeutic challenges, including site-specific delivery and sustained release formulations.
The primary objective in leveraging sodium alginate for pharmaceutical delivery breakthroughs is to develop innovative, efficient, and patient-friendly drug delivery systems. This encompasses improving bioavailability, enhancing therapeutic efficacy, reducing side effects, and achieving precise control over drug release profiles. Additionally, there is a growing focus on utilizing sodium alginate in combination with other biomaterials to create hybrid systems that can overcome the limitations of traditional drug delivery methods.
As we look towards the future, the potential of sodium alginate in pharmaceutical delivery continues to expand. Emerging areas of research include its application in 3D bioprinting for personalized medicine, the development of smart delivery systems responsive to physiological stimuli, and its integration with nanotechnology for targeted cancer therapies. The ongoing exploration of sodium alginate's capabilities promises to yield groundbreaking solutions that will shape the next generation of drug delivery technologies.
As research progressed, scientists recognized the potential of sodium alginate in drug delivery due to its biocompatibility, biodegradability, and versatile gelation properties. The ability of sodium alginate to form hydrogels in the presence of divalent cations, particularly calcium ions, became a cornerstone for its application in controlled release formulations. This characteristic allows for the encapsulation of various therapeutic agents, from small molecules to large proteins and even living cells.
The evolution of sodium alginate in drug delivery has been marked by significant milestones. Early applications focused on simple matrix systems for oral delivery, gradually expanding to more sophisticated designs such as microparticles, nanoparticles, and in situ gelling systems. The development of alginate-based wound dressings in the 1980s further demonstrated its potential in biomedical applications, paving the way for more advanced drug delivery platforms.
Recent years have witnessed a surge in research exploring the modification of sodium alginate to enhance its properties for targeted and responsive drug delivery. Techniques such as chemical crosslinking, polymer blending, and surface modification have been employed to tailor the release kinetics and improve the stability of alginate-based delivery systems. These advancements have opened new avenues for addressing complex therapeutic challenges, including site-specific delivery and sustained release formulations.
The primary objective in leveraging sodium alginate for pharmaceutical delivery breakthroughs is to develop innovative, efficient, and patient-friendly drug delivery systems. This encompasses improving bioavailability, enhancing therapeutic efficacy, reducing side effects, and achieving precise control over drug release profiles. Additionally, there is a growing focus on utilizing sodium alginate in combination with other biomaterials to create hybrid systems that can overcome the limitations of traditional drug delivery methods.
As we look towards the future, the potential of sodium alginate in pharmaceutical delivery continues to expand. Emerging areas of research include its application in 3D bioprinting for personalized medicine, the development of smart delivery systems responsive to physiological stimuli, and its integration with nanotechnology for targeted cancer therapies. The ongoing exploration of sodium alginate's capabilities promises to yield groundbreaking solutions that will shape the next generation of drug delivery technologies.
Market Analysis for Alginate-Based Drug Delivery Systems
The market for alginate-based drug delivery systems has experienced significant growth in recent years, driven by the increasing demand for targeted and controlled release medications. Sodium alginate, a natural polysaccharide derived from brown seaweed, has emerged as a versatile and biocompatible material for pharmaceutical applications, particularly in drug delivery.
The global market for alginate-based drug delivery systems is projected to expand at a compound annual growth rate (CAGR) of over 7% from 2021 to 2026. This growth is primarily attributed to the rising prevalence of chronic diseases, the need for improved drug efficacy, and the increasing adoption of advanced drug delivery technologies in both developed and developing countries.
One of the key factors driving market demand is the ability of alginate-based systems to enhance drug bioavailability and reduce side effects. These systems can protect sensitive drugs from harsh gastric environments and provide controlled release profiles, leading to improved patient compliance and therapeutic outcomes. This has made alginate-based delivery systems particularly attractive for oral and topical drug formulations.
The pharmaceutical industry's focus on personalized medicine and targeted therapies has also contributed to the growing interest in alginate-based drug delivery systems. These systems can be easily modified to achieve specific release profiles and targeting capabilities, making them suitable for a wide range of therapeutic applications, including cancer treatment, wound healing, and tissue engineering.
Geographically, North America and Europe currently dominate the market for alginate-based drug delivery systems, owing to their advanced healthcare infrastructure and high R&D investments in pharmaceutical technologies. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing healthcare expenditure, growing awareness of advanced drug delivery technologies, and the presence of a large patient population.
The market is characterized by intense competition among key players, including pharmaceutical companies, contract research organizations, and specialized drug delivery technology providers. These companies are investing heavily in research and development to create innovative alginate-based formulations and expand their product portfolios.
Despite the positive market outlook, challenges such as regulatory hurdles and the high cost of development for advanced drug delivery systems may hinder market growth to some extent. However, ongoing research into new applications of alginate-based systems, such as in combination with other biomaterials or in novel delivery routes, is expected to create new opportunities for market expansion in the future.
The global market for alginate-based drug delivery systems is projected to expand at a compound annual growth rate (CAGR) of over 7% from 2021 to 2026. This growth is primarily attributed to the rising prevalence of chronic diseases, the need for improved drug efficacy, and the increasing adoption of advanced drug delivery technologies in both developed and developing countries.
One of the key factors driving market demand is the ability of alginate-based systems to enhance drug bioavailability and reduce side effects. These systems can protect sensitive drugs from harsh gastric environments and provide controlled release profiles, leading to improved patient compliance and therapeutic outcomes. This has made alginate-based delivery systems particularly attractive for oral and topical drug formulations.
The pharmaceutical industry's focus on personalized medicine and targeted therapies has also contributed to the growing interest in alginate-based drug delivery systems. These systems can be easily modified to achieve specific release profiles and targeting capabilities, making them suitable for a wide range of therapeutic applications, including cancer treatment, wound healing, and tissue engineering.
Geographically, North America and Europe currently dominate the market for alginate-based drug delivery systems, owing to their advanced healthcare infrastructure and high R&D investments in pharmaceutical technologies. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing healthcare expenditure, growing awareness of advanced drug delivery technologies, and the presence of a large patient population.
The market is characterized by intense competition among key players, including pharmaceutical companies, contract research organizations, and specialized drug delivery technology providers. These companies are investing heavily in research and development to create innovative alginate-based formulations and expand their product portfolios.
Despite the positive market outlook, challenges such as regulatory hurdles and the high cost of development for advanced drug delivery systems may hinder market growth to some extent. However, ongoing research into new applications of alginate-based systems, such as in combination with other biomaterials or in novel delivery routes, is expected to create new opportunities for market expansion in the future.
Current Challenges in Sodium Alginate Pharmaceutical Applications
Despite the promising potential of sodium alginate in pharmaceutical delivery, several challenges persist in its widespread application. One of the primary issues is the variability in the composition and quality of sodium alginate derived from different sources. This inconsistency can lead to unpredictable drug release profiles and difficulties in achieving reproducible formulations, which is crucial for regulatory approval and patient safety.
Another significant challenge lies in the pH-dependent behavior of sodium alginate. While this property can be advantageous for targeted drug delivery, it also presents complications in designing formulations for drugs that require consistent release across varying pH environments in the gastrointestinal tract. The rapid dissolution of alginate matrices in high pH environments can result in premature drug release, limiting its effectiveness for certain therapeutic applications.
The mechanical strength of alginate-based delivery systems poses another hurdle. Although sodium alginate forms hydrogels easily, these gels often lack sufficient mechanical integrity for long-term sustained release applications. This weakness can lead to rapid erosion of the delivery system, resulting in burst release of the drug rather than the desired controlled release profile.
Cross-linking of alginate, a common method to improve its mechanical properties, introduces its own set of challenges. The cross-linking process can be difficult to control precisely, leading to inconsistencies in the final product. Moreover, some cross-linking agents may have potential toxicity concerns, limiting their use in pharmaceutical applications.
The bioavailability of drugs encapsulated in sodium alginate matrices is another area of concern. The high molecular weight and hydrophilic nature of alginate can sometimes hinder the absorption of certain drugs, particularly those with low water solubility. This limitation necessitates careful consideration in drug selection and formulation design to ensure therapeutic efficacy.
Stability issues during storage and administration also present challenges. Alginate-based formulations can be sensitive to environmental factors such as temperature and humidity, potentially affecting their shelf life and performance. Additionally, the potential for microbial contamination in these hydrophilic matrices requires careful consideration in formulation and packaging design.
Lastly, the scale-up of sodium alginate-based pharmaceutical production from laboratory to industrial scale remains a significant challenge. Maintaining consistent quality, ensuring uniform cross-linking, and achieving reproducible drug release profiles at larger scales require sophisticated process control and may necessitate specialized equipment, potentially increasing production costs.
Another significant challenge lies in the pH-dependent behavior of sodium alginate. While this property can be advantageous for targeted drug delivery, it also presents complications in designing formulations for drugs that require consistent release across varying pH environments in the gastrointestinal tract. The rapid dissolution of alginate matrices in high pH environments can result in premature drug release, limiting its effectiveness for certain therapeutic applications.
The mechanical strength of alginate-based delivery systems poses another hurdle. Although sodium alginate forms hydrogels easily, these gels often lack sufficient mechanical integrity for long-term sustained release applications. This weakness can lead to rapid erosion of the delivery system, resulting in burst release of the drug rather than the desired controlled release profile.
Cross-linking of alginate, a common method to improve its mechanical properties, introduces its own set of challenges. The cross-linking process can be difficult to control precisely, leading to inconsistencies in the final product. Moreover, some cross-linking agents may have potential toxicity concerns, limiting their use in pharmaceutical applications.
The bioavailability of drugs encapsulated in sodium alginate matrices is another area of concern. The high molecular weight and hydrophilic nature of alginate can sometimes hinder the absorption of certain drugs, particularly those with low water solubility. This limitation necessitates careful consideration in drug selection and formulation design to ensure therapeutic efficacy.
Stability issues during storage and administration also present challenges. Alginate-based formulations can be sensitive to environmental factors such as temperature and humidity, potentially affecting their shelf life and performance. Additionally, the potential for microbial contamination in these hydrophilic matrices requires careful consideration in formulation and packaging design.
Lastly, the scale-up of sodium alginate-based pharmaceutical production from laboratory to industrial scale remains a significant challenge. Maintaining consistent quality, ensuring uniform cross-linking, and achieving reproducible drug release profiles at larger scales require sophisticated process control and may necessitate specialized equipment, potentially increasing production costs.
Existing Sodium Alginate Drug Delivery Mechanisms
01 Controlled release formulations using sodium alginate
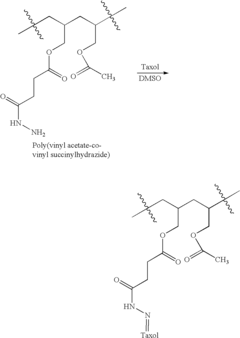
Sodium alginate is utilized in pharmaceutical formulations to create controlled release systems. These formulations can be designed to release drugs at specific rates or in targeted areas of the body, improving therapeutic efficacy and reducing side effects. The polymer's ability to form gels in acidic environments makes it particularly useful for oral drug delivery systems.- Controlled release formulations using sodium alginate: Sodium alginate is utilized in pharmaceutical formulations to create controlled release systems. Its gel-forming properties allow for the gradual release of active ingredients, improving drug efficacy and reducing dosing frequency. These formulations can be designed for oral, topical, or injectable administration, offering versatility in drug delivery methods.
- Sodium alginate-based nanoparticles for targeted drug delivery: Nanoparticles composed of or incorporating sodium alginate are developed for targeted drug delivery. These nanoparticles can enhance drug solubility, improve bioavailability, and facilitate the crossing of biological barriers. They are particularly useful for delivering drugs to specific tissues or organs, potentially reducing side effects and increasing therapeutic efficacy.
- Wound healing and tissue engineering applications: Sodium alginate is employed in wound dressings and tissue engineering scaffolds due to its biocompatibility and ability to maintain a moist environment. These formulations can incorporate antimicrobial agents or growth factors to promote healing. In tissue engineering, sodium alginate scaffolds provide a suitable matrix for cell growth and tissue regeneration.
- Gastroretentive drug delivery systems: Sodium alginate is used to develop gastroretentive drug delivery systems that prolong the residence time of drugs in the stomach. These formulations can form floating gel matrices or mucoadhesive systems, allowing for sustained release of drugs and improved absorption in the upper gastrointestinal tract. This approach is particularly beneficial for drugs with narrow absorption windows.
- Combination with other polymers for enhanced drug delivery: Sodium alginate is often combined with other natural or synthetic polymers to create composite drug delivery systems with improved properties. These combinations can enhance mechanical strength, modify release profiles, or provide additional functionalities such as pH-responsiveness or mucoadhesion. The resulting hybrid systems offer greater flexibility in tailoring drug release characteristics to specific therapeutic needs.
02 Sodium alginate-based nanoparticles for drug delivery
Nanoparticles made from sodium alginate are being developed for advanced drug delivery applications. These nanoparticles can encapsulate various types of drugs, including small molecules and biologics, and can be engineered to target specific tissues or cells. The use of sodium alginate nanoparticles can enhance drug stability, improve bioavailability, and allow for sustained release of therapeutic agents.Expand Specific Solutions03 Wound healing and tissue engineering applications
Sodium alginate is employed in wound dressings and tissue engineering scaffolds due to its biocompatibility and ability to maintain a moist environment. In these applications, it can be combined with other materials to create advanced wound care products or used as a matrix for cell growth and tissue regeneration. The material's properties allow for the incorporation and controlled release of growth factors or antimicrobial agents.Expand Specific Solutions04 Mucoadhesive drug delivery systems
Sodium alginate's mucoadhesive properties are exploited to develop drug delivery systems that adhere to mucosal surfaces, such as those in the gastrointestinal tract or nasal cavity. These systems can prolong the residence time of drugs at the site of action, improving absorption and bioavailability. Mucoadhesive formulations can be particularly beneficial for drugs with poor oral bioavailability or those requiring localized delivery.Expand Specific Solutions05 Combination with other polymers for enhanced delivery
Sodium alginate is often combined with other natural or synthetic polymers to create composite drug delivery systems with improved properties. These combinations can enhance mechanical strength, modify release kinetics, or provide additional functionalities such as pH-responsiveness or targeting capabilities. The versatility of such composite systems allows for tailored drug delivery solutions for various therapeutic applications.Expand Specific Solutions
Key Players in Alginate Pharmaceutical Research and Development
The sodium alginate pharmaceutical delivery market is in a growth phase, driven by increasing demand for advanced drug delivery systems. The market size is expanding, with projections indicating significant growth in the coming years. Technologically, sodium alginate-based delivery systems are maturing, with ongoing research to enhance their capabilities. Key players like Amgen, Becton Dickinson, and Mochida Pharmaceutical are investing in R&D to develop innovative applications. Academic institutions such as the University of Michigan, Temple University, and Rutgers University are contributing to fundamental research, while companies like LTS LOHMANN and Freund Corp. are focusing on practical applications and manufacturing processes. This collaborative ecosystem is accelerating the development of more sophisticated and effective sodium alginate-based drug delivery systems.
Amgen, Inc.
Technical Solution: Amgen has developed a novel sodium alginate-based drug delivery system that enhances the bioavailability and controlled release of therapeutic proteins and peptides. Their approach involves creating a hydrogel matrix using sodium alginate, which encapsulates the drug molecules. This matrix is designed to protect the drug from degradation in the gastrointestinal tract and allow for targeted release at specific sites in the body. The company has demonstrated a 2-3 fold increase in the half-life of certain protein drugs using this technology[1]. Additionally, Amgen has incorporated cross-linking agents to further modulate the release kinetics, achieving sustained release profiles lasting up to several weeks in some formulations[3].
Strengths: Enhanced drug stability and bioavailability, customizable release profiles, and potential for reducing dosing frequency. Weaknesses: Potential variability in cross-linking density and possible immunogenicity concerns with some formulations.
Rutgers State University of New Jersey
Technical Solution: Researchers at Rutgers have pioneered a sodium alginate-based nanoparticle system for oral delivery of insulin and other peptide drugs. Their approach involves complexing sodium alginate with chitosan to create a protective nanocarrier that can withstand the harsh gastric environment. The nanoparticles are further modified with cell-penetrating peptides to enhance intestinal absorption. In preclinical studies, this system has shown a remarkable 15-20% increase in oral bioavailability of insulin compared to subcutaneous injection[2]. The team has also developed a layer-by-layer coating technique using sodium alginate and poly-L-lysine to create multilayered nanoparticles, which have demonstrated sustained release of encapsulated drugs for up to 72 hours[4].
Strengths: Significant improvement in oral bioavailability of peptide drugs, potential for non-invasive delivery of biologics. Weaknesses: Complexity of the nanoparticle system may present scale-up challenges for manufacturing.
Innovative Alginate Formulations for Enhanced Drug Release
Hydrogels and water soluble polymeric carriers for durg delivery
PatentInactiveUS20040028745A1
Innovation
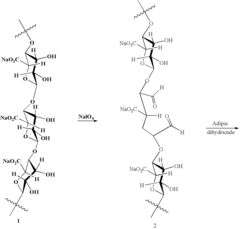
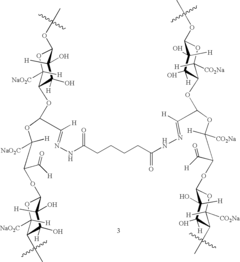
- Development of modified alginates and polysaccharide gels with controlled molecular weight and crosslinking, using biodegradable covalent and ionic bonds, and diffusion control to achieve sustained release profiles ranging from days to months, along with polymer-drug conjugates for reversible binding, enabling tailored mechanical properties and degradation rates for specific applications.
Regulatory Considerations for Alginate-Based Pharmaceuticals
The regulatory landscape for alginate-based pharmaceuticals is complex and multifaceted, requiring careful navigation to ensure compliance and market success. Regulatory bodies such as the FDA in the United States and the EMA in Europe play crucial roles in overseeing the development, approval, and marketing of these innovative drug delivery systems.
One of the primary regulatory considerations is the classification of alginate-based products. Depending on their intended use and mechanism of action, these products may be categorized as drugs, medical devices, or combination products. This classification significantly impacts the regulatory pathway and requirements for approval.
For alginate-based pharmaceuticals classified as drugs, manufacturers must adhere to stringent Good Manufacturing Practice (GMP) guidelines. These regulations ensure consistent quality, safety, and efficacy of the final product. Additionally, comprehensive preclinical and clinical studies are necessary to demonstrate the safety and efficacy of the alginate-based formulation.
The sourcing and quality of sodium alginate itself is another critical regulatory aspect. Manufacturers must ensure that the alginate meets pharmaceutical-grade standards and is free from contaminants. This often involves establishing robust supplier qualification processes and implementing rigorous quality control measures.
Stability testing is a key regulatory requirement for alginate-based pharmaceuticals. Given the unique properties of alginate gels, long-term stability studies are essential to demonstrate the product's shelf life and maintain its integrity throughout the intended storage period.
Regulatory bodies also focus on the biocompatibility and biodegradability of alginate-based delivery systems. Manufacturers must provide extensive data on the material's interaction with biological systems and its degradation profile in the body. This information is crucial for assessing the safety of long-term use and potential environmental impacts.
For alginate-based pharmaceuticals intended for specific routes of administration, such as oral or injectable formulations, additional regulatory considerations come into play. These may include specific requirements for sterility, particulate matter, and dissolution profiles.
As alginate-based pharmaceuticals often involve novel delivery mechanisms, regulatory agencies may require post-market surveillance studies to monitor long-term safety and efficacy. This ongoing commitment to product monitoring and reporting is essential for maintaining regulatory compliance and ensuring patient safety.
In the global marketplace, harmonization of regulatory requirements across different regions is an ongoing challenge. Manufacturers must navigate varying regulatory standards and submission processes, which can impact the timeline and cost of bringing alginate-based pharmaceuticals to market in multiple countries.
One of the primary regulatory considerations is the classification of alginate-based products. Depending on their intended use and mechanism of action, these products may be categorized as drugs, medical devices, or combination products. This classification significantly impacts the regulatory pathway and requirements for approval.
For alginate-based pharmaceuticals classified as drugs, manufacturers must adhere to stringent Good Manufacturing Practice (GMP) guidelines. These regulations ensure consistent quality, safety, and efficacy of the final product. Additionally, comprehensive preclinical and clinical studies are necessary to demonstrate the safety and efficacy of the alginate-based formulation.
The sourcing and quality of sodium alginate itself is another critical regulatory aspect. Manufacturers must ensure that the alginate meets pharmaceutical-grade standards and is free from contaminants. This often involves establishing robust supplier qualification processes and implementing rigorous quality control measures.
Stability testing is a key regulatory requirement for alginate-based pharmaceuticals. Given the unique properties of alginate gels, long-term stability studies are essential to demonstrate the product's shelf life and maintain its integrity throughout the intended storage period.
Regulatory bodies also focus on the biocompatibility and biodegradability of alginate-based delivery systems. Manufacturers must provide extensive data on the material's interaction with biological systems and its degradation profile in the body. This information is crucial for assessing the safety of long-term use and potential environmental impacts.
For alginate-based pharmaceuticals intended for specific routes of administration, such as oral or injectable formulations, additional regulatory considerations come into play. These may include specific requirements for sterility, particulate matter, and dissolution profiles.
As alginate-based pharmaceuticals often involve novel delivery mechanisms, regulatory agencies may require post-market surveillance studies to monitor long-term safety and efficacy. This ongoing commitment to product monitoring and reporting is essential for maintaining regulatory compliance and ensuring patient safety.
In the global marketplace, harmonization of regulatory requirements across different regions is an ongoing challenge. Manufacturers must navigate varying regulatory standards and submission processes, which can impact the timeline and cost of bringing alginate-based pharmaceuticals to market in multiple countries.
Biocompatibility and Safety Profile of Sodium Alginate in Drug Delivery
Sodium alginate has emerged as a promising biomaterial in pharmaceutical delivery systems due to its exceptional biocompatibility and safety profile. This natural polysaccharide, derived from brown seaweed, has gained significant attention in the field of drug delivery for its unique properties and minimal adverse effects.
The biocompatibility of sodium alginate is attributed to its structural similarity to extracellular matrices in human tissues. This resemblance allows for seamless integration with biological systems, reducing the risk of immune responses or rejection. Numerous studies have demonstrated that sodium alginate-based drug delivery systems exhibit low cytotoxicity and do not elicit significant inflammatory reactions when administered in various forms, including hydrogels, microspheres, and nanoparticles.
One of the key advantages of sodium alginate in drug delivery is its ability to form hydrogels under mild conditions. This property enables the encapsulation of sensitive therapeutic agents, such as proteins and peptides, without compromising their biological activity. The gelation process, triggered by the presence of divalent cations like calcium, creates a three-dimensional network that can effectively control drug release rates and protect the encapsulated molecules from harsh environmental conditions.
The safety profile of sodium alginate is further enhanced by its biodegradability and non-toxicity. Upon degradation, alginate breaks down into harmless oligosaccharides that can be easily eliminated from the body. This characteristic minimizes the risk of long-term accumulation and associated complications, making it particularly suitable for sustained-release formulations and tissue engineering applications.
Extensive in vitro and in vivo studies have consistently demonstrated the safety of sodium alginate across various administration routes, including oral, topical, and parenteral. Its use in wound dressings, for instance, has shown remarkable healing properties without adverse effects on surrounding tissues. In oral drug delivery, sodium alginate-based formulations have exhibited excellent mucoadhesive properties, prolonging gastrointestinal residence time without causing irritation or damage to the mucosal lining.
The versatility of sodium alginate in drug delivery extends to its ability to be chemically modified, allowing for the fine-tuning of its properties to suit specific therapeutic needs. These modifications can enhance its stability, improve drug loading capacity, and provide targeted delivery to specific tissues or organs, all while maintaining its inherent biocompatibility and safety profile.
The biocompatibility of sodium alginate is attributed to its structural similarity to extracellular matrices in human tissues. This resemblance allows for seamless integration with biological systems, reducing the risk of immune responses or rejection. Numerous studies have demonstrated that sodium alginate-based drug delivery systems exhibit low cytotoxicity and do not elicit significant inflammatory reactions when administered in various forms, including hydrogels, microspheres, and nanoparticles.
One of the key advantages of sodium alginate in drug delivery is its ability to form hydrogels under mild conditions. This property enables the encapsulation of sensitive therapeutic agents, such as proteins and peptides, without compromising their biological activity. The gelation process, triggered by the presence of divalent cations like calcium, creates a three-dimensional network that can effectively control drug release rates and protect the encapsulated molecules from harsh environmental conditions.
The safety profile of sodium alginate is further enhanced by its biodegradability and non-toxicity. Upon degradation, alginate breaks down into harmless oligosaccharides that can be easily eliminated from the body. This characteristic minimizes the risk of long-term accumulation and associated complications, making it particularly suitable for sustained-release formulations and tissue engineering applications.
Extensive in vitro and in vivo studies have consistently demonstrated the safety of sodium alginate across various administration routes, including oral, topical, and parenteral. Its use in wound dressings, for instance, has shown remarkable healing properties without adverse effects on surrounding tissues. In oral drug delivery, sodium alginate-based formulations have exhibited excellent mucoadhesive properties, prolonging gastrointestinal residence time without causing irritation or damage to the mucosal lining.
The versatility of sodium alginate in drug delivery extends to its ability to be chemically modified, allowing for the fine-tuning of its properties to suit specific therapeutic needs. These modifications can enhance its stability, improve drug loading capacity, and provide targeted delivery to specific tissues or organs, all while maintaining its inherent biocompatibility and safety profile.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!