How Sodium Alginate Enhances Sophisticated Drug Delivery Systems?
JUL 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Alginate in Drug Delivery: Background and Objectives
Sodium alginate has emerged as a pivotal component in the evolution of sophisticated drug delivery systems. This natural polysaccharide, derived from brown seaweed, has garnered significant attention in the pharmaceutical industry due to its unique properties and versatile applications. The journey of sodium alginate in drug delivery can be traced back to the mid-20th century, with its potential recognized in the 1950s for controlled release formulations.
Over the decades, the utilization of sodium alginate has expanded dramatically, driven by advancements in polymer science and a growing understanding of its molecular structure. Its ability to form hydrogels, coupled with its biocompatibility and biodegradability, has positioned it as a cornerstone material in modern drug delivery research. The evolution of sodium alginate-based systems has paralleled the broader trends in pharmaceutical technology, moving from simple matrix tablets to complex, multi-functional delivery platforms.
The primary objective in leveraging sodium alginate for drug delivery is to enhance therapeutic efficacy while minimizing side effects. This is achieved through controlled release mechanisms, targeted delivery, and improved drug stability. Researchers aim to exploit the polymer's responsive behavior to environmental stimuli such as pH, temperature, and ionic strength, enabling smart drug release profiles tailored to specific physiological conditions.
Another critical goal is to develop sodium alginate-based carriers capable of delivering a wide range of therapeutic agents, from small molecule drugs to large biologics and even gene therapies. This versatility is crucial in addressing the diverse challenges posed by different disease states and routes of administration. The ongoing research also focuses on improving the mechanical properties and stability of alginate-based formulations to ensure consistent performance in various physiological environments.
As the field progresses, there is a growing emphasis on combining sodium alginate with other polymers and nanomaterials to create hybrid systems with enhanced functionalities. These composite materials aim to overcome some of the limitations of pure alginate systems, such as rapid erosion in certain pH conditions or limited control over release kinetics. The integration of sodium alginate into advanced drug delivery platforms aligns with the broader trends in personalized medicine and targeted therapies, promising more effective and patient-friendly treatment options in the future.
Over the decades, the utilization of sodium alginate has expanded dramatically, driven by advancements in polymer science and a growing understanding of its molecular structure. Its ability to form hydrogels, coupled with its biocompatibility and biodegradability, has positioned it as a cornerstone material in modern drug delivery research. The evolution of sodium alginate-based systems has paralleled the broader trends in pharmaceutical technology, moving from simple matrix tablets to complex, multi-functional delivery platforms.
The primary objective in leveraging sodium alginate for drug delivery is to enhance therapeutic efficacy while minimizing side effects. This is achieved through controlled release mechanisms, targeted delivery, and improved drug stability. Researchers aim to exploit the polymer's responsive behavior to environmental stimuli such as pH, temperature, and ionic strength, enabling smart drug release profiles tailored to specific physiological conditions.
Another critical goal is to develop sodium alginate-based carriers capable of delivering a wide range of therapeutic agents, from small molecule drugs to large biologics and even gene therapies. This versatility is crucial in addressing the diverse challenges posed by different disease states and routes of administration. The ongoing research also focuses on improving the mechanical properties and stability of alginate-based formulations to ensure consistent performance in various physiological environments.
As the field progresses, there is a growing emphasis on combining sodium alginate with other polymers and nanomaterials to create hybrid systems with enhanced functionalities. These composite materials aim to overcome some of the limitations of pure alginate systems, such as rapid erosion in certain pH conditions or limited control over release kinetics. The integration of sodium alginate into advanced drug delivery platforms aligns with the broader trends in personalized medicine and targeted therapies, promising more effective and patient-friendly treatment options in the future.
Market Analysis for Advanced Drug Delivery Systems
The advanced drug delivery systems market has been experiencing significant growth, driven by the increasing demand for targeted and controlled release of therapeutic agents. This market segment is characterized by sophisticated technologies that enhance the efficacy and safety of drug administration, with sodium alginate emerging as a key player in this field.
The global market for advanced drug delivery systems is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) outpacing many other pharmaceutical sectors. This growth is fueled by several factors, including the rising prevalence of chronic diseases, the need for improved patient compliance, and the push for more effective treatments with fewer side effects.
Sodium alginate, a natural polysaccharide derived from brown seaweed, has gained considerable attention in the pharmaceutical industry due to its unique properties. Its ability to form hydrogels and its biocompatibility make it an ideal candidate for various drug delivery applications. The market for sodium alginate-based drug delivery systems is expected to grow rapidly, as it addresses many of the challenges faced in conventional drug delivery methods.
One of the key drivers for the adoption of sodium alginate in advanced drug delivery systems is its versatility. It can be used in various formulations, including microspheres, nanoparticles, and hydrogels, allowing for a wide range of applications across different therapeutic areas. This versatility has led to increased research and development activities, further propelling market growth.
The pharmaceutical industry's focus on personalized medicine and targeted therapies has also contributed to the rising demand for advanced drug delivery systems. Sodium alginate's ability to be modified and combined with other polymers enables the creation of tailored drug delivery platforms that can target specific tissues or release drugs in response to environmental stimuli.
Geographically, North America and Europe currently dominate the market for advanced drug delivery systems, including those utilizing sodium alginate. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing healthcare expenditure, growing awareness of advanced therapies, and improving regulatory frameworks.
Despite the promising outlook, the market faces challenges such as high development costs and stringent regulatory requirements. However, the potential benefits of sodium alginate-enhanced drug delivery systems, including improved drug efficacy, reduced side effects, and better patient outcomes, continue to drive investment and innovation in this field.
As the healthcare industry increasingly focuses on patient-centric approaches and value-based care, the demand for advanced drug delivery systems is expected to grow further. Sodium alginate's role in enhancing these systems positions it as a critical component in the future of drug delivery technology, with significant market potential in the years to come.
The global market for advanced drug delivery systems is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) outpacing many other pharmaceutical sectors. This growth is fueled by several factors, including the rising prevalence of chronic diseases, the need for improved patient compliance, and the push for more effective treatments with fewer side effects.
Sodium alginate, a natural polysaccharide derived from brown seaweed, has gained considerable attention in the pharmaceutical industry due to its unique properties. Its ability to form hydrogels and its biocompatibility make it an ideal candidate for various drug delivery applications. The market for sodium alginate-based drug delivery systems is expected to grow rapidly, as it addresses many of the challenges faced in conventional drug delivery methods.
One of the key drivers for the adoption of sodium alginate in advanced drug delivery systems is its versatility. It can be used in various formulations, including microspheres, nanoparticles, and hydrogels, allowing for a wide range of applications across different therapeutic areas. This versatility has led to increased research and development activities, further propelling market growth.
The pharmaceutical industry's focus on personalized medicine and targeted therapies has also contributed to the rising demand for advanced drug delivery systems. Sodium alginate's ability to be modified and combined with other polymers enables the creation of tailored drug delivery platforms that can target specific tissues or release drugs in response to environmental stimuli.
Geographically, North America and Europe currently dominate the market for advanced drug delivery systems, including those utilizing sodium alginate. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing healthcare expenditure, growing awareness of advanced therapies, and improving regulatory frameworks.
Despite the promising outlook, the market faces challenges such as high development costs and stringent regulatory requirements. However, the potential benefits of sodium alginate-enhanced drug delivery systems, including improved drug efficacy, reduced side effects, and better patient outcomes, continue to drive investment and innovation in this field.
As the healthcare industry increasingly focuses on patient-centric approaches and value-based care, the demand for advanced drug delivery systems is expected to grow further. Sodium alginate's role in enhancing these systems positions it as a critical component in the future of drug delivery technology, with significant market potential in the years to come.
Current Challenges in Sodium Alginate-based Drug Delivery
Despite the promising potential of sodium alginate in drug delivery systems, several challenges persist in its application. One of the primary issues is the variability in the composition and quality of sodium alginate derived from natural sources. This inconsistency can lead to unpredictable drug release profiles and difficulties in achieving reproducible results across different batches.
Another significant challenge is the rapid dissolution of sodium alginate in physiological fluids, which can result in premature drug release. This characteristic limits its effectiveness in sustained-release formulations, particularly for oral drug delivery systems where prolonged gastric retention is desired. Researchers are actively exploring various crosslinking methods and modifications to enhance the stability of alginate matrices, but achieving an optimal balance between stability and drug release kinetics remains a complex task.
The pH-dependent behavior of sodium alginate presents both opportunities and challenges. While it allows for targeted drug delivery in specific pH environments, it also complicates the design of delivery systems intended for multiple pH zones along the gastrointestinal tract. Developing formulations that can maintain their integrity across diverse pH ranges without compromising drug release efficiency is an ongoing area of research.
Encapsulation efficiency and drug loading capacity are additional concerns in sodium alginate-based systems. The hydrophilic nature of alginate can limit its ability to effectively encapsulate hydrophobic drugs, necessitating the development of composite systems or chemical modifications to improve drug-polymer interactions. Moreover, achieving high drug loading without compromising the structural integrity of the alginate matrix remains a delicate balance.
The scale-up of sodium alginate-based drug delivery systems from laboratory to industrial production poses significant challenges. Maintaining consistent quality, particle size distribution, and drug encapsulation efficiency during large-scale manufacturing requires careful process optimization and quality control measures. The development of standardized production protocols that can accommodate the inherent variability of natural alginate sources is crucial for commercial viability.
Regulatory considerations also present hurdles in the widespread adoption of sodium alginate-based drug delivery systems. Demonstrating the safety, efficacy, and consistency of these formulations to regulatory bodies requires extensive documentation and clinical trials. The natural origin of alginate, while advantageous in terms of biocompatibility, can introduce complexities in meeting stringent regulatory requirements for pharmaceutical products.
Another significant challenge is the rapid dissolution of sodium alginate in physiological fluids, which can result in premature drug release. This characteristic limits its effectiveness in sustained-release formulations, particularly for oral drug delivery systems where prolonged gastric retention is desired. Researchers are actively exploring various crosslinking methods and modifications to enhance the stability of alginate matrices, but achieving an optimal balance between stability and drug release kinetics remains a complex task.
The pH-dependent behavior of sodium alginate presents both opportunities and challenges. While it allows for targeted drug delivery in specific pH environments, it also complicates the design of delivery systems intended for multiple pH zones along the gastrointestinal tract. Developing formulations that can maintain their integrity across diverse pH ranges without compromising drug release efficiency is an ongoing area of research.
Encapsulation efficiency and drug loading capacity are additional concerns in sodium alginate-based systems. The hydrophilic nature of alginate can limit its ability to effectively encapsulate hydrophobic drugs, necessitating the development of composite systems or chemical modifications to improve drug-polymer interactions. Moreover, achieving high drug loading without compromising the structural integrity of the alginate matrix remains a delicate balance.
The scale-up of sodium alginate-based drug delivery systems from laboratory to industrial production poses significant challenges. Maintaining consistent quality, particle size distribution, and drug encapsulation efficiency during large-scale manufacturing requires careful process optimization and quality control measures. The development of standardized production protocols that can accommodate the inherent variability of natural alginate sources is crucial for commercial viability.
Regulatory considerations also present hurdles in the widespread adoption of sodium alginate-based drug delivery systems. Demonstrating the safety, efficacy, and consistency of these formulations to regulatory bodies requires extensive documentation and clinical trials. The natural origin of alginate, while advantageous in terms of biocompatibility, can introduce complexities in meeting stringent regulatory requirements for pharmaceutical products.
Existing Sodium Alginate Drug Delivery Mechanisms
01 Sodium alginate as a thickening and stabilizing agent
Sodium alginate is widely used in various formulations as a thickening and stabilizing agent. It can improve the viscosity and stability of products, enhancing their texture and shelf life. This property makes it particularly useful in cosmetic, pharmaceutical, and food applications.- Sodium alginate as a thickening and stabilizing agent: Sodium alginate is widely used in various formulations as a thickening and stabilizing agent. It can improve the texture, viscosity, and stability of products such as cosmetics, pharmaceuticals, and food items. Its ability to form gels and emulsions makes it valuable for enhancing the consistency and shelf life of different preparations.
- Sodium alginate in drug delivery systems: Sodium alginate is utilized in advanced drug delivery systems due to its biocompatibility and ability to form hydrogels. It can be used to encapsulate drugs, control their release, and improve their bioavailability. This application is particularly useful in developing sustained-release formulations and targeted drug delivery systems.
- Sodium alginate in wound healing and tissue engineering: The biocompatibility and gel-forming properties of sodium alginate make it suitable for wound healing applications and tissue engineering. It can be used to create wound dressings that maintain a moist environment, absorb exudates, and promote healing. In tissue engineering, it serves as a scaffold material for cell growth and tissue regeneration.
- Sodium alginate in food and beverage applications: In the food industry, sodium alginate is used as a thickener, emulsifier, and stabilizer. It can improve the texture of various food products, act as a gelling agent, and enhance the stability of emulsions. Its applications range from dairy products to beverages and processed foods, contributing to improved consistency and mouthfeel.
- Sodium alginate in environmental applications: Sodium alginate has potential applications in environmental remediation and water treatment. It can be used to create adsorbents for removing heavy metals and other pollutants from water. Additionally, it has been explored for its potential in creating biodegradable materials and packaging, contributing to more sustainable product development.
02 Sodium alginate in drug delivery systems
Sodium alginate is utilized in advanced drug delivery systems due to its biocompatibility and ability to form gels. It can be used to encapsulate drugs, control their release, and improve their efficacy. This application is particularly beneficial in targeted drug delivery and sustained release formulations.Expand Specific Solutions03 Sodium alginate in wound healing and tissue engineering
The biocompatibility and gel-forming properties of sodium alginate make it valuable in wound healing applications and tissue engineering. It can be used to create scaffolds for cell growth, promote tissue regeneration, and provide a moist environment for wound healing.Expand Specific Solutions04 Sodium alginate in food preservation and packaging
Sodium alginate is used in food preservation and packaging due to its film-forming abilities and natural origin. It can create edible coatings that extend the shelf life of fruits and vegetables, and can be used in biodegradable packaging materials, contributing to sustainable food packaging solutions.Expand Specific Solutions05 Sodium alginate in wastewater treatment
Sodium alginate has applications in wastewater treatment processes. It can be used as a flocculant to remove contaminants from water, and as a biosorbent for heavy metal removal. Its biodegradability makes it an environmentally friendly option for water purification technologies.Expand Specific Solutions
Key Players in Sodium Alginate Drug Delivery Research
The sodium alginate-enhanced drug delivery systems market is in a growth phase, driven by increasing demand for sophisticated drug delivery methods. The market size is expanding due to rising applications in pharmaceutical and biomedical fields. Technologically, the field is advancing rapidly, with companies like LTS LOHMANN Therapie-Systeme AG and Amgen, Inc. leading innovation in transdermal patches and biopharmaceuticals. Academic institutions such as the University of Michigan and Zhejiang University are contributing significant research. The technology's maturity is progressing, with established players like Becton, Dickinson & Co. and Novo Nordisk A/S incorporating sodium alginate in their product development, indicating a trend towards commercialization and widespread adoption in the pharmaceutical industry.
Amgen, Inc.
Technical Solution: Amgen has developed a sophisticated drug delivery system utilizing sodium alginate as a key component. Their approach involves encapsulating therapeutic proteins or peptides within sodium alginate-based microspheres. These microspheres are designed to protect the drug from degradation in the gastrointestinal tract and provide controlled release over time. The company has implemented a cross-linking process using calcium ions to enhance the stability of the alginate matrix, resulting in improved drug retention and release kinetics[1]. Additionally, Amgen has explored the use of alginate-based hydrogels for localized delivery of growth factors in tissue engineering applications, demonstrating the versatility of their sodium alginate-enhanced delivery systems[3].
Strengths: Improved drug stability, controlled release profiles, and versatility in various therapeutic applications. Weaknesses: Potential variability in cross-linking density and release rates depending on environmental conditions.
Becton, Dickinson & Co.
Technical Solution: Becton, Dickinson & Co. (BD) has developed advanced drug delivery systems incorporating sodium alginate, particularly focusing on parenteral and transdermal applications. Their technology utilizes alginate-based microparticles and nanoparticles for controlled release of various therapeutic agents. BD has engineered a novel crosslinking method that allows for fine-tuning of the alginate matrix porosity, enabling precise control over drug release kinetics[12]. The company has also developed alginate-based hydrogels for transdermal drug delivery, incorporating iontophoretic technology to enhance the penetration of charged drug molecules through the skin[13]. Additionally, BD has explored the use of alginate-coated microneedles for painless and efficient delivery of vaccines and other biologics[14]. Recent research has focused on combining alginate with synthetic polymers to create hybrid systems with improved mechanical properties and drug loading capacity[15].
Strengths: Versatile applications in parenteral and transdermal delivery, precise control over drug release, and potential for painless vaccine delivery. Weaknesses: Complexity in manufacturing processes and potential variability in drug release profiles across different patient populations.
Innovative Sodium Alginate Formulations and Patents
Hydrogels and water soluble polymeric carriers for durg delivery
PatentInactiveUS20040028745A1
Innovation
- Development of modified alginates and polysaccharide gels with controlled molecular weight and crosslinking, using biodegradable covalent and ionic bonds, and diffusion control to achieve sustained release profiles ranging from days to months, along with polymer-drug conjugates for reversible binding, enabling tailored mechanical properties and degradation rates for specific applications.
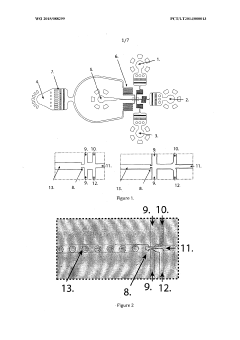
Method for production of biopolymer-based droplets and particles in a microfluidic system
PatentWO2015088299A1
Innovation
- A microfluidic device with three inlets and a specially designed cross-junction facilitates the production of monodisperse alginate particles of sizes similar to mammalian cells, using a flow-focusing junction and surfactants to stabilize droplets, allowing for controlled polymerization and release of biochemical compounds.
Regulatory Considerations for Alginate-based Drug Systems
The regulatory landscape for alginate-based drug delivery systems is complex and multifaceted, requiring careful consideration throughout the development and approval process. Regulatory bodies, such as the FDA in the United States and the EMA in Europe, have established specific guidelines for the evaluation of these novel drug delivery systems.
One of the primary regulatory considerations is the classification of alginate-based drug delivery systems. Depending on their specific characteristics and intended use, these systems may be categorized as drugs, medical devices, or combination products. This classification significantly impacts the regulatory pathway and requirements for approval.
Safety and biocompatibility are paramount concerns for regulatory agencies. Manufacturers must provide comprehensive data demonstrating the safety profile of alginate-based systems, including potential immunogenicity, toxicity, and long-term effects. This often involves extensive in vitro and in vivo studies to assess the biological interactions of the alginate material with the human body.
Efficacy and performance claims are subject to rigorous scrutiny. Regulatory bodies require robust clinical data to support the intended therapeutic benefits of alginate-based drug delivery systems. This includes demonstrating consistent drug release profiles, targeted delivery capabilities, and improved bioavailability compared to conventional formulations.
Quality control and manufacturing processes are critical aspects of regulatory compliance. Manufacturers must adhere to Good Manufacturing Practices (GMP) and implement stringent quality management systems. This includes establishing validated production methods, ensuring batch-to-batch consistency, and maintaining detailed documentation throughout the manufacturing process.
Stability testing is another crucial regulatory requirement. Manufacturers must provide data on the shelf life and storage conditions of alginate-based drug delivery systems, ensuring that the product maintains its integrity and efficacy over time. This often involves accelerated stability studies and real-time stability monitoring.
Environmental impact and disposal considerations are increasingly important in the regulatory landscape. Manufacturers may need to address the biodegradability and potential environmental effects of alginate-based systems, particularly for those designed for long-term use or implantation.
As alginate-based drug delivery systems often incorporate nanotechnology, additional regulatory guidelines specific to nanomaterials may apply. This includes addressing potential unique toxicological properties and biodistribution patterns associated with nanoparticle formulations.
Regulatory agencies also emphasize the importance of post-market surveillance for alginate-based drug delivery systems. Manufacturers are required to implement robust pharmacovigilance programs to monitor and report any adverse events or unexpected effects associated with these novel delivery systems in real-world clinical settings.
One of the primary regulatory considerations is the classification of alginate-based drug delivery systems. Depending on their specific characteristics and intended use, these systems may be categorized as drugs, medical devices, or combination products. This classification significantly impacts the regulatory pathway and requirements for approval.
Safety and biocompatibility are paramount concerns for regulatory agencies. Manufacturers must provide comprehensive data demonstrating the safety profile of alginate-based systems, including potential immunogenicity, toxicity, and long-term effects. This often involves extensive in vitro and in vivo studies to assess the biological interactions of the alginate material with the human body.
Efficacy and performance claims are subject to rigorous scrutiny. Regulatory bodies require robust clinical data to support the intended therapeutic benefits of alginate-based drug delivery systems. This includes demonstrating consistent drug release profiles, targeted delivery capabilities, and improved bioavailability compared to conventional formulations.
Quality control and manufacturing processes are critical aspects of regulatory compliance. Manufacturers must adhere to Good Manufacturing Practices (GMP) and implement stringent quality management systems. This includes establishing validated production methods, ensuring batch-to-batch consistency, and maintaining detailed documentation throughout the manufacturing process.
Stability testing is another crucial regulatory requirement. Manufacturers must provide data on the shelf life and storage conditions of alginate-based drug delivery systems, ensuring that the product maintains its integrity and efficacy over time. This often involves accelerated stability studies and real-time stability monitoring.
Environmental impact and disposal considerations are increasingly important in the regulatory landscape. Manufacturers may need to address the biodegradability and potential environmental effects of alginate-based systems, particularly for those designed for long-term use or implantation.
As alginate-based drug delivery systems often incorporate nanotechnology, additional regulatory guidelines specific to nanomaterials may apply. This includes addressing potential unique toxicological properties and biodistribution patterns associated with nanoparticle formulations.
Regulatory agencies also emphasize the importance of post-market surveillance for alginate-based drug delivery systems. Manufacturers are required to implement robust pharmacovigilance programs to monitor and report any adverse events or unexpected effects associated with these novel delivery systems in real-world clinical settings.
Biocompatibility and Safety of Sodium Alginate Carriers
Sodium alginate has emerged as a promising material for sophisticated drug delivery systems due to its exceptional biocompatibility and safety profile. This natural polysaccharide, derived from brown seaweed, has been extensively studied for its potential in pharmaceutical applications, particularly in controlled release formulations.
The biocompatibility of sodium alginate is attributed to its structural similarity to extracellular matrices in human tissues. This resemblance allows for seamless integration with biological systems, minimizing the risk of adverse reactions or immune responses. Numerous in vitro and in vivo studies have demonstrated the non-toxic nature of sodium alginate, further solidifying its position as a safe carrier for drug delivery.
One of the key advantages of sodium alginate in drug delivery systems is its ability to form hydrogels under mild conditions. This property enables the encapsulation of sensitive therapeutic agents, such as proteins and peptides, without compromising their biological activity. The gelation process, triggered by the presence of divalent cations like calcium, results in a three-dimensional network that can effectively control drug release rates.
The safety profile of sodium alginate is further enhanced by its biodegradability. As the polymer breaks down in the body, it is metabolized and excreted without accumulating in tissues or organs. This characteristic is particularly important for long-term drug delivery applications, where the potential for carrier-induced toxicity must be minimized.
In terms of regulatory approval, sodium alginate has gained recognition from various health authorities. The U.S. Food and Drug Administration (FDA) has classified sodium alginate as Generally Recognized as Safe (GRAS), facilitating its use in pharmaceutical formulations. This regulatory status streamlines the development process for new drug delivery systems incorporating sodium alginate.
Recent advancements in nanotechnology have expanded the potential applications of sodium alginate in drug delivery. Nanoparticles and nanofibers composed of sodium alginate have shown promising results in targeted drug delivery, particularly in cancer therapeutics. These nanostructures can be engineered to respond to specific physiological conditions, such as pH changes or enzymatic activity, enabling precise control over drug release at the target site.
While the biocompatibility and safety of sodium alginate are well-established, ongoing research continues to explore its long-term effects and potential interactions with various drug molecules. As the complexity of drug delivery systems increases, understanding the nuanced behavior of sodium alginate in different physiological environments remains a priority for researchers and pharmaceutical developers.
The biocompatibility of sodium alginate is attributed to its structural similarity to extracellular matrices in human tissues. This resemblance allows for seamless integration with biological systems, minimizing the risk of adverse reactions or immune responses. Numerous in vitro and in vivo studies have demonstrated the non-toxic nature of sodium alginate, further solidifying its position as a safe carrier for drug delivery.
One of the key advantages of sodium alginate in drug delivery systems is its ability to form hydrogels under mild conditions. This property enables the encapsulation of sensitive therapeutic agents, such as proteins and peptides, without compromising their biological activity. The gelation process, triggered by the presence of divalent cations like calcium, results in a three-dimensional network that can effectively control drug release rates.
The safety profile of sodium alginate is further enhanced by its biodegradability. As the polymer breaks down in the body, it is metabolized and excreted without accumulating in tissues or organs. This characteristic is particularly important for long-term drug delivery applications, where the potential for carrier-induced toxicity must be minimized.
In terms of regulatory approval, sodium alginate has gained recognition from various health authorities. The U.S. Food and Drug Administration (FDA) has classified sodium alginate as Generally Recognized as Safe (GRAS), facilitating its use in pharmaceutical formulations. This regulatory status streamlines the development process for new drug delivery systems incorporating sodium alginate.
Recent advancements in nanotechnology have expanded the potential applications of sodium alginate in drug delivery. Nanoparticles and nanofibers composed of sodium alginate have shown promising results in targeted drug delivery, particularly in cancer therapeutics. These nanostructures can be engineered to respond to specific physiological conditions, such as pH changes or enzymatic activity, enabling precise control over drug release at the target site.
While the biocompatibility and safety of sodium alginate are well-established, ongoing research continues to explore its long-term effects and potential interactions with various drug molecules. As the complexity of drug delivery systems increases, understanding the nuanced behavior of sodium alginate in different physiological environments remains a priority for researchers and pharmaceutical developers.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!