Sodium Alginate for Improved Liquid Stability in Pharmaceutical Preparations
JUL 14, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Alginate Background and Objectives
Sodium alginate, a naturally occurring polysaccharide derived from brown seaweed, has been a subject of significant interest in the pharmaceutical industry for decades. Its unique properties, including biocompatibility, biodegradability, and versatile gelation capabilities, have positioned it as a valuable excipient in various pharmaceutical formulations. The evolution of sodium alginate's applications in drug delivery systems has been marked by continuous research and development efforts aimed at harnessing its full potential.
The primary objective of this research is to explore and enhance the role of sodium alginate in improving liquid stability in pharmaceutical preparations. This goal is driven by the increasing demand for stable, long-lasting liquid formulations in the pharmaceutical sector. As the industry shifts towards more complex and sensitive drug molecules, the need for effective stabilizing agents becomes paramount. Sodium alginate's ability to form viscous solutions and its interactions with other components in liquid formulations make it a promising candidate for addressing stability challenges.
Historically, sodium alginate has been utilized in various forms within the pharmaceutical industry, ranging from simple thickening agents to more sophisticated controlled-release matrices. The progression of its applications has been closely tied to advancements in polymer science and a deeper understanding of its molecular structure and behavior in different environments. This research aims to build upon this foundation, focusing specifically on leveraging sodium alginate's properties to enhance the stability of liquid pharmaceutical preparations.
The technological trajectory in this field points towards developing more refined and tailored sodium alginate derivatives. These modifications are expected to offer improved functionality, such as enhanced stability in a wider pH range, better compatibility with a diverse array of active pharmaceutical ingredients, and increased resistance to degradation over time. The research will explore these avenues, aiming to push the boundaries of what is currently achievable with standard sodium alginate formulations.
Furthermore, this study will investigate the synergistic effects of combining sodium alginate with other excipients to create novel stabilizing systems. The goal is to develop innovative formulation strategies that not only improve liquid stability but also potentially enhance other aspects of pharmaceutical preparations, such as bioavailability and controlled release profiles. This multifaceted approach aligns with the broader trend in pharmaceutical research towards creating more efficient and patient-friendly drug delivery systems.
In pursuing these objectives, the research will employ cutting-edge analytical techniques to characterize the behavior of sodium alginate in various pharmaceutical liquid systems. This will involve studying its interactions at the molecular level, assessing its impact on rheological properties, and evaluating its long-term stability under different storage conditions. The findings from these investigations are expected to provide valuable insights that will guide the development of next-generation liquid pharmaceutical formulations with superior stability characteristics.
The primary objective of this research is to explore and enhance the role of sodium alginate in improving liquid stability in pharmaceutical preparations. This goal is driven by the increasing demand for stable, long-lasting liquid formulations in the pharmaceutical sector. As the industry shifts towards more complex and sensitive drug molecules, the need for effective stabilizing agents becomes paramount. Sodium alginate's ability to form viscous solutions and its interactions with other components in liquid formulations make it a promising candidate for addressing stability challenges.
Historically, sodium alginate has been utilized in various forms within the pharmaceutical industry, ranging from simple thickening agents to more sophisticated controlled-release matrices. The progression of its applications has been closely tied to advancements in polymer science and a deeper understanding of its molecular structure and behavior in different environments. This research aims to build upon this foundation, focusing specifically on leveraging sodium alginate's properties to enhance the stability of liquid pharmaceutical preparations.
The technological trajectory in this field points towards developing more refined and tailored sodium alginate derivatives. These modifications are expected to offer improved functionality, such as enhanced stability in a wider pH range, better compatibility with a diverse array of active pharmaceutical ingredients, and increased resistance to degradation over time. The research will explore these avenues, aiming to push the boundaries of what is currently achievable with standard sodium alginate formulations.
Furthermore, this study will investigate the synergistic effects of combining sodium alginate with other excipients to create novel stabilizing systems. The goal is to develop innovative formulation strategies that not only improve liquid stability but also potentially enhance other aspects of pharmaceutical preparations, such as bioavailability and controlled release profiles. This multifaceted approach aligns with the broader trend in pharmaceutical research towards creating more efficient and patient-friendly drug delivery systems.
In pursuing these objectives, the research will employ cutting-edge analytical techniques to characterize the behavior of sodium alginate in various pharmaceutical liquid systems. This will involve studying its interactions at the molecular level, assessing its impact on rheological properties, and evaluating its long-term stability under different storage conditions. The findings from these investigations are expected to provide valuable insights that will guide the development of next-generation liquid pharmaceutical formulations with superior stability characteristics.
Market Analysis for Stable Liquid Pharmaceuticals
The global market for stable liquid pharmaceuticals has been experiencing significant growth in recent years, driven by the increasing demand for convenient and easily administrable drug formulations. This trend is particularly evident in the areas of oral suspensions, injectable solutions, and topical preparations. The market size for stable liquid pharmaceuticals was valued at approximately $150 billion in 2022 and is projected to reach $200 billion by 2027, with a compound annual growth rate (CAGR) of 5.9%.
Several factors contribute to the expanding market for stable liquid pharmaceuticals. Firstly, the aging population in many developed countries has led to a higher prevalence of chronic diseases, necessitating long-term medication regimens. Liquid formulations offer advantages in terms of ease of swallowing and dose adjustability, making them particularly suitable for elderly patients and children.
Additionally, the growing focus on personalized medicine has increased the demand for flexible dosage forms, which liquid pharmaceuticals can readily accommodate. This trend is further supported by advancements in drug delivery technologies, enabling the development of more sophisticated and stable liquid formulations.
The pharmaceutical industry's shift towards biologics and large molecule drugs has also impacted the market for stable liquid pharmaceuticals. Many of these complex molecules require liquid formulations to maintain their stability and efficacy, driving innovation in this sector.
Geographically, North America and Europe currently dominate the market for stable liquid pharmaceuticals, accounting for approximately 60% of the global market share. However, emerging markets in Asia-Pacific and Latin America are expected to witness the highest growth rates in the coming years, driven by improving healthcare infrastructure and increasing healthcare expenditure.
Key players in the stable liquid pharmaceuticals market include multinational pharmaceutical companies such as Pfizer, Novartis, and Johnson & Johnson, as well as specialized formulation development companies and contract manufacturing organizations (CMOs). These companies are investing heavily in research and development to improve the stability, bioavailability, and shelf-life of liquid pharmaceutical products.
The use of novel excipients and stabilizing agents, such as sodium alginate, has become a focal point for many companies seeking to enhance the stability of their liquid formulations. This trend aligns with the growing consumer preference for natural and plant-derived ingredients, as sodium alginate is derived from seaweed.
In conclusion, the market for stable liquid pharmaceuticals presents significant opportunities for growth and innovation. As the demand for these formulations continues to rise, companies that can develop effective stabilization techniques and overcome formulation challenges are likely to gain a competitive edge in this expanding market.
Several factors contribute to the expanding market for stable liquid pharmaceuticals. Firstly, the aging population in many developed countries has led to a higher prevalence of chronic diseases, necessitating long-term medication regimens. Liquid formulations offer advantages in terms of ease of swallowing and dose adjustability, making them particularly suitable for elderly patients and children.
Additionally, the growing focus on personalized medicine has increased the demand for flexible dosage forms, which liquid pharmaceuticals can readily accommodate. This trend is further supported by advancements in drug delivery technologies, enabling the development of more sophisticated and stable liquid formulations.
The pharmaceutical industry's shift towards biologics and large molecule drugs has also impacted the market for stable liquid pharmaceuticals. Many of these complex molecules require liquid formulations to maintain their stability and efficacy, driving innovation in this sector.
Geographically, North America and Europe currently dominate the market for stable liquid pharmaceuticals, accounting for approximately 60% of the global market share. However, emerging markets in Asia-Pacific and Latin America are expected to witness the highest growth rates in the coming years, driven by improving healthcare infrastructure and increasing healthcare expenditure.
Key players in the stable liquid pharmaceuticals market include multinational pharmaceutical companies such as Pfizer, Novartis, and Johnson & Johnson, as well as specialized formulation development companies and contract manufacturing organizations (CMOs). These companies are investing heavily in research and development to improve the stability, bioavailability, and shelf-life of liquid pharmaceutical products.
The use of novel excipients and stabilizing agents, such as sodium alginate, has become a focal point for many companies seeking to enhance the stability of their liquid formulations. This trend aligns with the growing consumer preference for natural and plant-derived ingredients, as sodium alginate is derived from seaweed.
In conclusion, the market for stable liquid pharmaceuticals presents significant opportunities for growth and innovation. As the demand for these formulations continues to rise, companies that can develop effective stabilization techniques and overcome formulation challenges are likely to gain a competitive edge in this expanding market.
Current Challenges in Liquid Stability
The pharmaceutical industry faces significant challenges in maintaining the stability of liquid formulations, particularly those containing sodium alginate. One of the primary issues is the inherent viscosity of sodium alginate solutions, which can lead to difficulties in processing and administration. As the concentration of sodium alginate increases, the viscosity of the solution rises exponentially, potentially causing problems in manufacturing, packaging, and patient use.
Another critical challenge is the pH sensitivity of sodium alginate. The stability and functionality of sodium alginate are highly dependent on the pH of the solution. In acidic environments, sodium alginate tends to form insoluble alginic acid, which can precipitate out of solution, compromising the integrity and efficacy of the pharmaceutical preparation. Conversely, in highly alkaline conditions, the alginate chains may undergo degradation, leading to a loss of its desired properties.
The presence of divalent cations, such as calcium and magnesium, in the formulation or during storage can also pose significant stability issues. These ions can crosslink with the alginate molecules, forming gels or precipitates that alter the physical properties of the liquid preparation. This phenomenon can result in changes in viscosity, flow characteristics, and even the release profile of active pharmaceutical ingredients.
Microbial contamination is another concern in liquid formulations containing sodium alginate. The polysaccharide nature of alginate can provide a suitable environment for microbial growth, necessitating the use of preservatives. However, finding compatible preservatives that do not interact negatively with sodium alginate or other components of the formulation can be challenging.
Long-term stability is a persistent issue, particularly in terms of maintaining consistent rheological properties over the shelf life of the product. Factors such as temperature fluctuations, light exposure, and mechanical stress during transportation can all contribute to changes in the physical and chemical properties of sodium alginate-containing liquids over time.
The interaction between sodium alginate and other excipients or active pharmaceutical ingredients in the formulation can also lead to stability problems. These interactions may result in changes in drug solubility, bioavailability, or the formation of undesirable complexes that affect the overall performance of the pharmaceutical preparation.
Addressing these challenges requires a multifaceted approach, involving careful formulation design, selection of appropriate excipients, and the development of robust manufacturing processes. Researchers and formulators must consider innovative strategies to overcome these stability issues while maintaining the beneficial properties of sodium alginate in liquid pharmaceutical preparations.
Another critical challenge is the pH sensitivity of sodium alginate. The stability and functionality of sodium alginate are highly dependent on the pH of the solution. In acidic environments, sodium alginate tends to form insoluble alginic acid, which can precipitate out of solution, compromising the integrity and efficacy of the pharmaceutical preparation. Conversely, in highly alkaline conditions, the alginate chains may undergo degradation, leading to a loss of its desired properties.
The presence of divalent cations, such as calcium and magnesium, in the formulation or during storage can also pose significant stability issues. These ions can crosslink with the alginate molecules, forming gels or precipitates that alter the physical properties of the liquid preparation. This phenomenon can result in changes in viscosity, flow characteristics, and even the release profile of active pharmaceutical ingredients.
Microbial contamination is another concern in liquid formulations containing sodium alginate. The polysaccharide nature of alginate can provide a suitable environment for microbial growth, necessitating the use of preservatives. However, finding compatible preservatives that do not interact negatively with sodium alginate or other components of the formulation can be challenging.
Long-term stability is a persistent issue, particularly in terms of maintaining consistent rheological properties over the shelf life of the product. Factors such as temperature fluctuations, light exposure, and mechanical stress during transportation can all contribute to changes in the physical and chemical properties of sodium alginate-containing liquids over time.
The interaction between sodium alginate and other excipients or active pharmaceutical ingredients in the formulation can also lead to stability problems. These interactions may result in changes in drug solubility, bioavailability, or the formation of undesirable complexes that affect the overall performance of the pharmaceutical preparation.
Addressing these challenges requires a multifaceted approach, involving careful formulation design, selection of appropriate excipients, and the development of robust manufacturing processes. Researchers and formulators must consider innovative strategies to overcome these stability issues while maintaining the beneficial properties of sodium alginate in liquid pharmaceutical preparations.
Sodium Alginate-based Stabilization Techniques
01 pH adjustment for stability
Adjusting the pH of sodium alginate solutions can significantly improve their stability. Maintaining the pH within a specific range, typically between 4 and 7, helps prevent degradation and maintains the desired viscosity of the liquid. This approach is often combined with the addition of buffering agents to ensure consistent pH levels over time.- pH adjustment for stability: Adjusting the pH of sodium alginate solutions can significantly improve their stability. Maintaining the pH within a specific range, typically between 4 and 7, helps prevent degradation and maintains the desired viscosity of the liquid. This approach is often combined with the use of buffer systems to ensure long-term stability.
- Addition of preservatives: Incorporating preservatives into sodium alginate solutions enhances their stability by preventing microbial growth. Common preservatives used include potassium sorbate, sodium benzoate, and various natural antimicrobial agents. These additives help extend the shelf life of the liquid formulation without affecting its functional properties.
- Temperature control during processing and storage: Controlling temperature during the preparation and storage of sodium alginate solutions is crucial for maintaining stability. Avoiding extreme temperatures and rapid temperature fluctuations helps prevent degradation of the alginate molecules. Proper temperature management during processing and storage can significantly extend the product's shelf life.
- Use of stabilizing agents: Adding specific stabilizing agents to sodium alginate solutions can improve their long-term stability. These agents may include calcium salts, which can form cross-links with alginate molecules, or other polymers that enhance the overall stability of the solution. The choice of stabilizing agent depends on the intended application of the sodium alginate liquid.
- Optimized concentration and viscosity: Adjusting the concentration of sodium alginate and optimizing the viscosity of the solution can contribute to improved stability. Higher concentrations may lead to increased stability, but this needs to be balanced with the desired flow properties of the liquid. Finding the optimal concentration and viscosity for specific applications is key to ensuring long-term stability.
02 Addition of preservatives
Incorporating preservatives into sodium alginate solutions enhances their stability by preventing microbial growth. Common preservatives used include potassium sorbate, sodium benzoate, and various natural antimicrobial agents. These additives help extend the shelf life of the liquid formulation while maintaining its functional properties.Expand Specific Solutions03 Temperature control during processing and storage
Controlling temperature during the preparation and storage of sodium alginate solutions is crucial for maintaining stability. Avoiding extreme temperatures and rapid temperature fluctuations helps prevent degradation of the alginate molecules. Proper temperature management during processing and storage can significantly extend the shelf life of the liquid formulation.Expand Specific Solutions04 Use of stabilizing agents
Adding specific stabilizing agents to sodium alginate solutions can enhance their long-term stability. These agents may include calcium sequestrants, antioxidants, or other compounds that prevent cross-linking or degradation of the alginate molecules. The choice of stabilizing agent depends on the specific application and desired properties of the final product.Expand Specific Solutions05 Optimized concentration and viscosity
Adjusting the concentration of sodium alginate in the solution and optimizing its viscosity can contribute to improved stability. Higher concentrations may lead to increased stability in some cases, while lower concentrations may be more stable in others. Finding the optimal balance between concentration and viscosity is essential for maintaining the desired properties of the liquid formulation over time.Expand Specific Solutions
Key Players in Pharmaceutical Excipients
The research on sodium alginate for improved liquid stability in pharmaceutical preparations is in a mature stage of development, with a significant market presence and established applications. The global market for sodium alginate in pharmaceuticals is substantial, driven by its widespread use as a stabilizer and thickening agent. Technologically, the field is well-developed, with major players like Bausch & Lomb, Unilever, and Pfizer incorporating sodium alginate in various formulations. Academic institutions such as Ocean University of China and Osaka University contribute to ongoing research, while specialized companies like Qingdao Bright Moon Seaweed Group focus on production. The competitive landscape is diverse, with pharmaceutical giants, specialty chemical manufacturers, and research institutions all playing significant roles in advancing the technology and its applications.
Ocean University of China
Technical Solution: Ocean University of China has conducted academic research on sodium alginate for pharmaceutical applications, including liquid stability enhancement. Their studies have focused on understanding the molecular structure-function relationships of sodium alginate and how they impact liquid formulation stability[17]. The university has explored novel extraction and modification techniques to produce sodium alginate with tailored properties for specific pharmaceutical applications[18]. Their research has also investigated the use of sodium alginate in combination with other polymers to create advanced stabilizing systems for liquid pharmaceuticals[19]. The university's work has contributed to the fundamental understanding of sodium alginate behavior in complex liquid systems and its potential for improving the stability of various pharmaceutical preparations[20].
Strengths: Strong focus on fundamental research; Exploration of novel extraction and modification techniques. Weaknesses: May lack immediate commercial application; Research findings may require further development for industrial use.
Rhodia Operations SASU
Technical Solution: Rhodia Operations SASU, now part of Solvay Group, has conducted extensive research on sodium alginate for pharmaceutical applications, including liquid stability improvement. They have developed a proprietary process for producing ultra-pure sodium alginate with controlled molecular weight distribution, which is crucial for consistent performance in liquid pharmaceuticals[9]. Their technology focuses on enhancing the interaction between sodium alginate and other excipients to create stable, homogeneous liquid formulations[10]. Rhodia has also explored the use of chemically modified alginates to improve stability in challenging environments, such as highly acidic or high-electrolyte solutions[11]. Their research has shown promising results in stabilizing complex liquid formulations, including those containing poorly soluble drugs[12].
Strengths: Production of ultra-pure sodium alginate; Expertise in chemical modification of alginates. Weaknesses: Higher production costs for specialized grades; May require additional formulation expertise to optimize use in pharmaceuticals.
Innovative Sodium Alginate Formulations
Alginate liquid preparation
PatentActiveUS11969437B2
Innovation
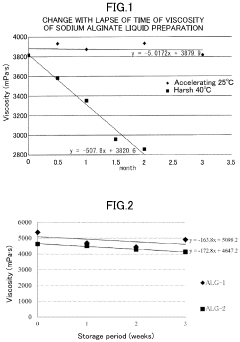
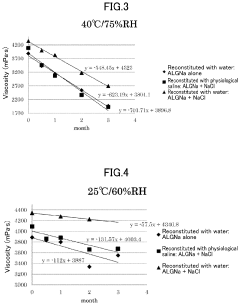
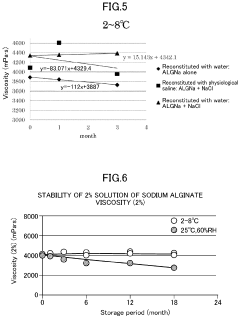
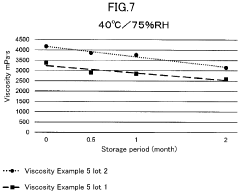
- A method involving the preparation of a low-concentration aqueous solution of monovalent metal alginate, dissolving a predetermined amount of a monovalent metal salt or ammonium salt, aseptic filtration, and concentration under a non-oxidizing atmosphere, followed by filling and sealing in a container or syringe, to create a stable, low-endotoxin solution with suppressed viscosity decrease.
Regulatory Considerations for Excipients
The regulatory landscape for excipients in pharmaceutical preparations is complex and dynamic, requiring careful consideration when incorporating sodium alginate for improved liquid stability. Regulatory bodies, such as the FDA in the United States and the EMA in Europe, have established guidelines and requirements for the use of excipients in drug formulations.
Sodium alginate, as an excipient, must comply with specific regulatory standards to ensure its safety and efficacy in pharmaceutical products. The FDA classifies sodium alginate as Generally Recognized as Safe (GRAS) for certain applications, but its use in pharmaceutical preparations may require additional scrutiny and documentation.
Manufacturers must adhere to Good Manufacturing Practices (GMP) when producing sodium alginate for pharmaceutical use. This includes maintaining consistent quality, purity, and stability of the excipient throughout the manufacturing process. Comprehensive documentation of the production process, quality control measures, and stability testing is essential for regulatory compliance.
Regulatory bodies often require detailed information on the sourcing of raw materials, including the algae species used and harvesting locations. This is crucial for ensuring the consistency and safety of the final product. Additionally, manufacturers must provide data on potential impurities, contaminants, and any processing aids used during production.
When incorporating sodium alginate into pharmaceutical formulations, developers must consider its impact on the overall stability, bioavailability, and performance of the drug product. Regulatory submissions should include comprehensive stability studies demonstrating the excipient's compatibility with active pharmaceutical ingredients and other formulation components.
Pharmacopoeial standards, such as those outlined in the United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.), provide specific quality requirements for sodium alginate. Compliance with these standards is often mandatory for regulatory approval. Manufacturers must conduct regular testing to ensure that their sodium alginate meets these specifications.
As regulations evolve, pharmaceutical companies must stay informed about changes in regulatory requirements for excipients. This includes monitoring updates to pharmacopoeial monographs, guidance documents, and regulatory policies. Proactive engagement with regulatory authorities through scientific advice meetings can help address potential concerns early in the development process.
In conclusion, navigating the regulatory landscape for sodium alginate as an excipient in pharmaceutical preparations requires a thorough understanding of current guidelines, meticulous documentation, and ongoing compliance efforts. By addressing these regulatory considerations, manufacturers can ensure the successful integration of sodium alginate for improved liquid stability in their pharmaceutical products.
Sodium alginate, as an excipient, must comply with specific regulatory standards to ensure its safety and efficacy in pharmaceutical products. The FDA classifies sodium alginate as Generally Recognized as Safe (GRAS) for certain applications, but its use in pharmaceutical preparations may require additional scrutiny and documentation.
Manufacturers must adhere to Good Manufacturing Practices (GMP) when producing sodium alginate for pharmaceutical use. This includes maintaining consistent quality, purity, and stability of the excipient throughout the manufacturing process. Comprehensive documentation of the production process, quality control measures, and stability testing is essential for regulatory compliance.
Regulatory bodies often require detailed information on the sourcing of raw materials, including the algae species used and harvesting locations. This is crucial for ensuring the consistency and safety of the final product. Additionally, manufacturers must provide data on potential impurities, contaminants, and any processing aids used during production.
When incorporating sodium alginate into pharmaceutical formulations, developers must consider its impact on the overall stability, bioavailability, and performance of the drug product. Regulatory submissions should include comprehensive stability studies demonstrating the excipient's compatibility with active pharmaceutical ingredients and other formulation components.
Pharmacopoeial standards, such as those outlined in the United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.), provide specific quality requirements for sodium alginate. Compliance with these standards is often mandatory for regulatory approval. Manufacturers must conduct regular testing to ensure that their sodium alginate meets these specifications.
As regulations evolve, pharmaceutical companies must stay informed about changes in regulatory requirements for excipients. This includes monitoring updates to pharmacopoeial monographs, guidance documents, and regulatory policies. Proactive engagement with regulatory authorities through scientific advice meetings can help address potential concerns early in the development process.
In conclusion, navigating the regulatory landscape for sodium alginate as an excipient in pharmaceutical preparations requires a thorough understanding of current guidelines, meticulous documentation, and ongoing compliance efforts. By addressing these regulatory considerations, manufacturers can ensure the successful integration of sodium alginate for improved liquid stability in their pharmaceutical products.
Environmental Impact of Sodium Alginate
The environmental impact of sodium alginate in pharmaceutical preparations is an important consideration in the context of sustainable drug development and production. Sodium alginate, derived from brown seaweed, is generally considered an eco-friendly and biodegradable material. However, its widespread use in the pharmaceutical industry necessitates a thorough examination of its environmental footprint.
The production of sodium alginate involves harvesting seaweed, which can have both positive and negative environmental implications. On one hand, seaweed cultivation can contribute to carbon sequestration and provide habitats for marine life. On the other hand, large-scale harvesting may disrupt local ecosystems if not managed sustainably. The processing of seaweed into sodium alginate also requires energy and chemical inputs, which should be evaluated for their environmental impact.
In pharmaceutical preparations, the use of sodium alginate as a stabilizer can potentially reduce the need for synthetic additives, thereby decreasing the overall environmental burden of drug formulations. Its ability to improve liquid stability may also lead to extended shelf life of pharmaceutical products, potentially reducing waste from expired medications.
The biodegradability of sodium alginate is a significant environmental advantage. When disposed of, it breaks down naturally without leaving harmful residues in the environment. This characteristic is particularly important in the context of pharmaceutical waste management, where concerns about drug residues in water systems are prevalent.
However, the increased demand for sodium alginate in pharmaceuticals may lead to intensified seaweed farming practices. This could potentially result in coastal habitat modification and impact marine biodiversity if not properly regulated. Additionally, the transportation of seaweed and processed sodium alginate contributes to the carbon footprint of pharmaceutical supply chains.
From a lifecycle perspective, the environmental impact of sodium alginate in pharmaceutical preparations extends beyond its production and use. The disposal of alginate-containing medications and their packaging must also be considered. While the alginate itself is biodegradable, it may be combined with other materials in drug formulations or packaging that are less environmentally friendly.
Research into the environmental impact of sodium alginate in pharmaceutical applications is ongoing. Studies are focusing on optimizing production processes to minimize energy consumption and chemical use, as well as exploring sustainable seaweed farming practices. Additionally, investigations into the fate of alginate-based pharmaceuticals in wastewater treatment systems and natural environments are crucial for a comprehensive environmental assessment.
The production of sodium alginate involves harvesting seaweed, which can have both positive and negative environmental implications. On one hand, seaweed cultivation can contribute to carbon sequestration and provide habitats for marine life. On the other hand, large-scale harvesting may disrupt local ecosystems if not managed sustainably. The processing of seaweed into sodium alginate also requires energy and chemical inputs, which should be evaluated for their environmental impact.
In pharmaceutical preparations, the use of sodium alginate as a stabilizer can potentially reduce the need for synthetic additives, thereby decreasing the overall environmental burden of drug formulations. Its ability to improve liquid stability may also lead to extended shelf life of pharmaceutical products, potentially reducing waste from expired medications.
The biodegradability of sodium alginate is a significant environmental advantage. When disposed of, it breaks down naturally without leaving harmful residues in the environment. This characteristic is particularly important in the context of pharmaceutical waste management, where concerns about drug residues in water systems are prevalent.
However, the increased demand for sodium alginate in pharmaceuticals may lead to intensified seaweed farming practices. This could potentially result in coastal habitat modification and impact marine biodiversity if not properly regulated. Additionally, the transportation of seaweed and processed sodium alginate contributes to the carbon footprint of pharmaceutical supply chains.
From a lifecycle perspective, the environmental impact of sodium alginate in pharmaceutical preparations extends beyond its production and use. The disposal of alginate-containing medications and their packaging must also be considered. While the alginate itself is biodegradable, it may be combined with other materials in drug formulations or packaging that are less environmentally friendly.
Research into the environmental impact of sodium alginate in pharmaceutical applications is ongoing. Studies are focusing on optimizing production processes to minimize energy consumption and chemical use, as well as exploring sustainable seaweed farming practices. Additionally, investigations into the fate of alginate-based pharmaceuticals in wastewater treatment systems and natural environments are crucial for a comprehensive environmental assessment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!