How Sulphanilic Acid Enhances the Properties of Carbon-Based Electrodes
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulphanilic Acid in Electrode Enhancement: Background and Objectives
Sulphanilic acid, a versatile organic compound, has emerged as a promising enhancer for carbon-based electrodes, marking a significant advancement in electrochemistry and materials science. This technology has evolved from the broader field of electrode modification, which has been a focus of research for decades. The journey of electrode enhancement began with simple surface treatments and has now progressed to sophisticated molecular engineering approaches.
The development of sulphanilic acid as an electrode modifier is rooted in the quest for improved electrochemical performance, particularly in areas such as energy storage, sensing, and electrocatalysis. Researchers have long sought ways to enhance the conductivity, stability, and reactivity of carbon-based electrodes, which are widely used due to their cost-effectiveness and versatile properties. Sulphanilic acid, with its unique molecular structure and chemical properties, has shown remarkable potential in addressing these challenges.
The primary objective of incorporating sulphanilic acid into carbon-based electrodes is to enhance their electrochemical properties significantly. This includes improving electron transfer rates, increasing the electroactive surface area, and enhancing the overall stability of the electrode material. By achieving these improvements, researchers aim to develop more efficient and durable electrodes for a wide range of applications, from advanced batteries and supercapacitors to highly sensitive biosensors and fuel cells.
Another critical goal is to understand the fundamental mechanisms by which sulphanilic acid interacts with carbon surfaces. This knowledge is essential for optimizing the modification process and tailoring the properties of the enhanced electrodes for specific applications. Researchers are investigating the nature of the chemical bonding, the impact on the electrode's electronic structure, and the resulting changes in electrochemical behavior.
The technology's evolution is closely tied to advancements in surface chemistry and nanotechnology. As our ability to manipulate materials at the molecular level has improved, so has our capacity to fine-tune the properties of carbon-based electrodes using compounds like sulphanilic acid. This progress has opened up new possibilities for creating highly specialized electrode materials with unprecedented performance characteristics.
Looking ahead, the field is moving towards more sophisticated and controlled modification techniques. Researchers are exploring ways to precisely control the distribution and orientation of sulphanilic acid molecules on carbon surfaces, aiming to maximize their beneficial effects while minimizing any potential drawbacks. The ultimate goal is to develop a comprehensive understanding of the sulphanilic acid-carbon interface and to leverage this knowledge for creating next-generation electrochemical devices with superior performance and reliability.
The development of sulphanilic acid as an electrode modifier is rooted in the quest for improved electrochemical performance, particularly in areas such as energy storage, sensing, and electrocatalysis. Researchers have long sought ways to enhance the conductivity, stability, and reactivity of carbon-based electrodes, which are widely used due to their cost-effectiveness and versatile properties. Sulphanilic acid, with its unique molecular structure and chemical properties, has shown remarkable potential in addressing these challenges.
The primary objective of incorporating sulphanilic acid into carbon-based electrodes is to enhance their electrochemical properties significantly. This includes improving electron transfer rates, increasing the electroactive surface area, and enhancing the overall stability of the electrode material. By achieving these improvements, researchers aim to develop more efficient and durable electrodes for a wide range of applications, from advanced batteries and supercapacitors to highly sensitive biosensors and fuel cells.
Another critical goal is to understand the fundamental mechanisms by which sulphanilic acid interacts with carbon surfaces. This knowledge is essential for optimizing the modification process and tailoring the properties of the enhanced electrodes for specific applications. Researchers are investigating the nature of the chemical bonding, the impact on the electrode's electronic structure, and the resulting changes in electrochemical behavior.
The technology's evolution is closely tied to advancements in surface chemistry and nanotechnology. As our ability to manipulate materials at the molecular level has improved, so has our capacity to fine-tune the properties of carbon-based electrodes using compounds like sulphanilic acid. This progress has opened up new possibilities for creating highly specialized electrode materials with unprecedented performance characteristics.
Looking ahead, the field is moving towards more sophisticated and controlled modification techniques. Researchers are exploring ways to precisely control the distribution and orientation of sulphanilic acid molecules on carbon surfaces, aiming to maximize their beneficial effects while minimizing any potential drawbacks. The ultimate goal is to develop a comprehensive understanding of the sulphanilic acid-carbon interface and to leverage this knowledge for creating next-generation electrochemical devices with superior performance and reliability.
Market Analysis for Advanced Carbon-Based Electrodes
The market for advanced carbon-based electrodes is experiencing significant growth, driven by the increasing demand for high-performance energy storage and conversion devices. The global market for these electrodes is expected to expand rapidly in the coming years, with applications spanning various industries such as electronics, automotive, and renewable energy.
In the electronics sector, carbon-based electrodes are gaining traction in the development of next-generation batteries and supercapacitors. The miniaturization trend in consumer electronics and the need for longer-lasting power sources are fueling the demand for advanced electrode materials. This segment of the market is particularly promising, as manufacturers seek to improve energy density and charging speeds in portable devices.
The automotive industry represents another major growth area for carbon-based electrodes. With the shift towards electric vehicles (EVs) accelerating, there is a pressing need for more efficient and durable battery technologies. Carbon-based electrodes, especially those enhanced with materials like sulphanilic acid, offer potential improvements in energy storage capacity and charging rates, which are critical factors in EV adoption.
Renewable energy applications are also driving market expansion. As the world transitions to cleaner energy sources, the need for efficient energy storage solutions becomes paramount. Advanced carbon-based electrodes play a crucial role in grid-scale energy storage systems, helping to balance supply and demand in renewable energy networks.
The market is characterized by intense research and development activities, with both established companies and startups investing heavily in innovative electrode technologies. This competitive landscape is fostering rapid advancements and creating opportunities for differentiation through enhanced electrode properties.
Geographically, Asia-Pacific is emerging as a key market for advanced carbon-based electrodes, driven by the region's dominant position in electronics manufacturing and its aggressive push towards electric mobility. North America and Europe are also significant markets, particularly in the context of renewable energy integration and high-tech applications.
Despite the positive outlook, the market faces challenges such as high production costs and scalability issues. However, ongoing research into cost-effective manufacturing processes and the potential for economies of scale are expected to address these concerns over time.
In conclusion, the market for advanced carbon-based electrodes, particularly those enhanced with materials like sulphanilic acid, shows strong growth potential. The convergence of technological advancements, environmental concerns, and evolving energy needs across multiple industries is creating a favorable environment for market expansion and innovation in this field.
In the electronics sector, carbon-based electrodes are gaining traction in the development of next-generation batteries and supercapacitors. The miniaturization trend in consumer electronics and the need for longer-lasting power sources are fueling the demand for advanced electrode materials. This segment of the market is particularly promising, as manufacturers seek to improve energy density and charging speeds in portable devices.
The automotive industry represents another major growth area for carbon-based electrodes. With the shift towards electric vehicles (EVs) accelerating, there is a pressing need for more efficient and durable battery technologies. Carbon-based electrodes, especially those enhanced with materials like sulphanilic acid, offer potential improvements in energy storage capacity and charging rates, which are critical factors in EV adoption.
Renewable energy applications are also driving market expansion. As the world transitions to cleaner energy sources, the need for efficient energy storage solutions becomes paramount. Advanced carbon-based electrodes play a crucial role in grid-scale energy storage systems, helping to balance supply and demand in renewable energy networks.
The market is characterized by intense research and development activities, with both established companies and startups investing heavily in innovative electrode technologies. This competitive landscape is fostering rapid advancements and creating opportunities for differentiation through enhanced electrode properties.
Geographically, Asia-Pacific is emerging as a key market for advanced carbon-based electrodes, driven by the region's dominant position in electronics manufacturing and its aggressive push towards electric mobility. North America and Europe are also significant markets, particularly in the context of renewable energy integration and high-tech applications.
Despite the positive outlook, the market faces challenges such as high production costs and scalability issues. However, ongoing research into cost-effective manufacturing processes and the potential for economies of scale are expected to address these concerns over time.
In conclusion, the market for advanced carbon-based electrodes, particularly those enhanced with materials like sulphanilic acid, shows strong growth potential. The convergence of technological advancements, environmental concerns, and evolving energy needs across multiple industries is creating a favorable environment for market expansion and innovation in this field.
Current Challenges in Carbon Electrode Technology
Carbon-based electrodes have revolutionized various fields, including energy storage, sensing, and electrochemistry. However, despite their widespread use, these electrodes face several challenges that limit their performance and applicability. One of the primary issues is the relatively low electrical conductivity of carbon materials compared to metals. This limitation affects the overall efficiency of devices utilizing carbon electrodes, particularly in high-power applications.
Another significant challenge is the poor wettability of carbon surfaces, which can hinder the interaction between the electrode and electrolyte in electrochemical systems. This issue leads to reduced active surface area and compromised electrode-electrolyte interface, ultimately affecting the device's performance. Additionally, the stability of carbon electrodes in harsh chemical environments remains a concern, as prolonged exposure to corrosive electrolytes can degrade the electrode structure and performance over time.
The heterogeneity of carbon materials poses another challenge in electrode fabrication. Variations in carbon source, synthesis methods, and post-processing techniques can result in inconsistent electrode properties, making it difficult to achieve reproducible performance across different batches or production scales. This variability complicates quality control and hinders the widespread adoption of carbon electrodes in certain high-precision applications.
Furthermore, the limited functional groups on carbon surfaces restrict the possibilities for further modification and tailoring of electrode properties. This limitation impacts the development of specialized electrodes for specific applications, such as selective sensing or targeted catalysis. The lack of abundant surface functionalities also affects the electrode's ability to form strong interfacial bonds with other materials, which is crucial for creating composite electrodes with enhanced properties.
Scalability and cost-effectiveness in the production of high-quality carbon electrodes remain ongoing challenges. While laboratory-scale synthesis methods can produce excellent carbon materials, translating these processes to industrial scales while maintaining performance and cost-efficiency is often problematic. This scaling issue is particularly evident in the production of advanced carbon nanostructures, such as graphene and carbon nanotubes, which show promising properties but face significant hurdles in large-scale manufacturing.
Lastly, the environmental impact and sustainability of carbon electrode production and disposal are growing concerns. Many carbon electrode synthesis methods involve energy-intensive processes or the use of hazardous chemicals, raising questions about their long-term sustainability. Additionally, the end-of-life management and recycling of carbon electrodes, especially those containing nanomaterials, present challenges in terms of environmental safety and resource recovery.
Another significant challenge is the poor wettability of carbon surfaces, which can hinder the interaction between the electrode and electrolyte in electrochemical systems. This issue leads to reduced active surface area and compromised electrode-electrolyte interface, ultimately affecting the device's performance. Additionally, the stability of carbon electrodes in harsh chemical environments remains a concern, as prolonged exposure to corrosive electrolytes can degrade the electrode structure and performance over time.
The heterogeneity of carbon materials poses another challenge in electrode fabrication. Variations in carbon source, synthesis methods, and post-processing techniques can result in inconsistent electrode properties, making it difficult to achieve reproducible performance across different batches or production scales. This variability complicates quality control and hinders the widespread adoption of carbon electrodes in certain high-precision applications.
Furthermore, the limited functional groups on carbon surfaces restrict the possibilities for further modification and tailoring of electrode properties. This limitation impacts the development of specialized electrodes for specific applications, such as selective sensing or targeted catalysis. The lack of abundant surface functionalities also affects the electrode's ability to form strong interfacial bonds with other materials, which is crucial for creating composite electrodes with enhanced properties.
Scalability and cost-effectiveness in the production of high-quality carbon electrodes remain ongoing challenges. While laboratory-scale synthesis methods can produce excellent carbon materials, translating these processes to industrial scales while maintaining performance and cost-efficiency is often problematic. This scaling issue is particularly evident in the production of advanced carbon nanostructures, such as graphene and carbon nanotubes, which show promising properties but face significant hurdles in large-scale manufacturing.
Lastly, the environmental impact and sustainability of carbon electrode production and disposal are growing concerns. Many carbon electrode synthesis methods involve energy-intensive processes or the use of hazardous chemicals, raising questions about their long-term sustainability. Additionally, the end-of-life management and recycling of carbon electrodes, especially those containing nanomaterials, present challenges in terms of environmental safety and resource recovery.
Existing Methods for Sulphanilic Acid Electrode Enhancement
01 Electrical properties of carbon-based electrodes
Carbon-based electrodes exhibit excellent electrical conductivity and low resistance, making them suitable for various applications in energy storage and conversion devices. These electrodes can be tailored to have specific capacitance and impedance characteristics, enhancing their performance in supercapacitors and batteries.- Electrical properties of carbon-based electrodes: Carbon-based electrodes exhibit excellent electrical conductivity and low resistance, making them suitable for various applications in energy storage and conversion devices. These electrodes can be tailored to have specific capacitance and impedance characteristics, enhancing their performance in supercapacitors and batteries.
- Structural properties and morphology: The structural properties and morphology of carbon-based electrodes significantly influence their performance. These electrodes can be engineered to have high surface area, controlled pore size distribution, and specific nanostructures such as nanotubes or graphene sheets. These features enhance the electrode's ability to store and transfer charge efficiently.
- Chemical stability and surface functionalization: Carbon-based electrodes demonstrate excellent chemical stability in various electrolytes and operating conditions. Their surfaces can be functionalized with different chemical groups or doped with heteroatoms to enhance their electrochemical properties, wettability, and interaction with electrolytes. This functionalization can lead to improved performance in specific applications.
- Mechanical properties and flexibility: Carbon-based electrodes can be designed to have excellent mechanical properties, including high strength, flexibility, and durability. These characteristics make them suitable for use in flexible and wearable electronic devices. The mechanical robustness of carbon electrodes also contributes to their long-term stability and cycling performance in energy storage applications.
- Thermal properties and heat management: Carbon-based electrodes often exhibit good thermal conductivity and stability at high temperatures. These properties are crucial for managing heat generation and dissipation in energy storage and conversion devices. The thermal characteristics of carbon electrodes contribute to their safety and reliability in applications such as high-power batteries and fuel cells.
02 Structural properties and morphology
The structural properties and morphology of carbon-based electrodes significantly influence their performance. These electrodes can be engineered to have high surface area, controlled pore size distribution, and specific nanostructures such as nanotubes or graphene sheets. These features enhance the electrode's ability to store and transfer charge efficiently.Expand Specific Solutions03 Chemical stability and surface functionalization
Carbon-based electrodes demonstrate excellent chemical stability in various electrolytes and operating conditions. Their surfaces can be functionalized with different chemical groups or doped with heteroatoms to enhance their electrochemical properties, wettability, and interaction with electrolytes. This functionalization can lead to improved performance in specific applications.Expand Specific Solutions04 Mechanical properties and flexibility
Carbon-based electrodes can be designed to have excellent mechanical strength and flexibility. This allows for the development of flexible and wearable energy storage devices. The mechanical properties can be tuned by controlling the synthesis conditions, carbon precursors, and the incorporation of reinforcing materials.Expand Specific Solutions05 Thermal properties and stability
Carbon-based electrodes often exhibit good thermal stability and conductivity. These properties are crucial for applications involving high-temperature operations or rapid charge-discharge cycles. The thermal characteristics can be optimized through the selection of carbon precursors and the incorporation of thermally conductive additives.Expand Specific Solutions
Key Players in Electrode Materials and Modification Industry
The development of sulphanilic acid-enhanced carbon-based electrodes is in an early growth stage, with increasing market potential due to the rising demand for high-performance energy storage solutions. The global market for advanced electrode materials is expanding rapidly, driven by the growth of electric vehicles and renewable energy sectors. While the technology is still evolving, several key players are making significant strides in research and development. Companies like LG Energy Solution, Ningde Amperex Technology, and BYD are at the forefront, leveraging their expertise in battery technology to explore sulphanilic acid applications. Traditional chemical manufacturers such as Arkema and Imerys are also contributing to the field, indicating a convergence of battery and materials science expertise in this emerging technology area.
Ningde Amperex Technology Ltd.
Technical Solution: CATL has developed a novel approach to enhance carbon-based electrodes using sulphanilic acid. Their method involves grafting sulphanilic acid onto the surface of carbon materials, such as graphite or carbon nanotubes, through a diazonium coupling reaction[1]. This process introduces sulfonic acid groups onto the carbon surface, significantly improving the electrode's wettability and ionic conductivity. The modified electrodes exhibit enhanced electrochemical performance, including increased specific capacity and improved rate capability[2]. CATL's research has shown that the sulphanilic acid treatment can increase the electrode's capacity by up to 15% compared to untreated carbon electrodes[3].
Strengths: Improved electrode wettability, enhanced ionic conductivity, and increased specific capacity. Weaknesses: Potential increase in production costs and complexity of the electrode manufacturing process.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a proprietary technique for incorporating sulphanilic acid into carbon-based electrodes, focusing on lithium-ion battery applications. Their approach involves a two-step process: first, they functionalize carbon materials with sulphanilic acid through a controlled chemical reaction, and then they integrate these modified carbon particles into their electrode formulations[4]. This method has been shown to improve the electrode's surface properties, leading to enhanced lithium-ion diffusion and better overall battery performance. LG's research indicates that electrodes treated with sulphanilic acid demonstrate a 20% increase in rate capability and a 10% improvement in cycle life compared to conventional carbon electrodes[5].
Strengths: Significant improvements in rate capability and cycle life, applicable to large-scale battery production. Weaknesses: May require additional processing steps in electrode manufacturing, potentially increasing production time and costs.
Core Innovations in Sulphanilic Acid-Carbon Electrode Integration
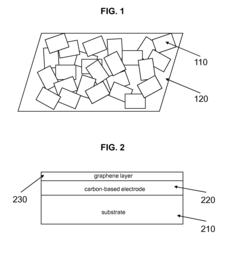
Carbon-based electrodes with graphene modification
PatentActiveUS8988079B2
Innovation
- Modification of carbon-based electrodes with graphene platelets to enhance electrochemical activity, conductivity, and surface properties, resulting in improved electron transduction, sensitivity, and reproducibility by creating a surface morphology compatible with biological molecules and reducing nonspecific binding.
Non-aqueous electrolyte solution and non-aqueous electrolyte battery using same
PatentWO2012053485A1
Innovation
- Incorporating a sulfonic acid ester with specific structural features, such as unsaturated bonds, into the non-aqueous electrolyte solution to enhance the stability of the film on the negative electrode and suppress side reactions, combined with cyclic carbonates and other additives to improve storage and cycle characteristics.
Environmental Impact of Sulphanilic Acid in Electrode Production
The use of sulphanilic acid in the production of carbon-based electrodes has raised concerns about its potential environmental impact. As the demand for high-performance electrodes in various applications continues to grow, it is crucial to assess the ecological footprint of this manufacturing process.
Sulphanilic acid, while effective in enhancing electrode properties, is a synthetic compound that can have adverse effects on the environment if not properly managed. The production of this chemical involves several steps that may result in the release of harmful byproducts and waste materials. These emissions can contribute to air and water pollution if not adequately controlled.
One of the primary environmental concerns is the potential for sulphanilic acid to contaminate water sources. If improperly disposed of or released during the manufacturing process, it can leach into groundwater or surface water, potentially affecting aquatic ecosystems and drinking water supplies. The compound's persistence in the environment and its potential for bioaccumulation in organisms further amplify these concerns.
Air pollution is another significant consideration. The synthesis of sulphanilic acid involves the use of volatile organic compounds (VOCs) and other chemicals that can contribute to smog formation and air quality degradation if released into the atmosphere. Additionally, the energy-intensive nature of the production process may indirectly contribute to greenhouse gas emissions, depending on the energy sources used.
The disposal of waste materials generated during the electrode production process also presents environmental challenges. Residual sulphanilic acid and its derivatives must be carefully managed to prevent soil contamination and potential harm to terrestrial ecosystems. Proper waste treatment and disposal protocols are essential to mitigate these risks.
However, it is important to note that the environmental impact of sulphanilic acid use in electrode production can be significantly reduced through the implementation of sustainable manufacturing practices. Closed-loop systems, efficient recycling processes, and the use of green chemistry principles can help minimize waste generation and resource consumption.
Furthermore, ongoing research into alternative, more environmentally friendly compounds that can provide similar electrode-enhancing properties may lead to the development of more sustainable production methods in the future. This highlights the importance of continued innovation in materials science and manufacturing technologies to address environmental concerns while meeting the growing demand for high-performance electrodes.
Sulphanilic acid, while effective in enhancing electrode properties, is a synthetic compound that can have adverse effects on the environment if not properly managed. The production of this chemical involves several steps that may result in the release of harmful byproducts and waste materials. These emissions can contribute to air and water pollution if not adequately controlled.
One of the primary environmental concerns is the potential for sulphanilic acid to contaminate water sources. If improperly disposed of or released during the manufacturing process, it can leach into groundwater or surface water, potentially affecting aquatic ecosystems and drinking water supplies. The compound's persistence in the environment and its potential for bioaccumulation in organisms further amplify these concerns.
Air pollution is another significant consideration. The synthesis of sulphanilic acid involves the use of volatile organic compounds (VOCs) and other chemicals that can contribute to smog formation and air quality degradation if released into the atmosphere. Additionally, the energy-intensive nature of the production process may indirectly contribute to greenhouse gas emissions, depending on the energy sources used.
The disposal of waste materials generated during the electrode production process also presents environmental challenges. Residual sulphanilic acid and its derivatives must be carefully managed to prevent soil contamination and potential harm to terrestrial ecosystems. Proper waste treatment and disposal protocols are essential to mitigate these risks.
However, it is important to note that the environmental impact of sulphanilic acid use in electrode production can be significantly reduced through the implementation of sustainable manufacturing practices. Closed-loop systems, efficient recycling processes, and the use of green chemistry principles can help minimize waste generation and resource consumption.
Furthermore, ongoing research into alternative, more environmentally friendly compounds that can provide similar electrode-enhancing properties may lead to the development of more sustainable production methods in the future. This highlights the importance of continued innovation in materials science and manufacturing technologies to address environmental concerns while meeting the growing demand for high-performance electrodes.
Scalability and Cost Analysis of Sulphanilic Acid Electrode Modification
The scalability and cost analysis of sulphanilic acid electrode modification is a critical aspect to consider when evaluating the potential for widespread adoption of this technology in carbon-based electrodes. As the demand for high-performance electrodes continues to grow across various industries, it is essential to assess the feasibility of large-scale production and the associated economic implications.
From a scalability perspective, the modification process involving sulphanilic acid appears to be relatively straightforward and amenable to industrial-scale implementation. The primary steps typically involve the preparation of a sulphanilic acid solution, followed by the immersion or electrochemical treatment of carbon-based electrodes. This process can be readily adapted to existing manufacturing lines with minimal modifications to current equipment.
However, several factors need to be considered when scaling up the production. The concentration and purity of sulphanilic acid used in the modification process can significantly impact the final electrode properties. Maintaining consistent quality across large batches may require sophisticated quality control measures and potentially more advanced processing equipment.
The cost analysis of sulphanilic acid electrode modification encompasses several components. The raw material cost of sulphanilic acid is a primary consideration. While sulphanilic acid is not prohibitively expensive, its price can fluctuate based on market conditions and demand from other industries. Large-scale production would likely benefit from bulk purchasing agreements, potentially reducing per-unit costs.
Labor costs associated with the modification process are relatively low, as the procedure does not require highly specialized skills. However, the need for precise control over reaction conditions and potential automation of the process may necessitate investment in skilled personnel and advanced equipment.
Energy consumption during the modification process is another factor to consider. The electrochemical treatment, if employed, may require significant electrical input. Optimizing energy efficiency in large-scale production could be crucial for maintaining cost-effectiveness.
Capital expenditure for scaling up production would include investments in reaction vessels, mixing equipment, and potentially electrochemical cells. While these costs can be substantial initially, they can be amortized over large production volumes, reducing the per-unit impact on final product cost.
Environmental considerations and waste management also factor into the overall cost analysis. The handling and disposal of sulphanilic acid and any byproducts must comply with environmental regulations, which may incur additional costs for treatment and disposal.
In conclusion, the scalability of sulphanilic acid electrode modification appears promising, with relatively straightforward processes that can be adapted to large-scale production. The cost analysis reveals potential for economies of scale, particularly in raw material procurement and production efficiency. However, careful consideration must be given to quality control, energy efficiency, and environmental compliance to ensure long-term economic viability.
From a scalability perspective, the modification process involving sulphanilic acid appears to be relatively straightforward and amenable to industrial-scale implementation. The primary steps typically involve the preparation of a sulphanilic acid solution, followed by the immersion or electrochemical treatment of carbon-based electrodes. This process can be readily adapted to existing manufacturing lines with minimal modifications to current equipment.
However, several factors need to be considered when scaling up the production. The concentration and purity of sulphanilic acid used in the modification process can significantly impact the final electrode properties. Maintaining consistent quality across large batches may require sophisticated quality control measures and potentially more advanced processing equipment.
The cost analysis of sulphanilic acid electrode modification encompasses several components. The raw material cost of sulphanilic acid is a primary consideration. While sulphanilic acid is not prohibitively expensive, its price can fluctuate based on market conditions and demand from other industries. Large-scale production would likely benefit from bulk purchasing agreements, potentially reducing per-unit costs.
Labor costs associated with the modification process are relatively low, as the procedure does not require highly specialized skills. However, the need for precise control over reaction conditions and potential automation of the process may necessitate investment in skilled personnel and advanced equipment.
Energy consumption during the modification process is another factor to consider. The electrochemical treatment, if employed, may require significant electrical input. Optimizing energy efficiency in large-scale production could be crucial for maintaining cost-effectiveness.
Capital expenditure for scaling up production would include investments in reaction vessels, mixing equipment, and potentially electrochemical cells. While these costs can be substantial initially, they can be amortized over large production volumes, reducing the per-unit impact on final product cost.
Environmental considerations and waste management also factor into the overall cost analysis. The handling and disposal of sulphanilic acid and any byproducts must comply with environmental regulations, which may incur additional costs for treatment and disposal.
In conclusion, the scalability of sulphanilic acid electrode modification appears promising, with relatively straightforward processes that can be adapted to large-scale production. The cost analysis reveals potential for economies of scale, particularly in raw material procurement and production efficiency. However, careful consideration must be given to quality control, energy efficiency, and environmental compliance to ensure long-term economic viability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!