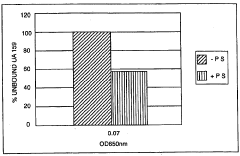
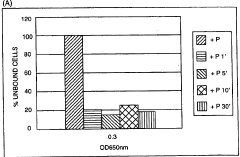
How Sulphanilic Acid Modulates Biofilm Formation in Bacteria
JUL 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulphanilic Acid and Biofilm Modulation: Background and Objectives
Sulphanilic acid, a synthetic organic compound, has emerged as a significant player in the field of bacterial biofilm modulation. The study of how this compound influences biofilm formation in bacteria has gained considerable attention in recent years, driven by the growing need to combat antibiotic resistance and develop novel strategies for controlling bacterial infections.
Biofilms are complex communities of microorganisms that adhere to surfaces and are encased in a self-produced extracellular matrix. These structures pose significant challenges in medical, industrial, and environmental settings due to their increased resistance to antimicrobial agents and host immune responses. Understanding the mechanisms by which sulphanilic acid interacts with and modulates biofilm formation is crucial for developing effective strategies to prevent or disrupt these bacterial communities.
The primary objective of this research is to elucidate the molecular and cellular mechanisms through which sulphanilic acid influences biofilm formation in various bacterial species. This includes investigating its effects on bacterial adhesion, extracellular matrix production, quorum sensing, and other key processes involved in biofilm development.
Historically, sulphanilic acid has been known for its applications in the dye industry and as a precursor in the synthesis of various pharmaceuticals. However, its potential role in modulating bacterial behavior, particularly in the context of biofilm formation, represents a relatively new and exciting area of exploration.
Recent advancements in microbiology, biochemistry, and molecular biology have provided researchers with powerful tools to investigate the intricate interactions between sulphanilic acid and bacterial cells. These technological developments have enabled more detailed studies of biofilm structure, composition, and the signaling pathways involved in their formation and maintenance.
The exploration of sulphanilic acid's effects on biofilm formation aligns with the broader trend in antimicrobial research towards finding alternative strategies to combat bacterial infections. As traditional antibiotics face increasing challenges due to resistance, compounds that can modulate bacterial behavior without directly killing the cells offer promising avenues for therapeutic interventions.
Understanding the mechanisms by which sulphanilic acid influences biofilm formation may also provide insights into fundamental biological processes, such as cell-cell communication and bacterial adaptation to environmental stresses. This knowledge could potentially be leveraged to develop new approaches for controlling biofilms in various applications, ranging from medical devices to industrial water systems.
As we delve deeper into this research area, it is essential to consider the potential implications of sulphanilic acid-based strategies on microbial ecology and the possible development of resistance mechanisms. This comprehensive approach will ensure that any future applications of sulphanilic acid in biofilm control are both effective and sustainable.
Biofilms are complex communities of microorganisms that adhere to surfaces and are encased in a self-produced extracellular matrix. These structures pose significant challenges in medical, industrial, and environmental settings due to their increased resistance to antimicrobial agents and host immune responses. Understanding the mechanisms by which sulphanilic acid interacts with and modulates biofilm formation is crucial for developing effective strategies to prevent or disrupt these bacterial communities.
The primary objective of this research is to elucidate the molecular and cellular mechanisms through which sulphanilic acid influences biofilm formation in various bacterial species. This includes investigating its effects on bacterial adhesion, extracellular matrix production, quorum sensing, and other key processes involved in biofilm development.
Historically, sulphanilic acid has been known for its applications in the dye industry and as a precursor in the synthesis of various pharmaceuticals. However, its potential role in modulating bacterial behavior, particularly in the context of biofilm formation, represents a relatively new and exciting area of exploration.
Recent advancements in microbiology, biochemistry, and molecular biology have provided researchers with powerful tools to investigate the intricate interactions between sulphanilic acid and bacterial cells. These technological developments have enabled more detailed studies of biofilm structure, composition, and the signaling pathways involved in their formation and maintenance.
The exploration of sulphanilic acid's effects on biofilm formation aligns with the broader trend in antimicrobial research towards finding alternative strategies to combat bacterial infections. As traditional antibiotics face increasing challenges due to resistance, compounds that can modulate bacterial behavior without directly killing the cells offer promising avenues for therapeutic interventions.
Understanding the mechanisms by which sulphanilic acid influences biofilm formation may also provide insights into fundamental biological processes, such as cell-cell communication and bacterial adaptation to environmental stresses. This knowledge could potentially be leveraged to develop new approaches for controlling biofilms in various applications, ranging from medical devices to industrial water systems.
As we delve deeper into this research area, it is essential to consider the potential implications of sulphanilic acid-based strategies on microbial ecology and the possible development of resistance mechanisms. This comprehensive approach will ensure that any future applications of sulphanilic acid in biofilm control are both effective and sustainable.
Market Analysis for Biofilm Control Solutions
The global market for biofilm control solutions has been experiencing significant growth, driven by increasing awareness of the detrimental effects of biofilms across various industries. The market is primarily segmented into healthcare, food and beverage, water treatment, and industrial applications. In the healthcare sector, the demand for biofilm control solutions is particularly high due to the rising incidence of hospital-acquired infections and the need for stringent hygiene protocols in medical devices and surfaces.
The food and beverage industry represents another major market segment, where biofilm formation can lead to product contamination and equipment damage. As food safety regulations become more stringent worldwide, companies are investing heavily in biofilm prevention and removal technologies. The water treatment sector is also a key driver of market growth, with biofilm control being crucial for maintaining water quality in both industrial and municipal systems.
Market analysts project a compound annual growth rate (CAGR) of 5-7% for the biofilm control solutions market over the next five years. This growth is attributed to factors such as technological advancements in antimicrobial coatings, increasing research and development activities, and the growing adoption of preventive maintenance strategies across industries.
The market landscape is characterized by a mix of established players and innovative startups. Major companies in this space are focusing on developing eco-friendly and cost-effective solutions to gain a competitive edge. There is a notable trend towards the development of multi-functional products that not only prevent biofilm formation but also offer additional benefits such as corrosion protection or enhanced surface properties.
Regionally, North America and Europe currently dominate the market, owing to stringent regulations and high awareness levels. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, increasing healthcare expenditure, and growing concerns over water quality in developing countries.
The potential of sulphanilic acid as a biofilm modulator presents an interesting opportunity in this market. If proven effective, it could offer a novel approach to biofilm control, potentially disrupting current market dynamics. The healthcare and water treatment sectors would likely be the primary beneficiaries of such an innovation, given the critical nature of biofilm control in these areas.
The food and beverage industry represents another major market segment, where biofilm formation can lead to product contamination and equipment damage. As food safety regulations become more stringent worldwide, companies are investing heavily in biofilm prevention and removal technologies. The water treatment sector is also a key driver of market growth, with biofilm control being crucial for maintaining water quality in both industrial and municipal systems.
Market analysts project a compound annual growth rate (CAGR) of 5-7% for the biofilm control solutions market over the next five years. This growth is attributed to factors such as technological advancements in antimicrobial coatings, increasing research and development activities, and the growing adoption of preventive maintenance strategies across industries.
The market landscape is characterized by a mix of established players and innovative startups. Major companies in this space are focusing on developing eco-friendly and cost-effective solutions to gain a competitive edge. There is a notable trend towards the development of multi-functional products that not only prevent biofilm formation but also offer additional benefits such as corrosion protection or enhanced surface properties.
Regionally, North America and Europe currently dominate the market, owing to stringent regulations and high awareness levels. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, increasing healthcare expenditure, and growing concerns over water quality in developing countries.
The potential of sulphanilic acid as a biofilm modulator presents an interesting opportunity in this market. If proven effective, it could offer a novel approach to biofilm control, potentially disrupting current market dynamics. The healthcare and water treatment sectors would likely be the primary beneficiaries of such an innovation, given the critical nature of biofilm control in these areas.
Current Challenges in Bacterial Biofilm Management
Bacterial biofilm management presents significant challenges in various fields, including healthcare, industrial processes, and environmental protection. The persistent nature of biofilms and their resistance to conventional treatments make them particularly difficult to eradicate. One of the primary challenges is the complex structure of biofilms, which consists of multiple layers of bacteria embedded in a self-produced extracellular matrix. This matrix acts as a protective barrier, hindering the penetration of antibiotics and other antimicrobial agents.
The development of antibiotic resistance within biofilms poses another major challenge. Bacteria in biofilms can exchange genetic material more easily, facilitating the spread of resistance genes. Additionally, the slow growth rate of bacteria within biofilms makes them less susceptible to antibiotics that target actively dividing cells. This phenomenon contributes to the persistence of biofilm-associated infections and the recurrence of symptoms even after seemingly successful treatment.
The heterogeneity of biofilms further complicates management strategies. Different regions within a biofilm may have varying metabolic states and gene expression profiles, leading to differential responses to treatment. This heterogeneity makes it challenging to develop a single, universally effective approach to biofilm eradication. Moreover, the ability of biofilms to form on a wide range of surfaces, including medical devices and industrial equipment, necessitates the development of surface-specific management strategies.
Another significant challenge is the lack of standardized methods for assessing biofilm formation and evaluating the efficacy of anti-biofilm treatments. The absence of universally accepted protocols makes it difficult to compare results across different studies and hinders the development of effective management strategies. Furthermore, the translation of laboratory findings to real-world applications remains a considerable hurdle, as the complex environmental conditions in which biofilms naturally occur are often difficult to replicate in controlled settings.
The emergence of multi-species biofilms presents an additional layer of complexity. These biofilms, composed of multiple bacterial species, can exhibit enhanced resistance and resilience compared to single-species biofilms. The interactions between different species within these communities can lead to synergistic effects that further complicate management efforts. Understanding and targeting these inter-species interactions represent a frontier in biofilm research and management.
In the context of sulphanilic acid's potential role in modulating biofilm formation, addressing these challenges becomes crucial. Investigating how sulphanilic acid interacts with the biofilm matrix, affects bacterial communication, or influences the expression of biofilm-related genes could provide valuable insights into novel management strategies. However, developing a comprehensive understanding of its mechanisms of action and potential applications in diverse biofilm scenarios remains a significant challenge in the field.
The development of antibiotic resistance within biofilms poses another major challenge. Bacteria in biofilms can exchange genetic material more easily, facilitating the spread of resistance genes. Additionally, the slow growth rate of bacteria within biofilms makes them less susceptible to antibiotics that target actively dividing cells. This phenomenon contributes to the persistence of biofilm-associated infections and the recurrence of symptoms even after seemingly successful treatment.
The heterogeneity of biofilms further complicates management strategies. Different regions within a biofilm may have varying metabolic states and gene expression profiles, leading to differential responses to treatment. This heterogeneity makes it challenging to develop a single, universally effective approach to biofilm eradication. Moreover, the ability of biofilms to form on a wide range of surfaces, including medical devices and industrial equipment, necessitates the development of surface-specific management strategies.
Another significant challenge is the lack of standardized methods for assessing biofilm formation and evaluating the efficacy of anti-biofilm treatments. The absence of universally accepted protocols makes it difficult to compare results across different studies and hinders the development of effective management strategies. Furthermore, the translation of laboratory findings to real-world applications remains a considerable hurdle, as the complex environmental conditions in which biofilms naturally occur are often difficult to replicate in controlled settings.
The emergence of multi-species biofilms presents an additional layer of complexity. These biofilms, composed of multiple bacterial species, can exhibit enhanced resistance and resilience compared to single-species biofilms. The interactions between different species within these communities can lead to synergistic effects that further complicate management efforts. Understanding and targeting these inter-species interactions represent a frontier in biofilm research and management.
In the context of sulphanilic acid's potential role in modulating biofilm formation, addressing these challenges becomes crucial. Investigating how sulphanilic acid interacts with the biofilm matrix, affects bacterial communication, or influences the expression of biofilm-related genes could provide valuable insights into novel management strategies. However, developing a comprehensive understanding of its mechanisms of action and potential applications in diverse biofilm scenarios remains a significant challenge in the field.
Existing Approaches to Biofilm Modulation
01 Biofilm inhibition using sulphanilic acid derivatives
Certain derivatives of sulphanilic acid have been found to inhibit biofilm formation. These compounds can be used in various applications to prevent or reduce the growth of biofilms on surfaces. The mechanism of action involves interfering with bacterial communication and adhesion processes.- Biofilm inhibition using sulphanilic acid derivatives: Certain derivatives of sulphanilic acid have been found to inhibit biofilm formation. These compounds can interfere with bacterial communication systems or disrupt the extracellular matrix, preventing the establishment and growth of biofilms. This approach is particularly useful in medical and industrial settings where biofilm formation is problematic.
- Sulphanilic acid in antimicrobial compositions: Sulphanilic acid and its derivatives are incorporated into antimicrobial compositions to prevent or reduce biofilm formation. These compositions can be applied to surfaces or used in treatment solutions, providing a protective barrier against bacterial adhesion and subsequent biofilm development. The antimicrobial properties of sulphanilic acid contribute to the overall efficacy of these formulations.
- Biofilm detection methods using sulphanilic acid: Sulphanilic acid is utilized in various analytical techniques for the detection and characterization of biofilms. These methods often involve colorimetric or spectrophotometric assays where sulphanilic acid reacts with specific components of the biofilm, allowing for quantitative analysis. Such detection methods are crucial for assessing the effectiveness of anti-biofilm strategies and monitoring biofilm formation in various environments.
- Combination therapy with sulphanilic acid for biofilm control: Sulphanilic acid is used in combination with other compounds or treatments to enhance biofilm control. This synergistic approach may involve pairing sulphanilic acid with enzymes, other antimicrobial agents, or physical removal methods. The combined effect often results in more effective biofilm prevention or eradication compared to single-agent treatments.
- Sulphanilic acid in biofilm-resistant materials: Sulphanilic acid and its derivatives are incorporated into materials to create surfaces resistant to biofilm formation. This approach is particularly useful in developing medical devices, industrial equipment, and consumer products that are less susceptible to bacterial colonization. The modified materials can maintain their anti-biofilm properties for extended periods, providing long-term protection against microbial attachment and growth.
02 Sulphanilic acid in antimicrobial compositions
Sulphanilic acid and its derivatives are incorporated into antimicrobial compositions to enhance their efficacy against biofilm-forming microorganisms. These compositions can be used in medical, industrial, and consumer products to combat biofilm-related issues.Expand Specific Solutions03 Detection and quantification of biofilms using sulphanilic acid
Methods have been developed to detect and quantify biofilm formation using sulphanilic acid-based reagents. These techniques allow for the assessment of biofilm growth and the evaluation of anti-biofilm strategies in various settings.Expand Specific Solutions04 Sulphanilic acid in biofilm removal formulations
Formulations containing sulphanilic acid or its derivatives have been developed for the removal of existing biofilms. These products are designed to break down the extracellular matrix of biofilms and facilitate their detachment from surfaces.Expand Specific Solutions05 Modification of surfaces with sulphanilic acid to prevent biofilm formation
Surface modification techniques using sulphanilic acid or its derivatives have been explored to create biofilm-resistant materials. These modified surfaces can be applied in various industries to prevent the initial attachment and subsequent growth of biofilms.Expand Specific Solutions
Key Players in Biofilm Research and Antimicrobial Industry
The research on sulphanilic acid's modulation of bacterial biofilm formation is in its early stages, indicating an emerging field with significant potential for growth. The market size for this technology is currently limited but expected to expand as applications in healthcare and industrial settings become more apparent. Technologically, the field is still developing, with academic institutions like Katholieke Universiteit Leuven, University of Southern California, and University of Rochester leading research efforts. Companies such as Medline Industries and Vertex Pharmaceuticals may be exploring practical applications, but commercial products are likely still in development. The involvement of diverse institutions suggests a competitive landscape with opportunities for breakthrough innovations in antimicrobial strategies and biofilm control.
Katholieke Universiteit Leuven
Technical Solution: Katholieke Universiteit Leuven has developed a novel approach to modulate biofilm formation using sulphanilic acid. Their research focuses on the molecular mechanisms by which sulphanilic acid interferes with bacterial communication and biofilm development. The team has identified specific pathways and genes affected by sulphanilic acid, leading to reduced biofilm formation. They have demonstrated that sulphanilic acid can inhibit quorum sensing systems in various bacterial species, effectively disrupting the coordinated behavior necessary for biofilm establishment [1][3]. Additionally, the university has explored the potential of combining sulphanilic acid with conventional antibiotics to enhance their efficacy against biofilm-associated infections.
Strengths: Comprehensive understanding of molecular mechanisms; potential for combination therapies. Weaknesses: May be limited to specific bacterial species; potential for bacterial resistance development.
University of Southern California
Technical Solution: The University of Southern California has conducted extensive research on the effects of sulphanilic acid on bacterial biofilm formation. Their approach involves studying the impact of sulphanilic acid on the extracellular polymeric substances (EPS) produced by bacteria during biofilm formation. The team has discovered that sulphanilic acid can alter the composition and structure of EPS, leading to weakened biofilm integrity [2][5]. They have also investigated the role of sulphanilic acid in modifying bacterial surface properties, which affects initial attachment and subsequent biofilm development. USC researchers have developed novel imaging techniques to visualize the real-time effects of sulphanilic acid on biofilm formation, providing valuable insights into the kinetics of biofilm disruption.
Strengths: Advanced imaging techniques; focus on EPS and surface interactions. Weaknesses: May require high concentrations of sulphanilic acid for effectiveness; potential environmental concerns.
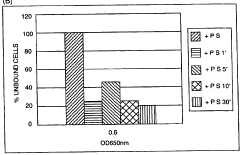
Sulphanilic Acid Mechanisms in Biofilm Formation
A method for depletion of caries-causing bacteria in the oral cavity
PatentWO2007146173A2
Innovation
- A family of non-toxic, insoluble compounds, specifically Sephadex-based dextran polymers, are used to bind selectively to S. mutans, allowing for their physical removal from the mouth without disturbing commensal bacteria, incorporated into chewing gum, toothpaste, or mouthwash for easy administration.
Methods and compositions for antimicrobial treatment
PatentActiveUS20210393815A1
Innovation
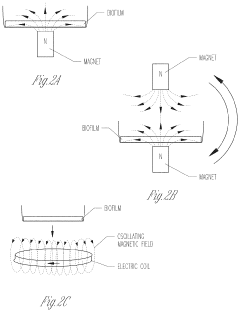
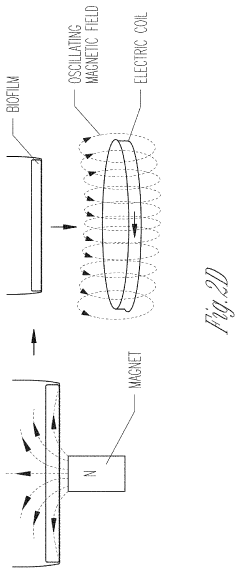
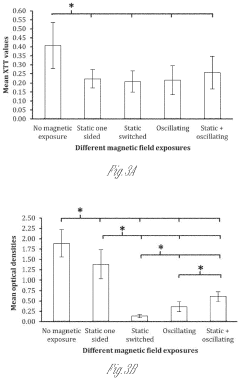
- Exposing biofilms to oscillating magnetic fields and contacting them with iron-based nanoparticles, such as Fe3O4, to disrupt the biofilm structure and enhance antibiotic penetration and efficacy.
Environmental Impact of Sulphanilic Acid Use
The use of sulphanilic acid in various industrial and consumer applications has raised concerns about its potential environmental impact. As a synthetic organic compound, sulphanilic acid can enter aquatic ecosystems through industrial effluents and wastewater treatment plants. Its presence in water bodies may have significant consequences for aquatic life and ecosystem balance.
One of the primary environmental concerns is the potential toxicity of sulphanilic acid to aquatic organisms. Studies have shown that exposure to elevated concentrations of sulphanilic acid can adversely affect fish, invertebrates, and algae. These effects may include reduced growth rates, impaired reproduction, and altered behavior patterns. The long-term ecological consequences of such impacts on aquatic populations and food webs remain a subject of ongoing research.
Furthermore, sulphanilic acid's persistence in the environment is a critical factor to consider. While it can undergo biodegradation under certain conditions, its rate of breakdown may vary depending on environmental factors such as temperature, pH, and the presence of specific microbial communities. Slow degradation rates could lead to accumulation in sediments and bioaccumulation in aquatic organisms, potentially magnifying its ecological impact over time.
The compound's ability to modulate biofilm formation in bacteria also raises questions about its broader ecological effects. Biofilms play crucial roles in aquatic ecosystems, including nutrient cycling and providing habitats for microorganisms. Alterations in biofilm formation due to sulphanilic acid exposure could disrupt these ecological processes, potentially leading to cascading effects throughout the ecosystem.
Additionally, the interaction of sulphanilic acid with other pollutants in aquatic environments is an area of concern. Synergistic effects with other contaminants may enhance toxicity or alter the compound's behavior in the environment. This complexity underscores the need for comprehensive environmental risk assessments that consider multiple stressors and their combined impacts on ecosystems.
Efforts to mitigate the environmental impact of sulphanilic acid use have focused on improving wastewater treatment technologies and implementing stricter regulations on industrial discharges. Advanced oxidation processes and biological treatment methods have shown promise in removing sulphanilic acid from wastewater, but their widespread adoption and effectiveness in real-world scenarios require further evaluation.
In conclusion, while sulphanilic acid serves important industrial functions, its potential environmental impact necessitates careful management and continued research. Balancing the compound's utility with ecological preservation remains a challenge that requires collaborative efforts from industry, researchers, and regulatory bodies to develop sustainable solutions and minimize environmental risks.
One of the primary environmental concerns is the potential toxicity of sulphanilic acid to aquatic organisms. Studies have shown that exposure to elevated concentrations of sulphanilic acid can adversely affect fish, invertebrates, and algae. These effects may include reduced growth rates, impaired reproduction, and altered behavior patterns. The long-term ecological consequences of such impacts on aquatic populations and food webs remain a subject of ongoing research.
Furthermore, sulphanilic acid's persistence in the environment is a critical factor to consider. While it can undergo biodegradation under certain conditions, its rate of breakdown may vary depending on environmental factors such as temperature, pH, and the presence of specific microbial communities. Slow degradation rates could lead to accumulation in sediments and bioaccumulation in aquatic organisms, potentially magnifying its ecological impact over time.
The compound's ability to modulate biofilm formation in bacteria also raises questions about its broader ecological effects. Biofilms play crucial roles in aquatic ecosystems, including nutrient cycling and providing habitats for microorganisms. Alterations in biofilm formation due to sulphanilic acid exposure could disrupt these ecological processes, potentially leading to cascading effects throughout the ecosystem.
Additionally, the interaction of sulphanilic acid with other pollutants in aquatic environments is an area of concern. Synergistic effects with other contaminants may enhance toxicity or alter the compound's behavior in the environment. This complexity underscores the need for comprehensive environmental risk assessments that consider multiple stressors and their combined impacts on ecosystems.
Efforts to mitigate the environmental impact of sulphanilic acid use have focused on improving wastewater treatment technologies and implementing stricter regulations on industrial discharges. Advanced oxidation processes and biological treatment methods have shown promise in removing sulphanilic acid from wastewater, but their widespread adoption and effectiveness in real-world scenarios require further evaluation.
In conclusion, while sulphanilic acid serves important industrial functions, its potential environmental impact necessitates careful management and continued research. Balancing the compound's utility with ecological preservation remains a challenge that requires collaborative efforts from industry, researchers, and regulatory bodies to develop sustainable solutions and minimize environmental risks.
Regulatory Framework for Antimicrobial Compounds
The regulatory framework for antimicrobial compounds plays a crucial role in governing the development, approval, and use of substances like sulphanilic acid in bacterial biofilm modulation. This framework encompasses a complex network of laws, guidelines, and standards set by various national and international regulatory bodies.
At the forefront of this regulatory landscape is the Food and Drug Administration (FDA) in the United States, which oversees the approval process for antimicrobial compounds. The FDA's Center for Drug Evaluation and Research (CDER) is responsible for evaluating the safety and efficacy of these substances before they can be marketed. Similarly, the European Medicines Agency (EMA) in the European Union provides guidelines and conducts assessments for antimicrobial agents.
These regulatory bodies require extensive preclinical and clinical studies to demonstrate the safety and efficacy of antimicrobial compounds. For substances like sulphanilic acid, which modulate biofilm formation, additional considerations may include their impact on microbial ecology and potential for resistance development.
The World Health Organization (WHO) also plays a significant role in shaping global policies on antimicrobial use. Their guidelines on the use of medically important antimicrobials in food-producing animals have implications for compounds that may find applications in both human and veterinary medicine.
Environmental regulations are another critical aspect of the framework. Agencies such as the Environmental Protection Agency (EPA) in the US and the European Environment Agency (EEA) set standards for the release of antimicrobial compounds into the environment, considering their potential ecological impacts.
In the context of biofilm modulation, regulatory bodies are increasingly recognizing the importance of alternative approaches to traditional antimicrobials. This has led to the development of specific guidelines for anti-biofilm agents, which may have different modes of action compared to conventional antibiotics.
The regulatory framework also addresses the manufacturing processes of antimicrobial compounds. Good Manufacturing Practices (GMP) guidelines ensure the quality and consistency of production, while pharmacovigilance regulations mandate ongoing monitoring of safety profiles post-market approval.
As research into biofilm modulation advances, regulatory bodies are adapting their frameworks to accommodate novel approaches. This includes the development of new testing methods to assess the efficacy of anti-biofilm agents and the establishment of appropriate endpoints for clinical trials involving biofilm-related infections.
At the forefront of this regulatory landscape is the Food and Drug Administration (FDA) in the United States, which oversees the approval process for antimicrobial compounds. The FDA's Center for Drug Evaluation and Research (CDER) is responsible for evaluating the safety and efficacy of these substances before they can be marketed. Similarly, the European Medicines Agency (EMA) in the European Union provides guidelines and conducts assessments for antimicrobial agents.
These regulatory bodies require extensive preclinical and clinical studies to demonstrate the safety and efficacy of antimicrobial compounds. For substances like sulphanilic acid, which modulate biofilm formation, additional considerations may include their impact on microbial ecology and potential for resistance development.
The World Health Organization (WHO) also plays a significant role in shaping global policies on antimicrobial use. Their guidelines on the use of medically important antimicrobials in food-producing animals have implications for compounds that may find applications in both human and veterinary medicine.
Environmental regulations are another critical aspect of the framework. Agencies such as the Environmental Protection Agency (EPA) in the US and the European Environment Agency (EEA) set standards for the release of antimicrobial compounds into the environment, considering their potential ecological impacts.
In the context of biofilm modulation, regulatory bodies are increasingly recognizing the importance of alternative approaches to traditional antimicrobials. This has led to the development of specific guidelines for anti-biofilm agents, which may have different modes of action compared to conventional antibiotics.
The regulatory framework also addresses the manufacturing processes of antimicrobial compounds. Good Manufacturing Practices (GMP) guidelines ensure the quality and consistency of production, while pharmacovigilance regulations mandate ongoing monitoring of safety profiles post-market approval.
As research into biofilm modulation advances, regulatory bodies are adapting their frameworks to accommodate novel approaches. This includes the development of new testing methods to assess the efficacy of anti-biofilm agents and the establishment of appropriate endpoints for clinical trials involving biofilm-related infections.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!