How to Analyze Stearic Acid's Electrostatic Properties
SEP 24, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Stearic Acid Electrostatic Analysis Background and Objectives
Stearic acid, a saturated fatty acid with the chemical formula C18H36O2, has been extensively studied since its isolation in the early 19th century. The understanding of its electrostatic properties has evolved significantly over the past century, from basic characterization to sophisticated analytical methods employing advanced computational modeling and spectroscopic techniques. The trajectory of research in this field has shifted from purely academic interest to practical applications across multiple industries, including pharmaceuticals, cosmetics, food processing, and materials science.
The electrostatic properties of stearic acid are fundamentally linked to its molecular structure, featuring a carboxylic acid head group and a long hydrocarbon tail. This amphipathic nature creates unique charge distribution patterns that influence its behavior at interfaces and in various chemical environments. Historical approaches to analyzing these properties relied primarily on macroscopic observations, but modern techniques have enabled investigations at the molecular and even atomic levels.
Recent technological advancements have significantly enhanced our ability to analyze the electrostatic characteristics of stearic acid with unprecedented precision. Techniques such as atomic force microscopy (AFM), Kelvin probe force microscopy (KPFM), and various spectroscopic methods have revolutionized how researchers approach this subject. Computational methods, including molecular dynamics simulations and density functional theory (DFT) calculations, have further expanded our theoretical understanding of charge distributions and interactions.
The primary objective of this technical research report is to comprehensively evaluate current methodologies for analyzing the electrostatic properties of stearic acid, identifying their strengths, limitations, and potential applications. We aim to establish a systematic framework for selecting appropriate analytical techniques based on specific research or industrial requirements, considering factors such as required precision, sample conditions, and available resources.
Additionally, this report seeks to identify emerging trends and innovative approaches in electrostatic analysis that may address current technological gaps. By examining cutting-edge research and cross-disciplinary applications, we intend to forecast potential breakthroughs that could transform our understanding and utilization of stearic acid's electrostatic properties in various fields.
Furthermore, we aim to explore how advances in this specific area might contribute to broader scientific understanding of molecular interactions and self-assembly processes. The insights gained from studying stearic acid's electrostatic properties have implications for fundamental research in physical chemistry, materials science, and biological systems, particularly in areas related to lipid membranes and protein-lipid interactions.
Finally, this report will establish clear metrics for evaluating the effectiveness of different analytical approaches, providing a valuable resource for researchers and industry professionals seeking to optimize their methodologies for specific applications involving stearic acid and similar fatty acids.
The electrostatic properties of stearic acid are fundamentally linked to its molecular structure, featuring a carboxylic acid head group and a long hydrocarbon tail. This amphipathic nature creates unique charge distribution patterns that influence its behavior at interfaces and in various chemical environments. Historical approaches to analyzing these properties relied primarily on macroscopic observations, but modern techniques have enabled investigations at the molecular and even atomic levels.
Recent technological advancements have significantly enhanced our ability to analyze the electrostatic characteristics of stearic acid with unprecedented precision. Techniques such as atomic force microscopy (AFM), Kelvin probe force microscopy (KPFM), and various spectroscopic methods have revolutionized how researchers approach this subject. Computational methods, including molecular dynamics simulations and density functional theory (DFT) calculations, have further expanded our theoretical understanding of charge distributions and interactions.
The primary objective of this technical research report is to comprehensively evaluate current methodologies for analyzing the electrostatic properties of stearic acid, identifying their strengths, limitations, and potential applications. We aim to establish a systematic framework for selecting appropriate analytical techniques based on specific research or industrial requirements, considering factors such as required precision, sample conditions, and available resources.
Additionally, this report seeks to identify emerging trends and innovative approaches in electrostatic analysis that may address current technological gaps. By examining cutting-edge research and cross-disciplinary applications, we intend to forecast potential breakthroughs that could transform our understanding and utilization of stearic acid's electrostatic properties in various fields.
Furthermore, we aim to explore how advances in this specific area might contribute to broader scientific understanding of molecular interactions and self-assembly processes. The insights gained from studying stearic acid's electrostatic properties have implications for fundamental research in physical chemistry, materials science, and biological systems, particularly in areas related to lipid membranes and protein-lipid interactions.
Finally, this report will establish clear metrics for evaluating the effectiveness of different analytical approaches, providing a valuable resource for researchers and industry professionals seeking to optimize their methodologies for specific applications involving stearic acid and similar fatty acids.
Market Applications and Demand for Stearic Acid Electrostatic Properties
The global market for stearic acid has been experiencing steady growth, with a significant portion of demand driven by applications that leverage its electrostatic properties. The market size for stearic acid was valued at approximately 9.4 billion USD in 2022, with projections indicating growth at a compound annual rate of 5.2% through 2030. Industries such as plastics, rubber, personal care, and pharmaceuticals represent the primary demand sectors where electrostatic properties play a crucial role.
In the plastics industry, which accounts for nearly 30% of stearic acid consumption, manufacturers utilize stearic acid as an anti-static agent and mold release compound. The growing demand for high-performance plastics in automotive and electronics sectors has intensified research into optimizing stearic acid's electrostatic behavior to improve product quality and processing efficiency.
The rubber manufacturing sector represents another significant market, where stearic acid functions as an activator in vulcanization processes. Here, understanding and controlling electrostatic properties is essential for ensuring proper dispersion of ingredients and preventing agglomeration during processing. With the global tire industry expanding at approximately 4% annually, demand for advanced stearic acid formulations with tailored electrostatic properties continues to rise.
Personal care and cosmetic applications constitute roughly 22% of the market, where stearic acid's electrostatic properties influence product texture, stability, and skin adhesion characteristics. Consumer preference for long-lasting cosmetics and improved sensory experiences has driven manufacturers to invest in research focused on manipulating stearic acid's electrostatic interactions with other formulation components.
Pharmaceutical applications represent a smaller but higher-value segment, where stearic acid's electrostatic properties affect drug formulation, tablet coating, and controlled release mechanisms. The pharmaceutical excipients market is growing at 6.7% annually, with increasing demand for excipients that offer precise electrostatic control to improve bioavailability and stability.
Regionally, Asia-Pacific dominates consumption, accounting for approximately 45% of global demand, followed by North America and Europe. The rapid industrialization in countries like China and India has significantly increased demand for stearic acid across multiple applications where electrostatic properties are critical performance factors.
Emerging applications in nanotechnology, 3D printing materials, and advanced coatings are creating new market opportunities that specifically leverage stearic acid's unique electrostatic characteristics. These high-growth sectors are expected to generate premium pricing for specialized grades of stearic acid with precisely engineered electrostatic properties, potentially expanding market value by an additional 15-20% over the next five years.
In the plastics industry, which accounts for nearly 30% of stearic acid consumption, manufacturers utilize stearic acid as an anti-static agent and mold release compound. The growing demand for high-performance plastics in automotive and electronics sectors has intensified research into optimizing stearic acid's electrostatic behavior to improve product quality and processing efficiency.
The rubber manufacturing sector represents another significant market, where stearic acid functions as an activator in vulcanization processes. Here, understanding and controlling electrostatic properties is essential for ensuring proper dispersion of ingredients and preventing agglomeration during processing. With the global tire industry expanding at approximately 4% annually, demand for advanced stearic acid formulations with tailored electrostatic properties continues to rise.
Personal care and cosmetic applications constitute roughly 22% of the market, where stearic acid's electrostatic properties influence product texture, stability, and skin adhesion characteristics. Consumer preference for long-lasting cosmetics and improved sensory experiences has driven manufacturers to invest in research focused on manipulating stearic acid's electrostatic interactions with other formulation components.
Pharmaceutical applications represent a smaller but higher-value segment, where stearic acid's electrostatic properties affect drug formulation, tablet coating, and controlled release mechanisms. The pharmaceutical excipients market is growing at 6.7% annually, with increasing demand for excipients that offer precise electrostatic control to improve bioavailability and stability.
Regionally, Asia-Pacific dominates consumption, accounting for approximately 45% of global demand, followed by North America and Europe. The rapid industrialization in countries like China and India has significantly increased demand for stearic acid across multiple applications where electrostatic properties are critical performance factors.
Emerging applications in nanotechnology, 3D printing materials, and advanced coatings are creating new market opportunities that specifically leverage stearic acid's unique electrostatic characteristics. These high-growth sectors are expected to generate premium pricing for specialized grades of stearic acid with precisely engineered electrostatic properties, potentially expanding market value by an additional 15-20% over the next five years.
Current Analytical Methods and Technical Challenges
The analysis of stearic acid's electrostatic properties currently employs several established methodologies, each with specific advantages and limitations. Surface charge measurement techniques, including zeta potential analysis and electrophoretic mobility measurements, provide quantitative data on the acid's surface charge distribution and magnitude. These methods typically utilize specialized instruments such as Zetasizers that track particle movement under applied electric fields, yielding valuable insights into colloidal stability and interfacial behavior.
Computational modeling has emerged as a powerful complementary approach, with molecular dynamics simulations and density functional theory calculations enabling researchers to visualize charge distributions at atomic resolution. These computational methods can predict electrostatic potential maps and charge transfer mechanisms, though they require significant computational resources and expertise in quantum chemistry principles.
Spectroscopic techniques, particularly Fourier-transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR), offer indirect insights into electrostatic properties by characterizing hydrogen bonding patterns and functional group interactions. These methods are widely accessible but provide less direct information about charge distribution compared to dedicated electrostatic measurement techniques.
Despite these advances, significant technical challenges persist in the comprehensive analysis of stearic acid's electrostatic properties. Environmental sensitivity represents a major obstacle, as temperature, pH, and ionic strength dramatically influence electrostatic interactions, necessitating precise control of experimental conditions. Many researchers report inconsistent results across different measurement platforms, highlighting issues with standardization and reproducibility.
Resolution limitations also hamper progress, as conventional techniques struggle to capture nanoscale charge heterogeneity across stearic acid molecules and their assemblies. This is particularly problematic when investigating complex interfaces or mixed systems where stearic acid interacts with other components.
The dynamic nature of electrostatic properties presents another challenge, as most analytical methods provide only static snapshots rather than capturing the temporal evolution of charge distributions. This limitation is especially relevant for understanding stearic acid behavior in biological systems or industrial processes where conditions fluctuate continuously.
Instrument accessibility remains a barrier for comprehensive analysis, with advanced techniques like Kelvin probe force microscopy or advanced computational resources often available only at specialized research facilities. This restricts widespread adoption of cutting-edge analytical approaches and limits cross-validation of results across different laboratories.
Computational modeling has emerged as a powerful complementary approach, with molecular dynamics simulations and density functional theory calculations enabling researchers to visualize charge distributions at atomic resolution. These computational methods can predict electrostatic potential maps and charge transfer mechanisms, though they require significant computational resources and expertise in quantum chemistry principles.
Spectroscopic techniques, particularly Fourier-transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR), offer indirect insights into electrostatic properties by characterizing hydrogen bonding patterns and functional group interactions. These methods are widely accessible but provide less direct information about charge distribution compared to dedicated electrostatic measurement techniques.
Despite these advances, significant technical challenges persist in the comprehensive analysis of stearic acid's electrostatic properties. Environmental sensitivity represents a major obstacle, as temperature, pH, and ionic strength dramatically influence electrostatic interactions, necessitating precise control of experimental conditions. Many researchers report inconsistent results across different measurement platforms, highlighting issues with standardization and reproducibility.
Resolution limitations also hamper progress, as conventional techniques struggle to capture nanoscale charge heterogeneity across stearic acid molecules and their assemblies. This is particularly problematic when investigating complex interfaces or mixed systems where stearic acid interacts with other components.
The dynamic nature of electrostatic properties presents another challenge, as most analytical methods provide only static snapshots rather than capturing the temporal evolution of charge distributions. This limitation is especially relevant for understanding stearic acid behavior in biological systems or industrial processes where conditions fluctuate continuously.
Instrument accessibility remains a barrier for comprehensive analysis, with advanced techniques like Kelvin probe force microscopy or advanced computational resources often available only at specialized research facilities. This restricts widespread adoption of cutting-edge analytical approaches and limits cross-validation of results across different laboratories.
Established Protocols for Stearic Acid Electrostatic Characterization
01 Electrostatic properties in polymer compositions
Stearic acid is used as an additive in polymer compositions to modify electrostatic properties. It functions as a charge control agent that can reduce static electricity buildup in polymeric materials. When incorporated into polymer matrices, stearic acid can alter the surface resistivity and triboelectric charging characteristics, making the resulting materials less prone to attracting dust or creating sparks. This property is particularly valuable in applications where static discharge could cause damage or safety hazards.- Electrostatic properties in polymer compositions: Stearic acid is used in polymer compositions to modify electrostatic properties. It acts as an anti-static agent by reducing surface resistivity and preventing charge accumulation. When incorporated into polymeric materials, stearic acid can improve the processing characteristics and final product performance by controlling static electricity generation. This is particularly important in applications where static discharge could cause damage or safety hazards.
- Surface modification and coating applications: Stearic acid is utilized in surface treatments and coatings to alter electrostatic properties of substrates. The amphiphilic nature of stearic acid, with its polar head group and non-polar tail, allows it to form organized layers on surfaces that can modify charge distribution. These coatings can provide anti-static properties, improve adhesion, or create hydrophobic barriers while controlling electrostatic interactions between the coated surface and its environment.
- Electrostatic stabilization in colloidal systems: Stearic acid plays a crucial role in stabilizing colloidal dispersions through electrostatic mechanisms. When added to particle suspensions, stearic acid molecules adsorb onto particle surfaces, creating charged layers that generate repulsive forces between particles. This electrostatic stabilization prevents agglomeration and sedimentation, maintaining the stability and performance of formulations such as emulsions, suspensions, and other dispersion systems.
- Triboelectric charging behavior: Stearic acid significantly influences triboelectric charging behavior in various materials. It can be incorporated into polymers and other substrates to control their position in the triboelectric series, which determines whether they tend to donate or accept electrons upon contact with other materials. This property is exploited in applications requiring controlled static generation or dissipation, such as in electrophotography, powder coating processes, and electronic components manufacturing.
- Electrostatic properties in pharmaceutical and cosmetic formulations: In pharmaceutical and cosmetic applications, stearic acid is used to modify electrostatic properties of formulations. It helps control the adhesion of powders to surfaces, improves flow properties, and reduces caking or agglomeration caused by electrostatic attraction. The ability of stearic acid to modify surface charge also affects the interaction of formulations with biological surfaces, influencing drug delivery efficiency or cosmetic product performance on skin or hair.
02 Antistatic coatings and treatments
Stearic acid is utilized in antistatic coatings and surface treatments to control electrostatic charge accumulation. When applied to surfaces, it forms a thin film that can dissipate static charges. These treatments are particularly important for electronic components, packaging materials, and textiles where static electricity can cause damage or interference. The polar carboxyl group of stearic acid contributes to its ability to modify surface conductivity and charge retention properties.Expand Specific Solutions03 Electrostatic properties in powder processing
Stearic acid is employed as a processing aid in powder technologies to control electrostatic properties. It serves as a surface modifier that can reduce particle agglomeration caused by electrostatic attraction. When powder particles are coated with stearic acid, their surface charge characteristics are altered, leading to improved flow properties and reduced adhesion. This is particularly beneficial in pharmaceutical formulations, cosmetic powders, and industrial powder processing where consistent flow and dispersion are critical.Expand Specific Solutions04 Electrostatic behavior in emulsions and dispersions
The electrostatic properties of stearic acid play a crucial role in stabilizing emulsions and dispersions. When ionized in aqueous systems, stearic acid forms carboxylate ions that contribute to the electrostatic repulsion between dispersed particles or droplets. This electrostatic stabilization mechanism prevents coalescence and phase separation. The zeta potential of stearic acid-stabilized systems can be adjusted by controlling pH and ionic strength, allowing for tailored stability profiles in various applications including cosmetics, pharmaceuticals, and food products.Expand Specific Solutions05 Modification of electrostatic properties through metal soaps
Stearic acid can be converted to metal stearates (soaps) that exhibit distinct electrostatic properties. These metal soaps, formed by reacting stearic acid with metal ions such as calcium, zinc, or aluminum, can function as electrostatic charge modifiers in various applications. The type of metal ion influences the resulting electrostatic behavior, allowing for customization based on specific requirements. Metal stearates are used in plastics, rubber, and lubricants to control static charge buildup and improve processing characteristics by modifying surface resistivity and charge dissipation rates.Expand Specific Solutions
Leading Research Institutions and Industrial Players
The electrostatic properties analysis of stearic acid represents an emerging technical field currently in its growth phase. The market is expanding steadily with an estimated global value of $300-400 million, driven by applications in electronics, pharmaceuticals, and materials science. Technologically, the field shows moderate maturity with established analytical methods but significant room for innovation. Leading players demonstrate varying levels of expertise: Sanyo Chemical Industries and Adeka Corp have developed proprietary surfactant technologies leveraging stearic acid's electrostatic properties; LG Energy Solution focuses on battery applications; while research institutions like Shandong University and Rensselaer Polytechnic Institute are advancing fundamental understanding through computational modeling. Phillips 66 and ExxonMobil Chemical Patents are integrating these analyses into industrial processes, indicating growing commercial relevance.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced molecular dynamics simulation techniques to analyze stearic acid's electrostatic properties at interfaces. Their approach combines quantum mechanical calculations with molecular dynamics simulations to quantify charge distribution and dipole moments in stearic acid molecules under various environmental conditions. The company utilizes Density Functional Theory (DFT) calculations to determine partial charges on atoms within stearic acid molecules, enabling precise mapping of electrostatic potential surfaces. Sinopec's methodology incorporates temperature and pressure variables to simulate realistic industrial conditions, particularly focusing on stearic acid behavior in petroleum processing and surfactant applications. Their proprietary software integrates these simulations with experimental validation through zeta potential measurements and surface charge density analyses.
Strengths: Comprehensive integration of computational and experimental methods; extensive experience with hydrocarbon compounds; industrial-scale validation capabilities. Weaknesses: Computational models may require significant processing resources; some aspects of their methodology remain proprietary and not fully disclosed in scientific literature.
Rensselaer Polytechnic Institute
Technical Solution: Rensselaer Polytechnic Institute has developed an innovative approach to analyzing stearic acid's electrostatic properties through advanced spectroscopic techniques combined with computational modeling. Their methodology employs sum-frequency generation (SFG) vibrational spectroscopy to probe the orientation and conformation of stearic acid molecules at interfaces, providing direct information about dipole alignment and charge distribution. RPI researchers have created custom software that integrates molecular dynamics simulations with experimental data to generate comprehensive electrostatic potential maps of stearic acid monolayers. Their approach includes systematic investigation of substrate effects on stearic acid's electrostatic properties, particularly focusing on metal oxide surfaces relevant to catalysis and materials science applications. The institute has pioneered the use of temperature-dependent electrostatic force microscopy to characterize how thermal fluctuations affect charge distribution in stearic acid films, with particular attention to phase transition temperatures where significant changes in electrostatic behavior occur.
Strengths: Cutting-edge spectroscopic techniques providing molecular-level insights; strong theoretical foundation; excellent integration of experimental and computational approaches. Weaknesses: Highly specialized equipment requirements; methodology may be more suitable for fundamental research than routine industrial analysis.
Key Scientific Principles and Measurement Technologies
Method for assessment of electrostatic properties of fibers or substrates
PatentInactiveUS8198901B2
Innovation
- A method involving placing samples of fibers or substrates in close proximity to particles capable of reacting to electrostatic charges, assessing the quantity of adhering particles, and using these assessments to compare treated and untreated samples or different compositions, allowing for direct visualization and effective demonstration of a composition's efficacy in minimizing particle accumulation.
Assessment of risk of hypertension and methods based thereon
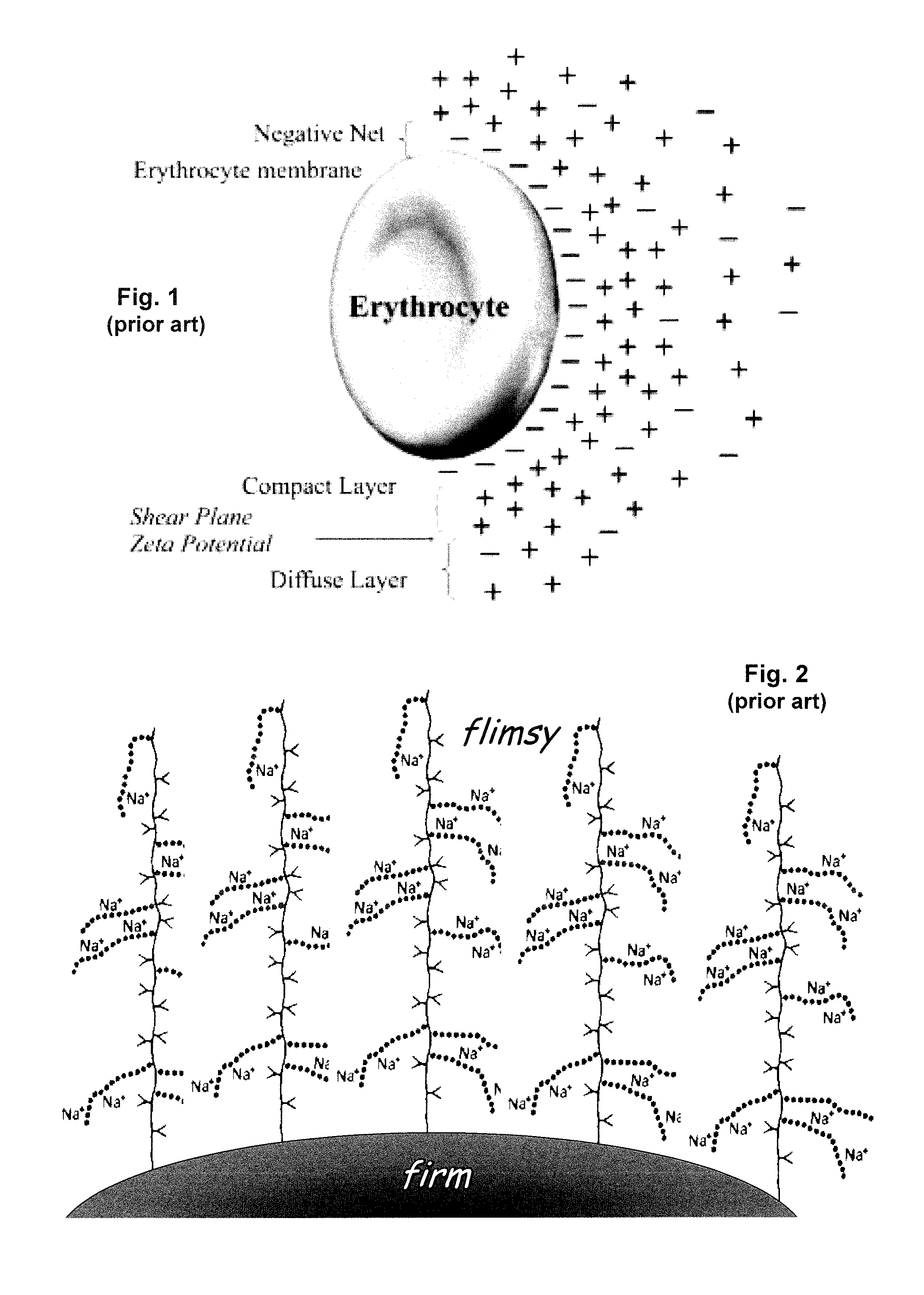
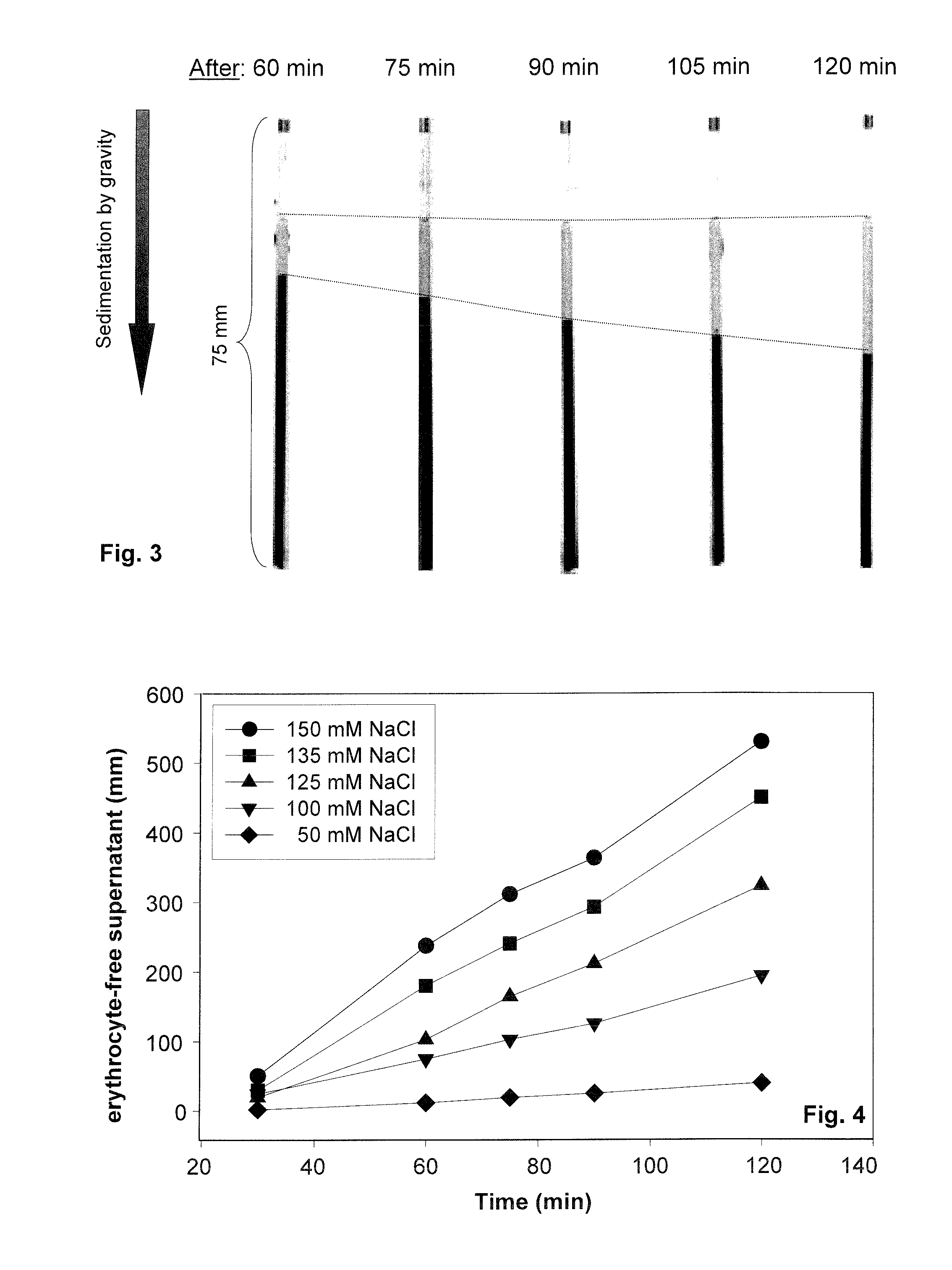
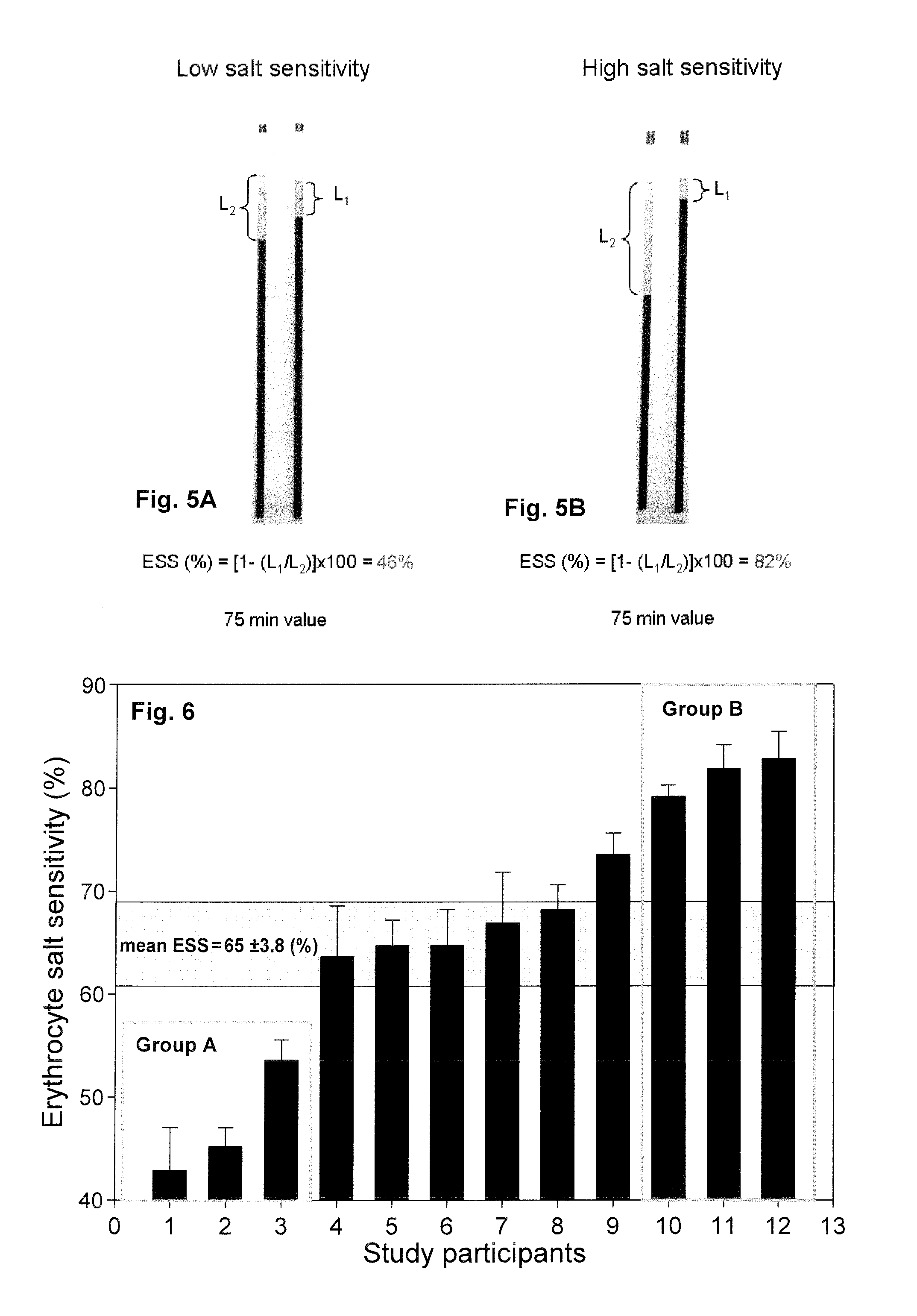
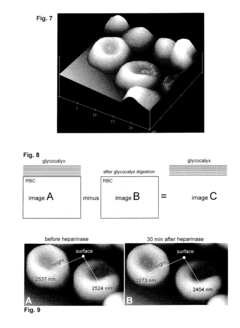
PatentInactiveUS20150308939A1
Innovation
- A method involving the analysis of erythrocyte glycocalyx thickness and zeta potential in response to varying sodium chloride concentrations to determine an individual's sensitivity to sodium intake, allowing for a quick and simple test to assess hypertension risk without requiring extensive equipment or expertise.
Environmental Factors Affecting Electrostatic Measurements
Environmental conditions play a crucial role in the accurate measurement and analysis of stearic acid's electrostatic properties. Temperature variations significantly impact the electrostatic behavior of stearic acid, as higher temperatures increase molecular mobility and affect charge distribution across the molecule's surface. Research indicates that measurements conducted at temperatures above 69.6°C (stearic acid's melting point) yield substantially different results compared to solid-state analyses, necessitating temperature-controlled environments for consistent data collection.
Humidity represents another critical environmental factor, as moisture content in the surrounding air can dramatically alter surface charge accumulation. High relative humidity (typically above 60%) leads to the formation of thin water films on stearic acid surfaces, which facilitate charge dissipation and reduce measurable electrostatic potential. Conversely, extremely dry conditions (below 20% RH) may promote excessive charge buildup, potentially skewing analytical results.
Atmospheric pressure variations, though less pronounced in their effects, can influence the interaction between stearic acid molecules and surrounding gas particles, thereby affecting charge transfer mechanisms. Barometric pressure fluctuations may require consideration when conducting highly sensitive electrostatic measurements, particularly in applications involving fine powders or aerosols containing stearic acid.
Light exposure constitutes an often overlooked environmental variable that can impact electrostatic measurements. Ultraviolet radiation may induce photochemical reactions in stearic acid, potentially altering its surface properties and charge distribution characteristics. Controlled lighting conditions are therefore recommended during analytical procedures to minimize this source of variability.
Air quality factors, including particulate matter concentration and gaseous contaminants, can significantly affect measurement accuracy. Airborne particles may adhere to stearic acid surfaces, introducing foreign charge carriers and distorting native electrostatic properties. Similarly, reactive gases can interact with stearic acid's carboxyl group, potentially neutralizing surface charges or creating new charge distribution patterns.
Electromagnetic interference from nearby equipment or power sources represents another environmental consideration. Even low-level electromagnetic fields can disrupt sensitive electrostatic measurements, necessitating proper shielding and isolation of analytical instruments. This is particularly relevant when utilizing field meters or charge detection devices for quantitative analysis of stearic acid's electrostatic behavior.
Standardized testing protocols typically recommend maintaining environmental conditions at 23±2°C and 50±5% relative humidity, with minimal air movement and controlled lighting. Advanced research facilities often employ environmental chambers capable of precisely regulating these parameters to ensure measurement reproducibility across different experimental sessions and laboratory locations.
Humidity represents another critical environmental factor, as moisture content in the surrounding air can dramatically alter surface charge accumulation. High relative humidity (typically above 60%) leads to the formation of thin water films on stearic acid surfaces, which facilitate charge dissipation and reduce measurable electrostatic potential. Conversely, extremely dry conditions (below 20% RH) may promote excessive charge buildup, potentially skewing analytical results.
Atmospheric pressure variations, though less pronounced in their effects, can influence the interaction between stearic acid molecules and surrounding gas particles, thereby affecting charge transfer mechanisms. Barometric pressure fluctuations may require consideration when conducting highly sensitive electrostatic measurements, particularly in applications involving fine powders or aerosols containing stearic acid.
Light exposure constitutes an often overlooked environmental variable that can impact electrostatic measurements. Ultraviolet radiation may induce photochemical reactions in stearic acid, potentially altering its surface properties and charge distribution characteristics. Controlled lighting conditions are therefore recommended during analytical procedures to minimize this source of variability.
Air quality factors, including particulate matter concentration and gaseous contaminants, can significantly affect measurement accuracy. Airborne particles may adhere to stearic acid surfaces, introducing foreign charge carriers and distorting native electrostatic properties. Similarly, reactive gases can interact with stearic acid's carboxyl group, potentially neutralizing surface charges or creating new charge distribution patterns.
Electromagnetic interference from nearby equipment or power sources represents another environmental consideration. Even low-level electromagnetic fields can disrupt sensitive electrostatic measurements, necessitating proper shielding and isolation of analytical instruments. This is particularly relevant when utilizing field meters or charge detection devices for quantitative analysis of stearic acid's electrostatic behavior.
Standardized testing protocols typically recommend maintaining environmental conditions at 23±2°C and 50±5% relative humidity, with minimal air movement and controlled lighting. Advanced research facilities often employ environmental chambers capable of precisely regulating these parameters to ensure measurement reproducibility across different experimental sessions and laboratory locations.
Computational Modeling and Simulation Methods
Computational modeling and simulation methods have become indispensable tools for analyzing the electrostatic properties of stearic acid. Molecular dynamics (MD) simulations offer a powerful approach to investigate the behavior of stearic acid molecules in various environments. These simulations typically employ force fields such as CHARMM, AMBER, or GROMOS, which contain parameters specifically optimized for lipid molecules. When conducting MD simulations for stearic acid, researchers must carefully consider the appropriate time scales, ranging from nanoseconds to microseconds, to capture relevant electrostatic interactions and conformational changes.
Density Functional Theory (DFT) calculations represent another valuable computational method for analyzing stearic acid's electrostatic properties at the quantum mechanical level. DFT approaches can accurately determine electron density distributions, dipole moments, and partial charges within the molecule. Common DFT functionals employed include B3LYP, PBE, and M06-2X, typically used with basis sets such as 6-31G(d,p) or cc-pVTZ. These calculations provide insights into the fundamental electronic structure that governs electrostatic interactions.
Monte Carlo (MC) simulations offer complementary capabilities by efficiently sampling configurational space to determine thermodynamic properties related to electrostatic interactions. MC methods are particularly useful for studying stearic acid in complex environments such as interfaces or within micelles, where electrostatic properties significantly influence molecular organization and function.
Coarse-grained modeling approaches have gained popularity for analyzing larger-scale systems containing stearic acid. Models such as MARTINI reduce computational complexity by representing groups of atoms as single interaction sites while preserving essential electrostatic characteristics. This approach enables simulations of systems containing thousands of stearic acid molecules, allowing researchers to investigate emergent properties arising from collective electrostatic interactions.
Continuum electrostatics models, including Poisson-Boltzmann (PB) and Generalized Born (GB) methods, provide efficient means to calculate electrostatic potentials around stearic acid molecules in solution. These approaches treat the solvent as a continuous dielectric medium rather than explicit molecules, significantly reducing computational costs while maintaining reasonable accuracy for many applications involving electrostatic properties.
Integration of multiple computational methods has emerged as a best practice for comprehensive analysis of stearic acid's electrostatic properties. For instance, quantum mechanical calculations might determine accurate partial charges, which are subsequently used in molecular dynamics simulations to explore dynamic behavior. This multi-scale modeling approach leverages the strengths of different computational techniques to provide a more complete understanding of stearic acid's electrostatic characteristics across different spatial and temporal scales.
Density Functional Theory (DFT) calculations represent another valuable computational method for analyzing stearic acid's electrostatic properties at the quantum mechanical level. DFT approaches can accurately determine electron density distributions, dipole moments, and partial charges within the molecule. Common DFT functionals employed include B3LYP, PBE, and M06-2X, typically used with basis sets such as 6-31G(d,p) or cc-pVTZ. These calculations provide insights into the fundamental electronic structure that governs electrostatic interactions.
Monte Carlo (MC) simulations offer complementary capabilities by efficiently sampling configurational space to determine thermodynamic properties related to electrostatic interactions. MC methods are particularly useful for studying stearic acid in complex environments such as interfaces or within micelles, where electrostatic properties significantly influence molecular organization and function.
Coarse-grained modeling approaches have gained popularity for analyzing larger-scale systems containing stearic acid. Models such as MARTINI reduce computational complexity by representing groups of atoms as single interaction sites while preserving essential electrostatic characteristics. This approach enables simulations of systems containing thousands of stearic acid molecules, allowing researchers to investigate emergent properties arising from collective electrostatic interactions.
Continuum electrostatics models, including Poisson-Boltzmann (PB) and Generalized Born (GB) methods, provide efficient means to calculate electrostatic potentials around stearic acid molecules in solution. These approaches treat the solvent as a continuous dielectric medium rather than explicit molecules, significantly reducing computational costs while maintaining reasonable accuracy for many applications involving electrostatic properties.
Integration of multiple computational methods has emerged as a best practice for comprehensive analysis of stearic acid's electrostatic properties. For instance, quantum mechanical calculations might determine accurate partial charges, which are subsequently used in molecular dynamics simulations to explore dynamic behavior. This multi-scale modeling approach leverages the strengths of different computational techniques to provide a more complete understanding of stearic acid's electrostatic characteristics across different spatial and temporal scales.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!