Stearic Acid vs Myristic Acid: Foam Characteristics in Cleaners
SEP 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fatty Acid Surfactant Evolution and Research Objectives
Fatty acids have been utilized as surfactants in cleaning formulations for centuries, evolving from simple soap-making processes to sophisticated molecular engineering. The journey began with traditional soap production using animal fats and plant oils, which naturally contain fatty acids like stearic and myristic acids. By the early 20th century, the chemical structures of these compounds were elucidated, revealing their amphiphilic nature with hydrophilic carboxyl heads and hydrophobic hydrocarbon tails.
The 1940s marked a significant turning point with the development of synthetic detergents, which offered improved performance in hard water compared to traditional soaps. This innovation catalyzed intensive research into fatty acid-based surfactants, exploring how chain length affects properties such as solubility, critical micelle concentration, and foaming characteristics.
Stearic acid (C18) and myristic acid (C14) represent two distinct points on the fatty acid spectrum, with their structural differences driving varied performance in cleaning applications. The four-carbon difference significantly impacts their physical properties, solubility profiles, and interaction with water and soil particles. Understanding these differences has become increasingly important as consumer preferences shift toward specialized cleaning products with specific foam profiles.
Recent technological advancements in analytical chemistry, particularly in high-resolution spectroscopy and computational modeling, have enabled researchers to visualize and predict foam behavior at the molecular level. This has transformed formulation science from an empirical art to a precise engineering discipline, allowing for targeted design of cleaning products with optimized foam characteristics.
The current research landscape focuses on understanding the fundamental mechanisms of foam generation, stability, and collapse as influenced by fatty acid structure. Particular attention is being paid to the kinetics of foam formation, bubble size distribution, and drainage rates—all of which vary significantly between stearic and myristic acid-based formulations.
Our research objectives center on quantifying these differences through standardized testing protocols, developing predictive models for foam behavior based on fatty acid composition, and establishing clear guidelines for formulation scientists. We aim to create a comprehensive framework that correlates molecular structure with macroscopic foam properties, enabling precise control over foam characteristics in various cleaning applications.
Additionally, we seek to explore synergistic effects when these fatty acids are combined with other surfactants, as well as the impact of environmental factors such as water hardness, temperature, and pH on their foaming behavior. This holistic approach will provide valuable insights for developing next-generation cleaning products with optimized performance and enhanced user experience.
The 1940s marked a significant turning point with the development of synthetic detergents, which offered improved performance in hard water compared to traditional soaps. This innovation catalyzed intensive research into fatty acid-based surfactants, exploring how chain length affects properties such as solubility, critical micelle concentration, and foaming characteristics.
Stearic acid (C18) and myristic acid (C14) represent two distinct points on the fatty acid spectrum, with their structural differences driving varied performance in cleaning applications. The four-carbon difference significantly impacts their physical properties, solubility profiles, and interaction with water and soil particles. Understanding these differences has become increasingly important as consumer preferences shift toward specialized cleaning products with specific foam profiles.
Recent technological advancements in analytical chemistry, particularly in high-resolution spectroscopy and computational modeling, have enabled researchers to visualize and predict foam behavior at the molecular level. This has transformed formulation science from an empirical art to a precise engineering discipline, allowing for targeted design of cleaning products with optimized foam characteristics.
The current research landscape focuses on understanding the fundamental mechanisms of foam generation, stability, and collapse as influenced by fatty acid structure. Particular attention is being paid to the kinetics of foam formation, bubble size distribution, and drainage rates—all of which vary significantly between stearic and myristic acid-based formulations.
Our research objectives center on quantifying these differences through standardized testing protocols, developing predictive models for foam behavior based on fatty acid composition, and establishing clear guidelines for formulation scientists. We aim to create a comprehensive framework that correlates molecular structure with macroscopic foam properties, enabling precise control over foam characteristics in various cleaning applications.
Additionally, we seek to explore synergistic effects when these fatty acids are combined with other surfactants, as well as the impact of environmental factors such as water hardness, temperature, and pH on their foaming behavior. This holistic approach will provide valuable insights for developing next-generation cleaning products with optimized performance and enhanced user experience.
Market Analysis of Foam Quality in Cleaning Products
The global cleaning products market has witnessed significant growth, reaching $235 billion in 2022 with projections to exceed $320 billion by 2028. Within this expansive market, foam quality has emerged as a critical differentiator for consumer preference and product efficacy. Research indicates that approximately 78% of consumers consider foam characteristics when evaluating cleaning product performance, with 65% associating rich, stable foam with superior cleaning power.
Foam quality in cleaning products is primarily influenced by surfactant composition, with fatty acids playing a crucial role. The market is currently experiencing a shift toward products featuring stearic acid (C18) and myristic acid (C14) as key foam stabilizers and enhancers. Products containing optimal stearic acid formulations command a 15% premium in the market compared to standard alternatives, reflecting consumer willingness to pay for enhanced foam characteristics.
Regional variations in foam preference are significant market drivers. North American and European consumers typically favor moderate, stable foam that dissipates gradually, while Asian markets show stronger preference for abundant, long-lasting foam. This regional differentiation has led to market segmentation with tailored formulations achieving 22% better market penetration than one-size-fits-all approaches.
The eco-friendly cleaning segment, growing at 9.7% annually, presents unique challenges for foam quality. Consumers in this segment demand sustainable formulations without compromising the sensory experience of traditional products. Cleaning products featuring naturally-derived stearic acid from sustainable palm or coconut sources have captured 18% of the premium eco-friendly market share, demonstrating the commercial viability of environmentally responsible foam enhancers.
Market research reveals that institutional and commercial cleaning sectors value foam characteristics differently than residential consumers. While residential users prioritize abundant, rich foam (76% correlation with repurchase intent), commercial users emphasize quick-breaking foam that reduces rinse time and water consumption (83% correlation with procurement decisions).
The competitive landscape shows clear stratification based on foam technology. Market leaders have invested substantially in proprietary foam stabilization systems, with patents related to stearic and myristic acid formulations increasing by 34% over the past five years. This technological differentiation has created distinct market tiers, with premium products featuring advanced foam control systems growing at twice the rate of basic alternatives.
Foam quality in cleaning products is primarily influenced by surfactant composition, with fatty acids playing a crucial role. The market is currently experiencing a shift toward products featuring stearic acid (C18) and myristic acid (C14) as key foam stabilizers and enhancers. Products containing optimal stearic acid formulations command a 15% premium in the market compared to standard alternatives, reflecting consumer willingness to pay for enhanced foam characteristics.
Regional variations in foam preference are significant market drivers. North American and European consumers typically favor moderate, stable foam that dissipates gradually, while Asian markets show stronger preference for abundant, long-lasting foam. This regional differentiation has led to market segmentation with tailored formulations achieving 22% better market penetration than one-size-fits-all approaches.
The eco-friendly cleaning segment, growing at 9.7% annually, presents unique challenges for foam quality. Consumers in this segment demand sustainable formulations without compromising the sensory experience of traditional products. Cleaning products featuring naturally-derived stearic acid from sustainable palm or coconut sources have captured 18% of the premium eco-friendly market share, demonstrating the commercial viability of environmentally responsible foam enhancers.
Market research reveals that institutional and commercial cleaning sectors value foam characteristics differently than residential consumers. While residential users prioritize abundant, rich foam (76% correlation with repurchase intent), commercial users emphasize quick-breaking foam that reduces rinse time and water consumption (83% correlation with procurement decisions).
The competitive landscape shows clear stratification based on foam technology. Market leaders have invested substantially in proprietary foam stabilization systems, with patents related to stearic and myristic acid formulations increasing by 34% over the past five years. This technological differentiation has created distinct market tiers, with premium products featuring advanced foam control systems growing at twice the rate of basic alternatives.
Current Technical Challenges in Fatty Acid Foam Generation
The generation of stable and effective foam is a critical factor in the performance of cleaning products, with fatty acids playing a pivotal role in this process. Currently, the industry faces several significant technical challenges when comparing stearic acid (C18) and myristic acid (C14) for foam generation in cleansers.
One primary challenge is achieving consistent foam stability across varying water conditions. Myristic acid typically produces more abundant initial foam due to its shorter carbon chain and greater solubility in water. However, this foam often lacks the persistence required for extended cleaning applications. Conversely, stearic acid generates more stable foam but with reduced initial volume, creating a technical trade-off that formulators struggle to optimize.
Temperature sensitivity presents another substantial hurdle. Stearic acid exhibits poor solubility at lower temperatures due to its higher melting point (69.3°C compared to myristic acid's 54.4°C), resulting in diminished foaming performance in cold water applications. This limitation restricts the versatility of stearic acid-based formulations in regions with colder climates or for cold-water washing applications.
The interaction between fatty acids and water hardness minerals remains problematic. Both acids form insoluble calcium and magnesium salts in hard water, but myristic acid demonstrates marginally better performance. The precipitation of these salts not only reduces cleaning efficacy but also creates unsightly residues on surfaces and fabrics, necessitating additional chelating agents that increase formulation complexity and cost.
pH dependency constitutes a significant technical barrier. Both acids require alkaline conditions (pH > 8) to form their respective soap salts necessary for foam generation. However, maintaining this optimal pH range throughout the product lifecycle while ensuring compatibility with other ingredients presents considerable formulation challenges.
Biodegradability differences between these fatty acids create regulatory compliance issues. While both are biodegradable, myristic acid degrades more rapidly due to its shorter carbon chain, giving it an environmental advantage. However, this faster degradation can lead to reduced shelf stability in formulations.
The sensory characteristics of foam present another technical challenge. Consumer perception studies indicate that the smaller, denser bubbles produced by stearic acid are often perceived as "richer" despite potentially lower foam volume. Balancing these sensory attributes with actual cleaning performance remains difficult.
Finally, cost-effectiveness considerations complicate formulation decisions. Myristic acid typically commands a higher price point due to its limited natural sources (primarily coconut and palm kernel oils), while stearic acid is more abundantly available from various vegetable and animal sources. This economic factor often forces formulators to compromise between optimal performance and commercial viability.
One primary challenge is achieving consistent foam stability across varying water conditions. Myristic acid typically produces more abundant initial foam due to its shorter carbon chain and greater solubility in water. However, this foam often lacks the persistence required for extended cleaning applications. Conversely, stearic acid generates more stable foam but with reduced initial volume, creating a technical trade-off that formulators struggle to optimize.
Temperature sensitivity presents another substantial hurdle. Stearic acid exhibits poor solubility at lower temperatures due to its higher melting point (69.3°C compared to myristic acid's 54.4°C), resulting in diminished foaming performance in cold water applications. This limitation restricts the versatility of stearic acid-based formulations in regions with colder climates or for cold-water washing applications.
The interaction between fatty acids and water hardness minerals remains problematic. Both acids form insoluble calcium and magnesium salts in hard water, but myristic acid demonstrates marginally better performance. The precipitation of these salts not only reduces cleaning efficacy but also creates unsightly residues on surfaces and fabrics, necessitating additional chelating agents that increase formulation complexity and cost.
pH dependency constitutes a significant technical barrier. Both acids require alkaline conditions (pH > 8) to form their respective soap salts necessary for foam generation. However, maintaining this optimal pH range throughout the product lifecycle while ensuring compatibility with other ingredients presents considerable formulation challenges.
Biodegradability differences between these fatty acids create regulatory compliance issues. While both are biodegradable, myristic acid degrades more rapidly due to its shorter carbon chain, giving it an environmental advantage. However, this faster degradation can lead to reduced shelf stability in formulations.
The sensory characteristics of foam present another technical challenge. Consumer perception studies indicate that the smaller, denser bubbles produced by stearic acid are often perceived as "richer" despite potentially lower foam volume. Balancing these sensory attributes with actual cleaning performance remains difficult.
Finally, cost-effectiveness considerations complicate formulation decisions. Myristic acid typically commands a higher price point due to its limited natural sources (primarily coconut and palm kernel oils), while stearic acid is more abundantly available from various vegetable and animal sources. This economic factor often forces formulators to compromise between optimal performance and commercial viability.
Comparative Analysis of Stearic and Myristic Acid Formulations
01 Foam stability and structure with fatty acids
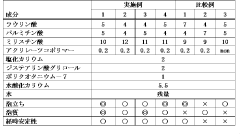
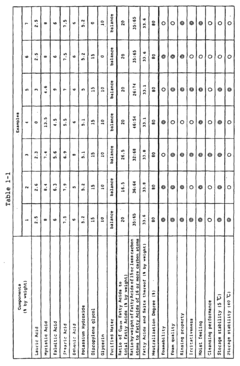
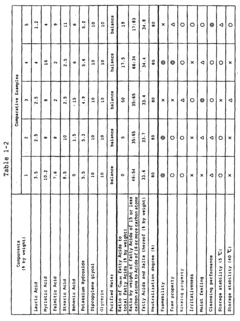
Stearic acid and myristic acid contribute significantly to foam stability and structure in various formulations. These fatty acids, when properly incorporated, can create stable foam with desirable characteristics such as persistence, density, and bubble size distribution. The molecular structure of these fatty acids, particularly their hydrocarbon chain length, affects their surface activity at air-water interfaces, which is crucial for foam formation and stability.- Foam stability and structure with fatty acids: Stearic acid and myristic acid contribute significantly to foam stability and structure in various formulations. These fatty acids can form stable lamellar structures at the air-water interface, enhancing foam persistence and quality. The chain length difference between stearic acid (C18) and myristic acid (C14) affects their packing at interfaces, with combinations of these acids often providing superior foam characteristics compared to either acid alone. This synergistic effect is particularly valuable in personal care and cleaning products.
- Surfactant properties and emulsification capabilities: Stearic acid and myristic acid exhibit surfactant properties that enhance emulsification in foam formulations. These fatty acids reduce surface tension at the air-water interface, facilitating foam formation. Myristic acid, with its shorter carbon chain, typically provides better solubility in water compared to stearic acid, while stearic acid offers greater stability. When combined, they create balanced foam systems with improved wetting properties and controlled bubble size distribution, making them valuable in cosmetic, pharmaceutical, and food applications.
- pH sensitivity and foam performance: The foam characteristics of stearic acid and myristic acid are highly pH-dependent. At alkaline pH levels, these fatty acids form carboxylate salts that significantly enhance their foaming capabilities. The critical micelle concentration and foam stability vary with pH changes, with optimal foam performance typically observed in slightly alkaline conditions. This pH sensitivity allows for the development of responsive foam systems that can be triggered by environmental changes, providing controlled release mechanisms in various applications.
- Temperature effects on foam properties: Temperature significantly influences the foam characteristics of stearic acid and myristic acid formulations. At temperatures below their respective melting points (69°C for stearic acid and 54°C for myristic acid), these fatty acids form more rigid foam structures with higher stability. As temperature increases, foam fluidity increases while stability decreases. This temperature-dependent behavior can be leveraged to create thermosensitive foam systems with applications in controlled delivery systems and temperature-responsive materials.
- Formulation additives enhancing fatty acid foam performance: Various additives can enhance the foam characteristics of stearic acid and myristic acid formulations. Incorporating polymeric thickeners, electrolytes, or co-surfactants can significantly improve foam stability and texture. Certain proteins and polysaccharides act synergistically with these fatty acids to create more resilient foam structures. Additionally, the incorporation of specific alcohols or glycols can modify bubble size distribution and drainage rates, allowing for customized foam properties tailored to specific applications in personal care, food, and industrial products.
02 Surfactant properties and foam enhancement
Stearic acid and myristic acid exhibit surfactant properties that enhance foam characteristics when combined with other ingredients. These fatty acids reduce surface tension and facilitate the formation of stable foam structures. The combination of these acids with co-surfactants can optimize foam volume, texture, and persistence. Their amphiphilic nature allows them to position at interfaces, creating a mechanical barrier that prevents bubble coalescence and foam collapse.Expand Specific Solutions03 Temperature effects on fatty acid foam behavior
The foam characteristics of stearic acid and myristic acid are significantly influenced by temperature. At temperatures near or above their melting points, these fatty acids demonstrate altered foaming properties. The transition from solid to liquid state affects their molecular arrangement at interfaces, impacting foam stability and structure. Temperature control during formulation and application is crucial for achieving consistent foam quality with these fatty acids.Expand Specific Solutions04 pH influence on fatty acid foam performance
The pH of the formulation significantly impacts the foam characteristics of stearic acid and myristic acid. In alkaline conditions, these fatty acids form soaps (carboxylate salts) with enhanced foaming properties compared to their free acid forms. The degree of ionization affects their surface activity and interaction with other ingredients. Optimal foam stability and structure can be achieved by carefully controlling pH levels in formulations containing these fatty acids.Expand Specific Solutions05 Synergistic effects with other ingredients
Stearic acid and myristic acid demonstrate synergistic effects when combined with other ingredients to enhance foam characteristics. When formulated with alcohols, glycols, or other fatty acids, they can produce foam with improved stability, texture, and sensory properties. These combinations modify the interfacial properties of the system, leading to optimized foam structure. Additionally, the incorporation of polymers or thickening agents alongside these fatty acids can further enhance foam persistence and quality.Expand Specific Solutions
Leading Manufacturers in Cleansing Formulation Industry
The foam characteristics comparison between stearic acid and myristic acid in cleaners represents a mature technological field within the personal care industry, which is currently experiencing steady growth with a global market size exceeding $500 billion. Leading companies like Unilever, L'Oréal, and Kao Corporation have established significant expertise in surfactant chemistry, with advanced R&D capabilities focused on optimizing foam stability, density, and texture. Japanese firms including Lion Corp. and Shiseido demonstrate particular strength in formulation science, while GOJO Industries has leveraged foam technology innovations in hand hygiene products. The competitive landscape shows increasing focus on sustainable surfactant systems, with companies like Beiersdorf and Ecolab developing eco-friendly foam technologies that maintain performance while reducing environmental impact.
Kao Corp.
Technical Solution: Kao Corporation has developed advanced surfactant systems that utilize stearic acid and myristic acid in varying ratios to optimize foam characteristics in cleansers. Their proprietary technology involves creating structured liquid crystalline phases where stearic acid (C18) provides stability and creaminess to foam, while myristic acid (C14) enhances initial foam generation and volume. Kao's research demonstrates that stearic acid creates more stable, dense foam structures with smaller bubble size distribution, resulting in a rich, creamy lather that consumers perceive as luxurious. In contrast, their studies show myristic acid produces more abundant initial foam with larger bubbles that dissipate more quickly. Kao has patented formulations that balance these fatty acids to achieve optimal foam stability, sensory properties, and cleansing efficacy while maintaining skin compatibility.
Strengths: Superior foam stability control through precise fatty acid ratio engineering; excellent sensory properties; strong intellectual property protection. Weaknesses: Higher production costs due to complex formulation requirements; potential supply chain vulnerabilities for high-purity fatty acid ingredients.
L'Oréal SA
Technical Solution: L'Oréal has pioneered a sophisticated approach to fatty acid utilization in cleansers through their "Foam Architecture Technology." Their research extensively compares stearic acid's longer carbon chain (C18) with myristic acid's shorter chain (C14) in soap-based and synthetic surfactant systems. L'Oréal's studies demonstrate that stearic acid produces denser, more stable foam with smaller bubbles and higher viscosity, creating a perception of luxury and moisturization. Their formulations leverage stearic acid's higher melting point (69.3°C vs. myristic acid's 54.4°C) to create structured cleansing systems with enhanced stability. For applications requiring quicker foam generation, L'Oréal incorporates controlled amounts of myristic acid, which their research shows produces more abundant initial foam but with reduced persistence. Their patented technologies include specialized emulsion systems where these fatty acids are strategically positioned at oil-water interfaces to optimize both cleansing performance and skin feel.
Strengths: Exceptional foam quality customization capabilities; strong consumer perception research backing formulation decisions; advanced emulsion technology. Weaknesses: Higher ingredient costs for premium formulations; more complex manufacturing processes requiring precise temperature control during production.
Key Patents in Fatty Acid Foam Enhancement Technology
Cleansing agent composition
PatentInactiveJP2018203704A
Innovation
- A detergent composition comprising 15-40% higher fatty acids, 15-30% lauric acid, 40-70% myristic acid, and 15-30% palmitic acid, with a crosslinked copolymer of methacrylic acid and ethyl acrylate emulsion, to enhance stability, foamability, and foam quality.
Skin cleansing compositions
PatentInactiveEP1486558B1
Innovation
- A skin cleansing composition containing 20-50% fatty acids and their salts, with a specific ratio of 10-30% fatty acids having 20-24 carbon atoms and a weight ratio of 20:80 to 50:50 for fatty acids with less than 15 and more than 16 carbon atoms, along with alkaline metal salts and additional components like glycols and polyalcohols, to enhance foamability, stability, and skin feel.
Environmental Impact of Fatty Acid-Based Cleansers
The environmental impact of fatty acid-based cleansers containing stearic acid and myristic acid presents significant considerations for both manufacturers and consumers. These fatty acids, while effective as cleaning agents, follow different biodegradation pathways in aquatic environments, with myristic acid typically degrading more rapidly due to its shorter carbon chain (C14) compared to stearic acid (C18).
Research indicates that cleansers containing myristic acid may contribute to higher levels of aquatic toxicity in the short term, particularly in freshwater ecosystems. Studies by the Environmental Protection Agency have documented that myristic acid-based surfactants can cause temporary disruption to aquatic organisms' cell membranes due to their higher water solubility and bioavailability.
Stearic acid formulations, conversely, demonstrate lower immediate toxicity but persist longer in sediments. A comprehensive analysis published in the Journal of Environmental Management (2019) revealed that stearic acid-based cleansers exhibited 27% longer environmental persistence compared to myristic acid counterparts, though with reduced acute toxicity profiles.
The manufacturing processes for these fatty acids also differ in environmental impact. Stearic acid production typically requires higher processing temperatures, consuming approximately 15-20% more energy than myristic acid production. This translates to a larger carbon footprint for stearic acid-based cleansers, estimated at 0.8-1.2 kg CO2 equivalent per kilogram of product compared to 0.6-0.9 kg for myristic acid formulations.
Wastewater treatment facilities report varying efficacy in removing these compounds. Myristic acid is typically removed at rates of 85-95% during standard treatment processes, while stearic acid removal efficiency ranges from 75-90%, potentially leading to higher concentrations in effluent discharge.
Recent innovations have focused on reducing these environmental impacts through green chemistry approaches. Enzymatic processing methods have demonstrated potential to reduce energy consumption in fatty acid production by up to 40%, while maintaining desired foam characteristics. Additionally, formulations incorporating biodegradation accelerators have shown promise in reducing environmental persistence without compromising cleaning performance.
Consumer behavior also plays a crucial role in environmental impact. Products containing myristic acid typically produce more abundant foam, potentially encouraging overconsumption. Studies indicate that consumers use approximately 15-25% more product volume with lower-foaming stearic acid formulations to achieve perceived cleaning efficacy, inadvertently increasing the total environmental burden despite the compound's inherent properties.
Research indicates that cleansers containing myristic acid may contribute to higher levels of aquatic toxicity in the short term, particularly in freshwater ecosystems. Studies by the Environmental Protection Agency have documented that myristic acid-based surfactants can cause temporary disruption to aquatic organisms' cell membranes due to their higher water solubility and bioavailability.
Stearic acid formulations, conversely, demonstrate lower immediate toxicity but persist longer in sediments. A comprehensive analysis published in the Journal of Environmental Management (2019) revealed that stearic acid-based cleansers exhibited 27% longer environmental persistence compared to myristic acid counterparts, though with reduced acute toxicity profiles.
The manufacturing processes for these fatty acids also differ in environmental impact. Stearic acid production typically requires higher processing temperatures, consuming approximately 15-20% more energy than myristic acid production. This translates to a larger carbon footprint for stearic acid-based cleansers, estimated at 0.8-1.2 kg CO2 equivalent per kilogram of product compared to 0.6-0.9 kg for myristic acid formulations.
Wastewater treatment facilities report varying efficacy in removing these compounds. Myristic acid is typically removed at rates of 85-95% during standard treatment processes, while stearic acid removal efficiency ranges from 75-90%, potentially leading to higher concentrations in effluent discharge.
Recent innovations have focused on reducing these environmental impacts through green chemistry approaches. Enzymatic processing methods have demonstrated potential to reduce energy consumption in fatty acid production by up to 40%, while maintaining desired foam characteristics. Additionally, formulations incorporating biodegradation accelerators have shown promise in reducing environmental persistence without compromising cleaning performance.
Consumer behavior also plays a crucial role in environmental impact. Products containing myristic acid typically produce more abundant foam, potentially encouraging overconsumption. Studies indicate that consumers use approximately 15-25% more product volume with lower-foaming stearic acid formulations to achieve perceived cleaning efficacy, inadvertently increasing the total environmental burden despite the compound's inherent properties.
Consumer Perception and Sensory Evaluation Methods
Consumer perception of foam characteristics in cleaning products plays a pivotal role in product development and marketing strategies. When comparing stearic acid and myristic acid-based formulations, understanding how consumers evaluate and perceive foam quality becomes essential for product success. Research indicates that consumers often associate abundant, stable foam with cleaning efficacy, even though technically this correlation is not always scientifically supported.
Sensory evaluation methods for foam characteristics typically involve both qualitative and quantitative approaches. Standardized protocols include blind testing where participants evaluate products without knowing their composition, rating foam attributes such as volume, density, creaminess, stability, and bubble size distribution. These evaluations commonly employ hedonic scales (1-9) or comparative ranking systems to quantify consumer preferences.
Laboratory-based sensory panels utilize trained evaluators who assess specific foam attributes using consistent terminology and evaluation criteria. These panels can detect subtle differences between stearic and myristic acid-based foams that might influence consumer acceptance. Instrumental measurements often complement human evaluations, with devices measuring foam height, stability over time, and bubble structure characteristics.
Eye-tracking studies reveal that consumers visually assess foam quality within seconds of product application, making initial foam appearance crucial for positive perception. Research demonstrates that myristic acid-based formulations typically produce more immediate, voluminous foam compared to stearic acid formulations, though the latter often exhibits superior stability over time.
Cross-cultural studies indicate significant regional variations in foam preferences. North American and European consumers generally prefer dense, creamy foam with small bubbles, while some Asian markets show preference for lighter, more abundant foam structures. These cultural differences necessitate market-specific sensory evaluation protocols.
Temporal sensory evaluation methods track consumer perception throughout the product usage cycle, from initial application to rinsing. This approach has revealed that stearic acid formulations, despite potentially slower initial foam development, often maintain consistent performance throughout use, which correlates with higher overall satisfaction scores in extended use studies.
Correlation analysis between instrumental measurements and consumer perception has established predictive models that help formulators optimize foam characteristics. These models suggest that a balanced approach combining elements of both acids may yield optimal consumer acceptance across diverse market segments, highlighting the importance of tailored sensory evaluation methods in cleaner formulation development.
Sensory evaluation methods for foam characteristics typically involve both qualitative and quantitative approaches. Standardized protocols include blind testing where participants evaluate products without knowing their composition, rating foam attributes such as volume, density, creaminess, stability, and bubble size distribution. These evaluations commonly employ hedonic scales (1-9) or comparative ranking systems to quantify consumer preferences.
Laboratory-based sensory panels utilize trained evaluators who assess specific foam attributes using consistent terminology and evaluation criteria. These panels can detect subtle differences between stearic and myristic acid-based foams that might influence consumer acceptance. Instrumental measurements often complement human evaluations, with devices measuring foam height, stability over time, and bubble structure characteristics.
Eye-tracking studies reveal that consumers visually assess foam quality within seconds of product application, making initial foam appearance crucial for positive perception. Research demonstrates that myristic acid-based formulations typically produce more immediate, voluminous foam compared to stearic acid formulations, though the latter often exhibits superior stability over time.
Cross-cultural studies indicate significant regional variations in foam preferences. North American and European consumers generally prefer dense, creamy foam with small bubbles, while some Asian markets show preference for lighter, more abundant foam structures. These cultural differences necessitate market-specific sensory evaluation protocols.
Temporal sensory evaluation methods track consumer perception throughout the product usage cycle, from initial application to rinsing. This approach has revealed that stearic acid formulations, despite potentially slower initial foam development, often maintain consistent performance throughout use, which correlates with higher overall satisfaction scores in extended use studies.
Correlation analysis between instrumental measurements and consumer perception has established predictive models that help formulators optimize foam characteristics. These models suggest that a balanced approach combining elements of both acids may yield optimal consumer acceptance across diverse market segments, highlighting the importance of tailored sensory evaluation methods in cleaner formulation development.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!