How to Detect Aggregation in Proteins Using Dynamic Light Scattering
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DLS Protein Aggregation Detection Background & Objectives
Protein aggregation represents a critical challenge in biopharmaceutical development, affecting product stability, efficacy, and safety. Dynamic Light Scattering (DLS) has emerged as a powerful analytical technique for detecting and characterizing protein aggregation phenomena across various stages of drug development. The evolution of DLS technology spans several decades, beginning with fundamental light scattering principles established in the early 20th century and advancing to today's sophisticated instrumentation capable of detecting nanoscale particles in complex biological solutions.
The technological trajectory of DLS has been marked by significant improvements in detection sensitivity, data processing algorithms, and instrument miniaturization. Recent innovations have focused on enhancing the technique's ability to distinguish between different types of protein aggregates, improving measurement reproducibility, and enabling real-time monitoring capabilities. These advancements align with the biopharmaceutical industry's growing emphasis on comprehensive protein characterization and quality control throughout the product lifecycle.
The primary objective of employing DLS for protein aggregation detection is to establish a reliable, non-destructive method for early identification of aggregation events before they progress to levels that compromise product quality. This technology aims to provide quantitative insights into aggregate size distribution, formation kinetics, and morphological characteristics under various environmental conditions relevant to manufacturing, storage, and administration.
Additionally, DLS technology seeks to address the multifaceted nature of protein aggregation by offering complementary data to other analytical techniques, thereby contributing to a more holistic understanding of aggregation mechanisms. This comprehensive approach is essential for developing effective mitigation strategies and establishing meaningful specifications for protein-based therapeutics.
Current technological goals include improving DLS sensitivity for detecting sub-visible particles in the 100nm-1μm range, enhancing measurement precision at low protein concentrations, and developing standardized protocols for data interpretation across different instrument platforms. There is also significant interest in adapting DLS for high-throughput screening applications to accelerate formulation development and stability assessment processes.
The evolution of regulatory expectations regarding protein aggregation characterization has further driven technological innovation in this field. Regulatory agencies increasingly require comprehensive aggregate profiling throughout product development, necessitating analytical methods that can reliably detect and characterize aggregates across multiple size ranges and under conditions that simulate real-world handling and storage scenarios.
The technological trajectory of DLS has been marked by significant improvements in detection sensitivity, data processing algorithms, and instrument miniaturization. Recent innovations have focused on enhancing the technique's ability to distinguish between different types of protein aggregates, improving measurement reproducibility, and enabling real-time monitoring capabilities. These advancements align with the biopharmaceutical industry's growing emphasis on comprehensive protein characterization and quality control throughout the product lifecycle.
The primary objective of employing DLS for protein aggregation detection is to establish a reliable, non-destructive method for early identification of aggregation events before they progress to levels that compromise product quality. This technology aims to provide quantitative insights into aggregate size distribution, formation kinetics, and morphological characteristics under various environmental conditions relevant to manufacturing, storage, and administration.
Additionally, DLS technology seeks to address the multifaceted nature of protein aggregation by offering complementary data to other analytical techniques, thereby contributing to a more holistic understanding of aggregation mechanisms. This comprehensive approach is essential for developing effective mitigation strategies and establishing meaningful specifications for protein-based therapeutics.
Current technological goals include improving DLS sensitivity for detecting sub-visible particles in the 100nm-1μm range, enhancing measurement precision at low protein concentrations, and developing standardized protocols for data interpretation across different instrument platforms. There is also significant interest in adapting DLS for high-throughput screening applications to accelerate formulation development and stability assessment processes.
The evolution of regulatory expectations regarding protein aggregation characterization has further driven technological innovation in this field. Regulatory agencies increasingly require comprehensive aggregate profiling throughout product development, necessitating analytical methods that can reliably detect and characterize aggregates across multiple size ranges and under conditions that simulate real-world handling and storage scenarios.
Market Analysis for Protein Aggregation Detection Technologies
The protein aggregation detection market is experiencing robust growth, driven by increasing demand in biopharmaceutical development, quality control processes, and research applications. The global market for protein analysis technologies was valued at approximately $3.6 billion in 2022 and is projected to reach $5.7 billion by 2027, with protein aggregation detection technologies representing a significant segment of this market.
Dynamic Light Scattering (DLS) technology holds a substantial market share within the protein aggregation detection segment due to its non-destructive nature, minimal sample requirements, and ability to provide real-time measurements. The DLS-specific market for protein characterization was estimated at $320 million in 2022, with a compound annual growth rate (CAGR) of 7.8% expected through 2028.
Biopharmaceutical companies constitute the largest end-user segment, accounting for approximately 65% of the market demand. This dominance stems from stringent regulatory requirements for protein-based therapeutics, where aggregation can significantly impact drug efficacy and safety profiles. The FDA and EMA have increasingly emphasized the importance of comprehensive aggregation analysis during drug development and manufacturing processes.
Academic and research institutions represent the second-largest market segment at 20%, followed by contract research organizations at 10%. Geographically, North America leads with 42% market share, followed by Europe (28%), Asia-Pacific (22%), and rest of the world (8%). The Asia-Pacific region is expected to witness the highest growth rate of 9.5% annually, primarily driven by expanding biopharmaceutical manufacturing capabilities in China, India, and South Korea.
Key market drivers include the growing pipeline of protein-based therapeutics, increasing regulatory scrutiny, technological advancements in detection sensitivity, and rising adoption of quality-by-design approaches in biopharmaceutical development. The COVID-19 pandemic has further accelerated market growth due to the rapid development of protein-based vaccines and therapeutics.
Market challenges include high instrument costs, technical expertise requirements, and competition from alternative technologies such as size exclusion chromatography and analytical ultracentrifugation. However, recent innovations in automated sample handling, improved data analysis algorithms, and multi-angle DLS systems are addressing these limitations and expanding market opportunities.
The competitive landscape features established analytical instrument manufacturers like Malvern Panalytical, Wyatt Technology, and Horiba Scientific dominating with approximately 70% combined market share. Emerging players offering specialized solutions for specific applications are gaining traction, particularly in niche segments like high-throughput screening and in-line manufacturing process monitoring.
Dynamic Light Scattering (DLS) technology holds a substantial market share within the protein aggregation detection segment due to its non-destructive nature, minimal sample requirements, and ability to provide real-time measurements. The DLS-specific market for protein characterization was estimated at $320 million in 2022, with a compound annual growth rate (CAGR) of 7.8% expected through 2028.
Biopharmaceutical companies constitute the largest end-user segment, accounting for approximately 65% of the market demand. This dominance stems from stringent regulatory requirements for protein-based therapeutics, where aggregation can significantly impact drug efficacy and safety profiles. The FDA and EMA have increasingly emphasized the importance of comprehensive aggregation analysis during drug development and manufacturing processes.
Academic and research institutions represent the second-largest market segment at 20%, followed by contract research organizations at 10%. Geographically, North America leads with 42% market share, followed by Europe (28%), Asia-Pacific (22%), and rest of the world (8%). The Asia-Pacific region is expected to witness the highest growth rate of 9.5% annually, primarily driven by expanding biopharmaceutical manufacturing capabilities in China, India, and South Korea.
Key market drivers include the growing pipeline of protein-based therapeutics, increasing regulatory scrutiny, technological advancements in detection sensitivity, and rising adoption of quality-by-design approaches in biopharmaceutical development. The COVID-19 pandemic has further accelerated market growth due to the rapid development of protein-based vaccines and therapeutics.
Market challenges include high instrument costs, technical expertise requirements, and competition from alternative technologies such as size exclusion chromatography and analytical ultracentrifugation. However, recent innovations in automated sample handling, improved data analysis algorithms, and multi-angle DLS systems are addressing these limitations and expanding market opportunities.
The competitive landscape features established analytical instrument manufacturers like Malvern Panalytical, Wyatt Technology, and Horiba Scientific dominating with approximately 70% combined market share. Emerging players offering specialized solutions for specific applications are gaining traction, particularly in niche segments like high-throughput screening and in-line manufacturing process monitoring.
Current DLS Technology Status and Challenges
Dynamic Light Scattering (DLS) technology has evolved significantly over the past decades, becoming a cornerstone method for protein aggregation detection in biopharmaceutical research and quality control. Currently, commercial DLS instruments offer high sensitivity, capable of detecting particles ranging from 0.3 nm to 10 μm in diameter, making them suitable for monitoring various stages of protein aggregation from early oligomers to larger visible particles.
The fundamental principle of modern DLS relies on measuring the Brownian motion of particles in solution and correlating this to particle size through the Stokes-Einstein equation. Contemporary systems typically employ photon correlation spectroscopy with laser light sources (usually 633 nm He-Ne lasers) and avalanche photodiode detectors that offer superior quantum efficiency and low noise characteristics.
Despite technological advancements, DLS faces several significant challenges when applied to protein aggregation detection. The technique's inherent bias toward larger particles presents a major limitation, as the scattered light intensity is proportional to the sixth power of particle diameter (Rayleigh scattering). Consequently, even a small number of large aggregates can dominate the signal, potentially masking the presence of smaller species that may be critical in understanding aggregation mechanisms.
Sample preparation remains another substantial challenge, as dust particles or air bubbles can severely interfere with measurements. This necessitates careful filtration procedures that might inadvertently remove relevant protein aggregates, creating a paradoxical situation where sample preparation may alter the very phenomenon being studied.
Data interpretation presents further complications, particularly in polydisperse samples containing multiple aggregate populations. The mathematical algorithms used to convert correlation functions to size distributions (such as CONTIN or NNLS) often struggle with resolving closely sized populations, leading to limited resolution where peaks differing by less than a factor of 3-5 in size cannot be reliably distinguished.
Temperature control and stability represent additional technical challenges, as even minor fluctuations can significantly affect Brownian motion and consequently the calculated particle sizes. Most modern instruments incorporate precise temperature control systems, but maintaining stability during measurements remains crucial for reproducible results.
The non-specific nature of DLS measurements constitutes another limitation, as the technique cannot differentiate between protein aggregates and other particulates of similar size. This necessitates complementary analytical techniques for comprehensive characterization of protein aggregation states.
Recent technological developments have focused on addressing these challenges through multi-angle DLS, machine learning algorithms for improved data processing, and integration with other analytical methods in hybrid systems. However, fundamental physical limitations of light scattering phenomena continue to constrain the technique's resolution and specificity for complex protein solutions.
The fundamental principle of modern DLS relies on measuring the Brownian motion of particles in solution and correlating this to particle size through the Stokes-Einstein equation. Contemporary systems typically employ photon correlation spectroscopy with laser light sources (usually 633 nm He-Ne lasers) and avalanche photodiode detectors that offer superior quantum efficiency and low noise characteristics.
Despite technological advancements, DLS faces several significant challenges when applied to protein aggregation detection. The technique's inherent bias toward larger particles presents a major limitation, as the scattered light intensity is proportional to the sixth power of particle diameter (Rayleigh scattering). Consequently, even a small number of large aggregates can dominate the signal, potentially masking the presence of smaller species that may be critical in understanding aggregation mechanisms.
Sample preparation remains another substantial challenge, as dust particles or air bubbles can severely interfere with measurements. This necessitates careful filtration procedures that might inadvertently remove relevant protein aggregates, creating a paradoxical situation where sample preparation may alter the very phenomenon being studied.
Data interpretation presents further complications, particularly in polydisperse samples containing multiple aggregate populations. The mathematical algorithms used to convert correlation functions to size distributions (such as CONTIN or NNLS) often struggle with resolving closely sized populations, leading to limited resolution where peaks differing by less than a factor of 3-5 in size cannot be reliably distinguished.
Temperature control and stability represent additional technical challenges, as even minor fluctuations can significantly affect Brownian motion and consequently the calculated particle sizes. Most modern instruments incorporate precise temperature control systems, but maintaining stability during measurements remains crucial for reproducible results.
The non-specific nature of DLS measurements constitutes another limitation, as the technique cannot differentiate between protein aggregates and other particulates of similar size. This necessitates complementary analytical techniques for comprehensive characterization of protein aggregation states.
Recent technological developments have focused on addressing these challenges through multi-angle DLS, machine learning algorithms for improved data processing, and integration with other analytical methods in hybrid systems. However, fundamental physical limitations of light scattering phenomena continue to constrain the technique's resolution and specificity for complex protein solutions.
Current DLS Methodologies for Protein Aggregation Analysis
01 Basic principles and apparatus for DLS aggregation detection
Dynamic Light Scattering (DLS) is a technique used to detect particle aggregation by measuring the scattered light from particles in suspension. The basic principles involve analyzing the intensity fluctuations of scattered light to determine particle size distribution. Specialized apparatus for DLS includes laser light sources, detectors, and correlators that process the scattered light signals. These systems can detect aggregation by identifying changes in particle size distribution over time.- Basic principles and apparatus for DLS aggregation detection: Dynamic Light Scattering (DLS) is a technique used to detect particle aggregation by measuring the scattered light from particles in suspension. The basic principles involve analyzing the time-dependent fluctuations in scattered light intensity caused by Brownian motion of particles. These fluctuations provide information about particle size distribution, with larger aggregates showing different scattering patterns than individual particles. Specialized apparatus with laser light sources, detectors, and signal processing components are used to perform these measurements with high sensitivity.
- Advanced data analysis methods for aggregation monitoring: Advanced computational methods enhance the accuracy of aggregation detection using DLS. These include algorithms for analyzing autocorrelation functions, multi-angle measurements, and statistical approaches to distinguish between monomers and aggregates. Machine learning and artificial intelligence techniques are increasingly applied to process complex DLS data, enabling more sensitive detection of early-stage aggregation and differentiation between various types of aggregates. These methods improve the reliability of measurements in complex biological samples and polydisperse systems.
- Application of DLS for protein and biopharmaceutical aggregation detection: DLS is widely used for monitoring protein aggregation in biopharmaceutical development and quality control. The technique allows for real-time, non-destructive assessment of protein stability and aggregation under various conditions such as temperature, pH, and concentration changes. This application is particularly important for therapeutic proteins and antibodies, where aggregation can affect efficacy and safety. DLS enables detection of submicron aggregates that may not be visible by other methods, providing critical information for formulation development and stability studies.
- Combination of DLS with other analytical techniques: Combining DLS with complementary analytical techniques creates powerful hybrid approaches for comprehensive aggregation analysis. Integration with size exclusion chromatography, multi-angle light scattering, or mass spectrometry provides multi-dimensional characterization of aggregates. These combined methods offer enhanced resolution across different size ranges and additional information about aggregate composition and structure. Such integrated approaches are particularly valuable for complex samples where DLS alone might have limitations in resolving heterogeneous aggregate populations.
- Innovations in DLS for high-throughput and in-line monitoring: Recent innovations have focused on adapting DLS for high-throughput screening and continuous in-line monitoring applications. These developments include miniaturized DLS systems, automated sample handling, and integration with manufacturing processes. Such advancements enable real-time aggregation monitoring during biopharmaceutical production, formulation development, and stability testing. High-throughput DLS platforms allow for rapid screening of multiple conditions, accelerating development timelines and improving quality control in industrial settings.
02 Advanced algorithms and data processing for aggregation analysis
Advanced algorithms and data processing techniques enhance the accuracy and sensitivity of DLS aggregation detection. These include correlation functions, mathematical models for particle size distribution, and statistical methods to analyze scattered light data. Machine learning and artificial intelligence approaches can be applied to improve data interpretation and detect subtle changes in aggregation states. Signal processing techniques help filter noise and extract meaningful information from raw DLS measurements.Expand Specific Solutions03 Application-specific DLS methods for protein and biological sample analysis
Specialized DLS methods have been developed for detecting aggregation in biological samples, particularly proteins and biomolecules. These methods account for the unique characteristics of biological materials, such as their sensitivity to environmental conditions and complex aggregation behaviors. Techniques include temperature-controlled measurements, pH monitoring, and integration with other analytical methods to provide comprehensive characterization of biological aggregation processes.Expand Specific Solutions04 Real-time and in-situ monitoring of aggregation processes
Real-time and in-situ DLS monitoring systems allow for continuous observation of aggregation processes as they occur. These systems incorporate flow cells, automated sampling, and rapid data acquisition to track dynamic changes in particle size distribution. Applications include pharmaceutical manufacturing, quality control processes, and research settings where understanding the kinetics of aggregation is critical. These methods can detect early stages of aggregation before visible precipitation occurs.Expand Specific Solutions05 Multi-angle and multi-wavelength DLS techniques for enhanced detection
Multi-angle and multi-wavelength DLS techniques provide enhanced detection capabilities for complex aggregation phenomena. By measuring scattered light at multiple angles or using different wavelengths, these methods can distinguish between different types of aggregates and provide more detailed characterization of particle size distributions. These advanced techniques are particularly useful for polydisperse samples containing particles of varying sizes and compositions, offering improved resolution compared to conventional single-angle DLS.Expand Specific Solutions
Key Industry Players in DLS Instrumentation
The protein aggregation detection market using Dynamic Light Scattering (DLS) is in a growth phase, with increasing adoption across biopharmaceutical research and development. The global market size is expanding steadily as protein-based therapeutics gain prominence, creating demand for reliable aggregation monitoring technologies. Technologically, DLS has reached moderate maturity with established players like Malvern Panalytical Ltd. leading commercial applications, while companies such as Wyatt Technology, Fluence Analytics, and Konica Minolta continue to innovate with advanced instrumentation. Research institutions including Wisconsin Alumni Research Foundation, Dana-Farber Cancer Institute, and National University of Singapore are driving fundamental advancements. Pharmaceutical companies like Amgen and Merck are integrating these technologies into their development pipelines, indicating growing industry acceptance and technological refinement.
Malvern Panalytical Ltd.
Technical Solution: Malvern Panalytical has developed advanced DLS systems specifically optimized for protein aggregation detection. Their Zetasizer series employs non-invasive backscatter (NIBS) technology with 173° detection optics that significantly reduces multiple scattering effects and increases sensitivity for detecting small aggregates. The company's instruments incorporate proprietary adaptive correlation algorithms that automatically optimize measurement parameters based on sample characteristics, enabling accurate size distribution analysis across a wide range of protein concentrations (0.1mg/mL to >100mg/mL). Their systems feature temperature control modules (4-90°C) with 0.1°C precision to study temperature-dependent aggregation behaviors and automated titration accessories for pH-dependent studies. Malvern's software suite provides specialized protein analysis tools including molecular weight estimation, thermal trend analysis, and data quality assessment metrics to identify artifacts.
Strengths: Industry-leading sensitivity for detecting aggregates down to 0.3nm; comprehensive software specifically designed for protein applications; high reproducibility with minimal sample volume requirements (as little as 2μL). Weaknesses: Higher cost compared to simpler DLS systems; complex data interpretation requiring specialized training; potential overestimation of large aggregate populations in polydisperse samples.
Olympus Corp.
Technical Solution: Olympus Corporation has developed specialized DLS instrumentation for protein aggregation analysis through their Life Science Division. Their approach integrates DLS technology with advanced optical systems leveraging their expertise in microscopy and imaging. Olympus' DLS platforms feature proprietary confocal detection optics that significantly reduce background scatter and improve signal-to-noise ratios when analyzing dilute protein samples. Their systems employ avalanche photodiode detectors with single-photon counting capabilities, enabling detection of early-stage aggregation events with high sensitivity. The company has developed temperature-controlled sample chambers (4-70°C) with minimal thermal gradients to ensure accurate characterization of temperature-dependent aggregation behaviors. Olympus' analytical software incorporates regularization algorithms specifically optimized for protein samples, capable of resolving multimodal size distributions even in complex formulations. Their technology also features automated quality control measures that identify and compensate for common artifacts such as dust contamination or laser fluctuations.
Strengths: Exceptional optical design leveraging microscopy expertise; high sensitivity for early aggregation detection; robust temperature control systems; user-friendly software with automated quality checks. Weaknesses: More limited market presence in dedicated protein characterization compared to specialized competitors; fewer protein-specific analysis tools; higher cost relative to performance for protein-specific applications.
Critical Technical Innovations in DLS for Proteins
Light Scattering Detector
PatentInactiveEP1884762A3
Innovation
- A hybrid light scattering detector with two light sources emitting different wavelengths for simultaneous static and dynamic light scattering measurements, combined using a light combiner and processed using a mathematical processor to perform both methods concurrently, allowing for accurate measurement of particles across a wide size range.
Methods for diagnosing a neurodegenerative condition
PatentInactiveUS20070038127A1
Innovation
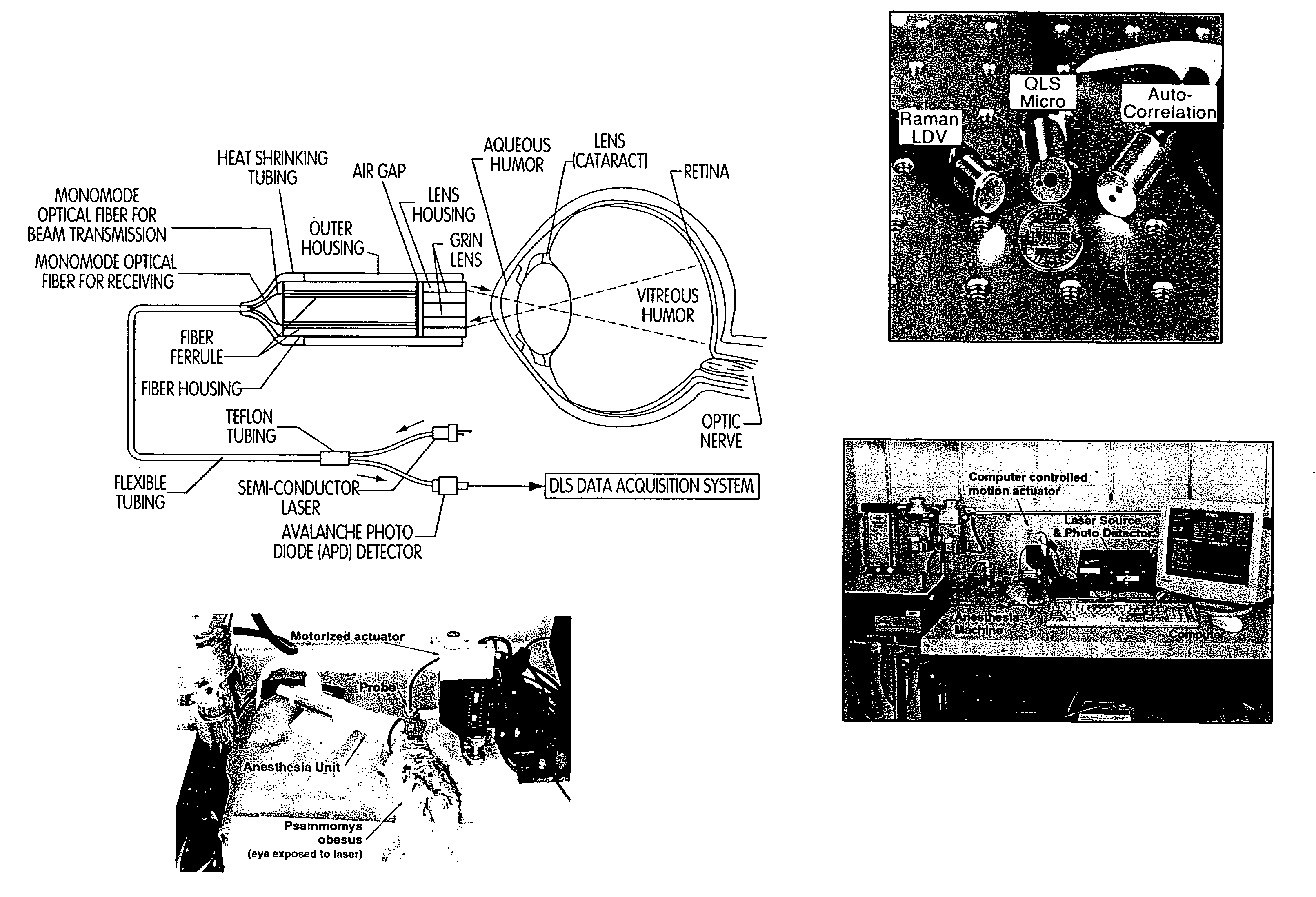
- A non-invasive method using dynamic light scattering (DLS), Raman spectroscopy, and other optical instrumentation to detect morphological changes in the eye, specifically in the cortical and supranuclear regions, to identify amyloid protein aggregates associated with AD, allowing for early detection and monitoring of the disease.
Regulatory Considerations for Protein Characterization Methods
Regulatory frameworks governing protein characterization methods have evolved significantly in response to the growing complexity of biopharmaceutical products. For dynamic light scattering (DLS) as a protein aggregation detection method, compliance with regulatory guidelines is essential for method validation and acceptance in pharmaceutical development and quality control processes.
The U.S. Food and Drug Administration (FDA) has established specific requirements through ICH Q6B guidelines that address the characterization of biotechnology products, including protein aggregation assessment. These guidelines emphasize the importance of using orthogonal methods, with DLS recognized as a valuable complementary technique for detecting protein aggregates across different size ranges. FDA guidance documents specifically mention DLS as an appropriate method for monitoring protein stability and aggregation propensity during formulation development.
European Medicines Agency (EMA) regulations similarly acknowledge DLS in their guidelines on quality of biological medicinal products. The EMA emphasizes method validation parameters that are particularly relevant to DLS, including specificity, precision, and robustness when applied to protein aggregation studies. Manufacturers must demonstrate that their DLS protocols can reliably detect clinically relevant aggregates in their specific protein products.
The International Council for Harmonisation (ICH) provides additional guidance through Q2(R1) for analytical procedure validation applicable to DLS methods. When implementing DLS for regulatory submissions, pharmaceutical companies must address validation parameters including accuracy, precision, specificity, detection limit, and linearity within the context of protein aggregation detection.
Regulatory bodies increasingly require comprehensive characterization of the size distribution of protein aggregates throughout product development and manufacturing. DLS data is often expected in regulatory filings to demonstrate product consistency and stability, particularly in comparability studies following manufacturing changes. The method's non-destructive nature makes it especially valuable for in-process testing and stability studies.
Recent regulatory trends indicate growing acceptance of DLS as a release test for certain protein products when properly validated. However, regulators typically require correlation with other orthogonal methods such as size-exclusion chromatography or analytical ultracentrifugation to overcome the inherent limitations of DLS, particularly its bias toward larger particles and limited resolution of closely sized species.
Pharmaceutical companies implementing DLS for protein aggregation detection must develop standard operating procedures that address sample preparation, instrument qualification, data analysis, and interpretation in alignment with current good manufacturing practices (cGMP) requirements. Documentation of these procedures and their validation is critical for successful regulatory submissions and inspections.
The U.S. Food and Drug Administration (FDA) has established specific requirements through ICH Q6B guidelines that address the characterization of biotechnology products, including protein aggregation assessment. These guidelines emphasize the importance of using orthogonal methods, with DLS recognized as a valuable complementary technique for detecting protein aggregates across different size ranges. FDA guidance documents specifically mention DLS as an appropriate method for monitoring protein stability and aggregation propensity during formulation development.
European Medicines Agency (EMA) regulations similarly acknowledge DLS in their guidelines on quality of biological medicinal products. The EMA emphasizes method validation parameters that are particularly relevant to DLS, including specificity, precision, and robustness when applied to protein aggregation studies. Manufacturers must demonstrate that their DLS protocols can reliably detect clinically relevant aggregates in their specific protein products.
The International Council for Harmonisation (ICH) provides additional guidance through Q2(R1) for analytical procedure validation applicable to DLS methods. When implementing DLS for regulatory submissions, pharmaceutical companies must address validation parameters including accuracy, precision, specificity, detection limit, and linearity within the context of protein aggregation detection.
Regulatory bodies increasingly require comprehensive characterization of the size distribution of protein aggregates throughout product development and manufacturing. DLS data is often expected in regulatory filings to demonstrate product consistency and stability, particularly in comparability studies following manufacturing changes. The method's non-destructive nature makes it especially valuable for in-process testing and stability studies.
Recent regulatory trends indicate growing acceptance of DLS as a release test for certain protein products when properly validated. However, regulators typically require correlation with other orthogonal methods such as size-exclusion chromatography or analytical ultracentrifugation to overcome the inherent limitations of DLS, particularly its bias toward larger particles and limited resolution of closely sized species.
Pharmaceutical companies implementing DLS for protein aggregation detection must develop standard operating procedures that address sample preparation, instrument qualification, data analysis, and interpretation in alignment with current good manufacturing practices (cGMP) requirements. Documentation of these procedures and their validation is critical for successful regulatory submissions and inspections.
Data Analysis Algorithms and Software Solutions for DLS
The evolution of data analysis algorithms for Dynamic Light Scattering (DLS) has significantly enhanced protein aggregation detection capabilities. Traditional algorithms relied on the autocorrelation function and cumulant analysis, which provided basic size distribution information but struggled with polydisperse samples containing protein aggregates. Modern algorithms have overcome these limitations through advanced mathematical approaches including CONTIN, non-negative least squares (NNLS), and maximum entropy methods, enabling more accurate resolution of multimodal distributions typical in aggregating protein solutions.
Machine learning integration represents the cutting edge in DLS data processing, with neural networks and support vector machines improving signal-to-noise ratios and enabling automated classification of aggregation states. These AI-enhanced algorithms can detect subtle changes in scattering patterns that might indicate early-stage aggregation, providing valuable predictive capabilities for pharmaceutical formulation development.
Commercial software solutions have evolved to incorporate these advanced algorithms while simplifying user interaction. Industry leaders like Malvern Panalytical's Zetasizer series, Wyatt Technology's DynaPro, and Brookhaven Instruments' NanoBrook offer comprehensive packages with intuitive interfaces that guide users through data collection, processing, and interpretation. These platforms typically include visualization tools that present size distributions, correlation functions, and trend analyses in accessible formats.
Open-source alternatives have gained traction in research environments, with packages like DynamicLightScattering.jl (Julia), DLSanalysis (Python), and openDLS providing cost-effective options with customizable analysis pipelines. These solutions enable researchers to modify algorithms for specific protein aggregation studies and integrate DLS data with other analytical techniques.
Real-time analysis capabilities represent a significant advancement in DLS software, allowing continuous monitoring of protein solutions during stress testing or formulation development. These systems can trigger alerts when aggregation parameters exceed predefined thresholds, enabling immediate intervention in manufacturing processes or research protocols.
Cloud-based DLS data processing platforms have emerged as collaborative solutions for pharmaceutical research teams, offering centralized data storage, remote access capabilities, and standardized analysis protocols. These platforms facilitate consistent interpretation across multiple instruments and sites, addressing a critical need in multi-center protein formulation studies and regulatory submissions where reproducibility is paramount.
Machine learning integration represents the cutting edge in DLS data processing, with neural networks and support vector machines improving signal-to-noise ratios and enabling automated classification of aggregation states. These AI-enhanced algorithms can detect subtle changes in scattering patterns that might indicate early-stage aggregation, providing valuable predictive capabilities for pharmaceutical formulation development.
Commercial software solutions have evolved to incorporate these advanced algorithms while simplifying user interaction. Industry leaders like Malvern Panalytical's Zetasizer series, Wyatt Technology's DynaPro, and Brookhaven Instruments' NanoBrook offer comprehensive packages with intuitive interfaces that guide users through data collection, processing, and interpretation. These platforms typically include visualization tools that present size distributions, correlation functions, and trend analyses in accessible formats.
Open-source alternatives have gained traction in research environments, with packages like DynamicLightScattering.jl (Julia), DLSanalysis (Python), and openDLS providing cost-effective options with customizable analysis pipelines. These solutions enable researchers to modify algorithms for specific protein aggregation studies and integrate DLS data with other analytical techniques.
Real-time analysis capabilities represent a significant advancement in DLS software, allowing continuous monitoring of protein solutions during stress testing or formulation development. These systems can trigger alerts when aggregation parameters exceed predefined thresholds, enabling immediate intervention in manufacturing processes or research protocols.
Cloud-based DLS data processing platforms have emerged as collaborative solutions for pharmaceutical research teams, offering centralized data storage, remote access capabilities, and standardized analysis protocols. These platforms facilitate consistent interpretation across multiple instruments and sites, addressing a critical need in multi-center protein formulation studies and regulatory submissions where reproducibility is paramount.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!