How to Detect Early Aggregation with Dynamic Light Scattering
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DLS Technology Background and Objectives
Dynamic Light Scattering (DLS) emerged in the 1960s as a non-invasive technique for measuring particle size in solutions. Initially applied in polymer science and colloid research, DLS has evolved significantly over the past six decades to become a cornerstone analytical method in various scientific and industrial applications. The technology leverages the Brownian motion of particles and the resulting fluctuations in scattered light intensity to determine particle size distributions in solution.
The fundamental principle behind DLS relies on the correlation between particle size and diffusion speed - smaller particles move faster than larger ones in solution. By analyzing the time-dependent fluctuations in scattered light intensity, DLS can provide valuable information about particle size, molecular weight, and aggregation states of biomolecules, nanoparticles, and colloidal systems.
Recent technological advancements have dramatically improved DLS capabilities, including enhanced sensitivity, reduced sample volume requirements, and sophisticated data analysis algorithms. Modern DLS instruments can detect particles ranging from sub-nanometer to several micrometers in diameter, making the technique particularly valuable for studying protein aggregation phenomena in biopharmaceutical development.
Protein aggregation represents a critical challenge in biopharmaceutical development, affecting product stability, efficacy, and safety. Early detection of protein aggregation is essential for ensuring drug quality and preventing potential immunogenic responses in patients. Traditional methods for detecting aggregation often require large sample volumes or can only detect aggregates after substantial formation has occurred.
The primary objective of employing DLS for early aggregation detection is to identify the initial stages of protein self-association before visible aggregates form. This capability allows researchers and manufacturers to implement preventive measures, optimize formulation conditions, and ensure product quality throughout the development pipeline. Early intervention based on DLS data can significantly reduce development costs and accelerate time-to-market for biopharmaceutical products.
Another key goal is to establish standardized protocols for DLS measurements that enable reliable, reproducible detection of early aggregation events across different laboratory settings and instrument platforms. This standardization would facilitate better comparison of results between research groups and regulatory submissions, ultimately benefiting the entire biopharmaceutical industry.
The technology also aims to distinguish between different types of aggregation pathways, providing insights into the mechanisms underlying protein instability. Understanding these mechanisms is crucial for developing targeted strategies to prevent or mitigate aggregation in therapeutic proteins and other biomolecular systems.
The fundamental principle behind DLS relies on the correlation between particle size and diffusion speed - smaller particles move faster than larger ones in solution. By analyzing the time-dependent fluctuations in scattered light intensity, DLS can provide valuable information about particle size, molecular weight, and aggregation states of biomolecules, nanoparticles, and colloidal systems.
Recent technological advancements have dramatically improved DLS capabilities, including enhanced sensitivity, reduced sample volume requirements, and sophisticated data analysis algorithms. Modern DLS instruments can detect particles ranging from sub-nanometer to several micrometers in diameter, making the technique particularly valuable for studying protein aggregation phenomena in biopharmaceutical development.
Protein aggregation represents a critical challenge in biopharmaceutical development, affecting product stability, efficacy, and safety. Early detection of protein aggregation is essential for ensuring drug quality and preventing potential immunogenic responses in patients. Traditional methods for detecting aggregation often require large sample volumes or can only detect aggregates after substantial formation has occurred.
The primary objective of employing DLS for early aggregation detection is to identify the initial stages of protein self-association before visible aggregates form. This capability allows researchers and manufacturers to implement preventive measures, optimize formulation conditions, and ensure product quality throughout the development pipeline. Early intervention based on DLS data can significantly reduce development costs and accelerate time-to-market for biopharmaceutical products.
Another key goal is to establish standardized protocols for DLS measurements that enable reliable, reproducible detection of early aggregation events across different laboratory settings and instrument platforms. This standardization would facilitate better comparison of results between research groups and regulatory submissions, ultimately benefiting the entire biopharmaceutical industry.
The technology also aims to distinguish between different types of aggregation pathways, providing insights into the mechanisms underlying protein instability. Understanding these mechanisms is crucial for developing targeted strategies to prevent or mitigate aggregation in therapeutic proteins and other biomolecular systems.
Market Applications for Early Aggregation Detection
The early detection of protein aggregation using Dynamic Light Scattering (DLS) has significant market applications across multiple industries, with biopharmaceuticals representing the largest and most immediate beneficiary. In this sector, protein-based therapeutics such as monoclonal antibodies, vaccines, and recombinant proteins require stringent quality control throughout their development and manufacturing processes. Early aggregation detection enables pharmaceutical companies to optimize formulation conditions, extend product shelf-life, and ensure batch-to-batch consistency, ultimately reducing production costs and minimizing product failures.
The global biopharmaceutical market, valued at over $325 billion, continues to grow at approximately 8% annually, creating substantial demand for advanced analytical technologies like DLS-based aggregation detection systems. Companies implementing these technologies can achieve significant cost savings by identifying problematic formulations earlier in development cycles, potentially saving millions in late-stage failures.
Food and beverage industries represent another substantial market for early aggregation detection technologies. Protein-rich products such as dairy, plant-based alternatives, and functional foods benefit from aggregation monitoring to ensure consistent texture, appearance, and stability. The global protein ingredients market exceeds $50 billion, with manufacturers increasingly adopting advanced quality control measures to differentiate their products in competitive markets.
Academic and research institutions constitute a growing market segment, utilizing DLS for fundamental protein science, material development, and nanotechnology applications. This sector drives innovation in methodology and expands potential applications across interdisciplinary fields.
Cosmetics and personal care products incorporating bioactive proteins and peptides benefit from aggregation monitoring to ensure product efficacy and stability. With the premium skincare market growing at 6% annually, manufacturers are investing in advanced analytical techniques to support claims of product effectiveness and longevity.
Emerging applications include environmental monitoring, where DLS can detect protein aggregates in water systems as indicators of contamination or biological activity. Additionally, the growing field of nanomedicine utilizes DLS to monitor nanoparticle stability and protein corona formation, critical for drug delivery system development.
The instrumentation market for DLS systems shows steady growth, with increasing demand for automated, high-throughput systems that can integrate with existing pharmaceutical manufacturing processes. Companies offering comprehensive solutions that combine hardware, software, and analytical services are particularly well-positioned in this evolving market landscape.
The global biopharmaceutical market, valued at over $325 billion, continues to grow at approximately 8% annually, creating substantial demand for advanced analytical technologies like DLS-based aggregation detection systems. Companies implementing these technologies can achieve significant cost savings by identifying problematic formulations earlier in development cycles, potentially saving millions in late-stage failures.
Food and beverage industries represent another substantial market for early aggregation detection technologies. Protein-rich products such as dairy, plant-based alternatives, and functional foods benefit from aggregation monitoring to ensure consistent texture, appearance, and stability. The global protein ingredients market exceeds $50 billion, with manufacturers increasingly adopting advanced quality control measures to differentiate their products in competitive markets.
Academic and research institutions constitute a growing market segment, utilizing DLS for fundamental protein science, material development, and nanotechnology applications. This sector drives innovation in methodology and expands potential applications across interdisciplinary fields.
Cosmetics and personal care products incorporating bioactive proteins and peptides benefit from aggregation monitoring to ensure product efficacy and stability. With the premium skincare market growing at 6% annually, manufacturers are investing in advanced analytical techniques to support claims of product effectiveness and longevity.
Emerging applications include environmental monitoring, where DLS can detect protein aggregates in water systems as indicators of contamination or biological activity. Additionally, the growing field of nanomedicine utilizes DLS to monitor nanoparticle stability and protein corona formation, critical for drug delivery system development.
The instrumentation market for DLS systems shows steady growth, with increasing demand for automated, high-throughput systems that can integrate with existing pharmaceutical manufacturing processes. Companies offering comprehensive solutions that combine hardware, software, and analytical services are particularly well-positioned in this evolving market landscape.
Current Challenges in DLS Aggregation Detection
Despite significant advancements in Dynamic Light Scattering (DLS) technology, several critical challenges persist in early aggregation detection that limit its effectiveness in various applications. The fundamental physical limitation of DLS relates to its sensitivity threshold, which typically cannot reliably detect aggregates smaller than approximately 1-2 nm in diameter or distinguish particles that differ in size by less than a factor of three. This creates a significant blind spot in early-stage aggregation monitoring, particularly for protein therapeutics and nanomaterials.
Signal-to-noise ratio presents another substantial challenge, especially when attempting to detect small quantities of aggregates in the presence of predominant monomeric species. The scattering intensity being proportional to the sixth power of particle diameter means that even a small number of large aggregates can overwhelm the signal from numerous smaller particles, potentially masking critical early aggregation events.
Sample preparation inconsistencies further complicate reliable detection. Factors such as dust contamination, buffer composition variations, and concentration differences can significantly alter DLS measurements, leading to false positives or negatives in aggregation detection. This challenge is particularly pronounced in industrial settings where high-throughput analysis is required.
Data interpretation complexity represents a significant hurdle in DLS analysis. The mathematical algorithms used to convert correlation functions to size distributions involve assumptions that may not hold true for complex, polydisperse systems. Different analysis methods (cumulants analysis, CONTIN, maximum entropy) can yield substantially different results from the same raw data, creating interpretation ambiguities.
Temperature control during measurement presents another technical challenge. Even minor temperature fluctuations can significantly affect particle Brownian motion and consequently DLS measurements. This is particularly problematic for temperature-sensitive samples where the measurement process itself might induce aggregation.
The non-specificity of DLS measurements also limits detailed characterization. While DLS can detect size changes, it cannot provide information about the nature, composition, or structure of the aggregates formed, necessitating complementary analytical techniques for comprehensive characterization.
Batch-to-batch reproducibility remains problematic, with variations in instrument calibration, laser stability, and detector sensitivity contributing to measurement inconsistencies. This challenge is particularly acute when comparing data across different instruments or laboratories, hampering standardization efforts in pharmaceutical and material science industries.
Signal-to-noise ratio presents another substantial challenge, especially when attempting to detect small quantities of aggregates in the presence of predominant monomeric species. The scattering intensity being proportional to the sixth power of particle diameter means that even a small number of large aggregates can overwhelm the signal from numerous smaller particles, potentially masking critical early aggregation events.
Sample preparation inconsistencies further complicate reliable detection. Factors such as dust contamination, buffer composition variations, and concentration differences can significantly alter DLS measurements, leading to false positives or negatives in aggregation detection. This challenge is particularly pronounced in industrial settings where high-throughput analysis is required.
Data interpretation complexity represents a significant hurdle in DLS analysis. The mathematical algorithms used to convert correlation functions to size distributions involve assumptions that may not hold true for complex, polydisperse systems. Different analysis methods (cumulants analysis, CONTIN, maximum entropy) can yield substantially different results from the same raw data, creating interpretation ambiguities.
Temperature control during measurement presents another technical challenge. Even minor temperature fluctuations can significantly affect particle Brownian motion and consequently DLS measurements. This is particularly problematic for temperature-sensitive samples where the measurement process itself might induce aggregation.
The non-specificity of DLS measurements also limits detailed characterization. While DLS can detect size changes, it cannot provide information about the nature, composition, or structure of the aggregates formed, necessitating complementary analytical techniques for comprehensive characterization.
Batch-to-batch reproducibility remains problematic, with variations in instrument calibration, laser stability, and detector sensitivity contributing to measurement inconsistencies. This challenge is particularly acute when comparing data across different instruments or laboratories, hampering standardization efforts in pharmaceutical and material science industries.
Current DLS Methods for Early Aggregation Detection
01 DLS techniques for protein aggregation detection
Dynamic Light Scattering (DLS) can be used to detect early protein aggregation by measuring the size distribution of particles in solution. This technique allows for real-time monitoring of protein stability and can identify the formation of small aggregates before they become visible. The method is particularly valuable in biopharmaceutical development for quality control of protein-based drugs, where early detection of aggregation can prevent product failure.- Basic principles and apparatus for DLS aggregation detection: Dynamic Light Scattering (DLS) is a technique used to detect early aggregation in various substances by measuring the scattered light from particles in solution. The basic apparatus includes a light source (typically a laser), a sample holder, and a detector that measures the intensity fluctuations of scattered light. These fluctuations are analyzed to determine particle size distribution, which can indicate the presence of aggregates before they become visible to the naked eye. This early detection capability is crucial for quality control in pharmaceutical and biological sample analysis.
- Protein and biopharmaceutical aggregation monitoring: DLS is particularly valuable for monitoring aggregation in protein solutions and biopharmaceutical products. The technique can detect early-stage aggregation of therapeutic proteins, antibodies, and other biological molecules, which is critical for ensuring product stability and efficacy. By continuously monitoring these solutions using DLS, researchers can identify conditions that promote aggregation and develop formulations that minimize this process. This application is essential in the development and quality control of biopharmaceuticals, where protein aggregation can affect drug safety and efficacy.
- Advanced data analysis algorithms for early aggregation detection: Advanced algorithms and computational methods enhance the sensitivity and specificity of DLS for early aggregation detection. These algorithms process the raw scattering data to extract meaningful information about particle size distribution and aggregation kinetics. Machine learning approaches can identify subtle patterns in DLS data that indicate the onset of aggregation before traditional analysis methods would detect changes. Real-time data processing allows for immediate feedback on sample stability, enabling researchers to intervene before significant aggregation occurs.
- Integration with other analytical techniques: Combining DLS with complementary analytical techniques provides more comprehensive characterization of aggregation phenomena. Integrated systems may incorporate static light scattering, size exclusion chromatography, viscosity measurements, or spectroscopic methods alongside DLS. This multi-modal approach allows researchers to correlate changes in particle size with structural alterations, chemical modifications, or functional properties. The integration of multiple techniques enhances the reliability of early aggregation detection and provides deeper insights into the mechanisms of aggregate formation.
- Automated high-throughput screening systems: Automated DLS systems enable high-throughput screening for aggregation propensity across multiple samples and conditions. These systems incorporate robotics for sample handling, temperature control modules, and automated data acquisition and analysis. They allow researchers to systematically evaluate the effects of various formulation parameters, environmental conditions, and stress factors on aggregation behavior. High-throughput DLS screening accelerates formulation development and stability testing for pharmaceuticals, biologics, and other colloidal systems by efficiently identifying optimal conditions that minimize aggregation.
02 Advanced data analysis algorithms for DLS measurements
Sophisticated algorithms and data processing techniques enhance the sensitivity and accuracy of DLS for early aggregation detection. These computational methods can filter noise, perform correlation analysis, and apply mathematical models to extract meaningful information from light scattering data. Machine learning approaches can also be integrated to improve pattern recognition in complex DLS datasets, enabling more reliable detection of aggregation onset.Expand Specific Solutions03 Instrumentation innovations for improved DLS sensitivity
Advancements in DLS instrumentation have significantly improved the sensitivity for early aggregation detection. These innovations include enhanced laser sources, more sensitive detectors, temperature control systems, and specialized sample cells. Automated measurement protocols allow for continuous monitoring of samples over time, capturing the earliest stages of aggregation. Miniaturized and integrated systems have also been developed for high-throughput screening applications.Expand Specific Solutions04 Multi-angle and multi-wavelength DLS approaches
Multi-angle and multi-wavelength DLS techniques provide more comprehensive characterization of aggregating systems. By collecting scattering data at multiple angles or using different wavelengths of light, these methods can distinguish between different types of aggregates and provide information about their shape and internal structure. This approach is particularly useful for complex biological samples where various aggregation pathways may occur simultaneously.Expand Specific Solutions05 Integration of DLS with complementary analytical techniques
Combining DLS with other analytical methods creates powerful hybrid approaches for comprehensive aggregation monitoring. These integrated systems may incorporate techniques such as size exclusion chromatography, mass spectrometry, or spectroscopic methods to provide multi-dimensional characterization of aggregating samples. Such combinations overcome the limitations of individual techniques and provide more detailed information about aggregation mechanisms and kinetics.Expand Specific Solutions
Key Industry Players in DLS Instrumentation
Dynamic Light Scattering (DLS) technology for early aggregation detection is evolving rapidly in a growing market driven by biopharmaceutical development and quality control needs. The industry is in a growth phase with increasing adoption across pharmaceutical, biotechnology, and academic research sectors. Key players include specialized analytical instrument manufacturers like Malvern Panalytical and Wyatt Technology, who lead with advanced DLS solutions specifically designed for protein aggregation detection. Established scientific instrument companies such as Shimadzu, Bio-Rad, and Bruker AXS have integrated DLS capabilities into their broader analytical portfolios. The technology has reached moderate maturity with standardized methodologies, but innovation continues in sensitivity improvements, automation, and data analysis algorithms to detect increasingly smaller aggregates at earlier formation stages.
Malvern Panalytical Ltd.
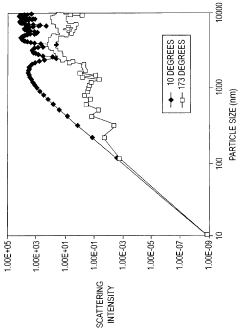
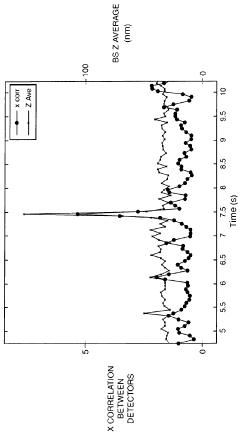
Technical Solution: Malvern Panalytical has developed advanced Dynamic Light Scattering (DLS) systems specifically designed for early aggregation detection in various samples. Their Zetasizer series incorporates Non-Invasive Back Scatter (NIBS) technology that optimizes the detection angle (typically 173°) to minimize multiple scattering effects and maximize signal quality when measuring aggregation-prone samples. The company's instruments employ proprietary algorithms that can distinguish between monomers and early-stage aggregates by analyzing the correlation function and size distribution with high resolution. Their adaptive correlation technology automatically adjusts measurement parameters based on sample characteristics, enabling detection of aggregates even in the presence of larger particles. Malvern's systems also integrate temperature control modules (from 0°C to 90°C) that can perform temperature ramps to study aggregation kinetics and identify onset temperatures for aggregation processes, critical for understanding protein stability.
Strengths: Industry-leading sensitivity for detecting early-stage aggregates as small as 0.3nm; sophisticated software with specialized algorithms for distinguishing between different particle populations; temperature-dependent measurement capabilities for studying aggregation mechanisms. Weaknesses: Higher cost compared to simpler DLS systems; requires skilled operators for optimal data interpretation; measurements can be affected by dust contamination requiring careful sample preparation.
Wyatt Technology LLC
Technical Solution: Wyatt Technology has pioneered multi-angle dynamic light scattering (MADLS) technology for early aggregation detection through their DynaPro® NanoStar® and Mobius® instruments. Their approach combines traditional DLS with multi-angle light scattering (MALS) to provide enhanced resolution and characterization capabilities. The DynaPro® platform utilizes a high-power laser (typically 100mW) and advanced photon counting technology to achieve exceptional sensitivity for detecting early-stage aggregates. Their proprietary DYNAMICS® software employs regularization algorithms that can deconvolute complex mixtures of monomers and early aggregates with superior resolution compared to conventional DLS systems. Wyatt's instruments feature automated batch measurement capabilities with temperature control from 4°C to 70°C, allowing for automated stability studies that can identify aggregation onset conditions. The company has also developed specialized sample cells with minimal volume requirements (as low as 1.25 μL) that reduce sample consumption while maintaining measurement quality for precious biological samples.
Strengths: Exceptional resolution for separating closely-sized particle populations; integrated MALS capabilities provide additional structural information beyond traditional DLS; automated batch processing enables high-throughput screening. Weaknesses: Premium pricing positions these instruments at the higher end of the market; complex data analysis may require specialized training; limited compatibility with highly concentrated or turbid samples.
Advanced Algorithms for DLS Data Analysis
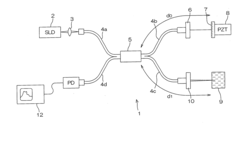
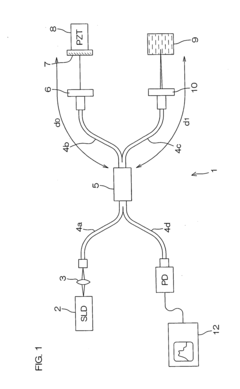
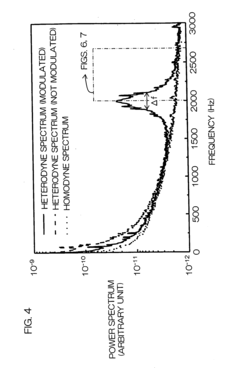
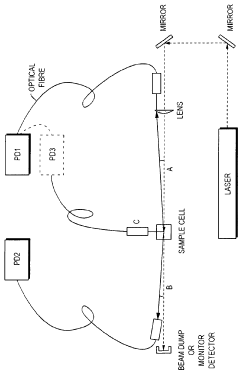
Dynamic light scattering measurement apparatus using phase modulation interference method
PatentInactiveUS20050122528A1
Innovation
- A dynamic light scattering measurement apparatus utilizing a low coherence light source, phase modulation, and a specific light path length normalization (s/L ≤ 3) to selectively extract the single-scattered spectrum component from multiple scattering media, enabling precise measurement of particle dynamics.
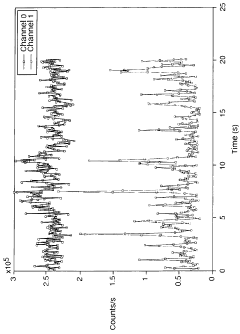
Light scattering measurements using simultaneous detection
PatentWO2009090562A2
Innovation
- A detection scheme utilizing two or more photon-counting detectors and simultaneous detection logic, allowing for the measurement of particle size and characteristics by acquiring light scattered at different angles, effectively gating out the interference from larger contaminants through cross-correlation and digital signal processing.
Regulatory Standards for Aggregation Analysis
Regulatory frameworks governing protein aggregation analysis have evolved significantly over the past decade, reflecting the growing understanding of aggregation's impact on biopharmaceutical safety and efficacy. The International Conference on Harmonisation (ICH) guidelines, particularly ICH Q6B, establish foundational requirements for characterizing protein products, including the need to assess aggregation as a critical quality attribute. These guidelines emphasize that manufacturers must implement appropriate analytical methods, including Dynamic Light Scattering (DLS), to detect and quantify protein aggregates throughout product development and manufacturing.
The United States Food and Drug Administration (FDA) has published specific guidance documents addressing protein aggregation in biological products. The FDA's "Guidance for Industry: Immunogenicity Assessment for Therapeutic Protein Products" explicitly recognizes aggregation as a risk factor for immunogenicity and requires comprehensive aggregation analysis during product development. For DLS applications, the FDA recommends validation protocols that demonstrate method sensitivity, specificity, and reproducibility for early aggregation detection.
European Medicines Agency (EMA) regulations similarly emphasize aggregation control through their "Guideline on Development, Production, Characterisation and Specifications for Monoclonal Antibodies and Related Products." The EMA specifically acknowledges DLS as an acceptable technique for size distribution analysis and early aggregation detection, provided that appropriate method validation is performed and limitations are understood.
The United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) have established specific monographs and general chapters addressing particulate matter in injectable products. USP <788> and Ph. Eur. 2.9.19 set limits for visible and sub-visible particles, while newer chapters like USP <1787> provide guidance on measurement techniques including light scattering methods. These standards are increasingly recognizing the importance of detecting smaller aggregates in the nanometer range, where DLS excels.
Japanese Pharmacopoeia (JP) requirements align with international standards but include additional specifications for protein aggregation testing. The JP emphasizes the need for orthogonal methods to confirm DLS findings, particularly for regulatory submissions.
Industry standards organizations, including ASTM International and ISO, have developed technical standards for particle characterization using light scattering techniques. ASTM E2578 provides guidelines for using DLS for particle size analysis, while ISO 22412:2017 establishes standardized protocols for DLS measurements, ensuring consistency across laboratories and facilitating regulatory compliance.
Regulatory bodies increasingly require manufacturers to implement a comprehensive aggregation control strategy that includes multiple orthogonal methods. While DLS is recognized for its sensitivity to early-stage aggregation, regulators typically require confirmation with complementary techniques such as size-exclusion chromatography or analytical ultracentrifugation for comprehensive characterization.
The United States Food and Drug Administration (FDA) has published specific guidance documents addressing protein aggregation in biological products. The FDA's "Guidance for Industry: Immunogenicity Assessment for Therapeutic Protein Products" explicitly recognizes aggregation as a risk factor for immunogenicity and requires comprehensive aggregation analysis during product development. For DLS applications, the FDA recommends validation protocols that demonstrate method sensitivity, specificity, and reproducibility for early aggregation detection.
European Medicines Agency (EMA) regulations similarly emphasize aggregation control through their "Guideline on Development, Production, Characterisation and Specifications for Monoclonal Antibodies and Related Products." The EMA specifically acknowledges DLS as an acceptable technique for size distribution analysis and early aggregation detection, provided that appropriate method validation is performed and limitations are understood.
The United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) have established specific monographs and general chapters addressing particulate matter in injectable products. USP <788> and Ph. Eur. 2.9.19 set limits for visible and sub-visible particles, while newer chapters like USP <1787> provide guidance on measurement techniques including light scattering methods. These standards are increasingly recognizing the importance of detecting smaller aggregates in the nanometer range, where DLS excels.
Japanese Pharmacopoeia (JP) requirements align with international standards but include additional specifications for protein aggregation testing. The JP emphasizes the need for orthogonal methods to confirm DLS findings, particularly for regulatory submissions.
Industry standards organizations, including ASTM International and ISO, have developed technical standards for particle characterization using light scattering techniques. ASTM E2578 provides guidelines for using DLS for particle size analysis, while ISO 22412:2017 establishes standardized protocols for DLS measurements, ensuring consistency across laboratories and facilitating regulatory compliance.
Regulatory bodies increasingly require manufacturers to implement a comprehensive aggregation control strategy that includes multiple orthogonal methods. While DLS is recognized for its sensitivity to early-stage aggregation, regulators typically require confirmation with complementary techniques such as size-exclusion chromatography or analytical ultracentrifugation for comprehensive characterization.
Sample Preparation Optimization for DLS
Sample preparation represents a critical foundation for accurate and reliable Dynamic Light Scattering (DLS) measurements, particularly when detecting early protein aggregation. The quality of DLS data directly correlates with proper sample handling and preparation techniques, which must be carefully optimized to avoid introducing artifacts that could be misinterpreted as aggregation events.
Protein concentration plays a fundamental role in DLS measurements. For early aggregation detection, concentrations typically between 0.1-2.0 mg/mL provide optimal signal-to-noise ratios while minimizing multiple scattering effects. Higher concentrations may accelerate aggregation processes artificially, while excessively dilute samples might fail to capture early aggregation events due to insufficient signal intensity.
Buffer composition significantly impacts protein stability and aggregation kinetics. Ionic strength, pH, and specific buffer components must be carefully selected to maintain native protein conformation while enabling sensitive detection of aggregation onset. Phosphate buffers at physiological pH (7.2-7.4) often serve as suitable starting points, though optimization for specific proteins remains essential.
Filtration protocols represent another critical aspect of sample preparation. Pre-measurement filtration through 0.02-0.22 μm filters removes dust particles and pre-existing large aggregates that would otherwise dominate scattering signals and mask early aggregation events. However, excessive filtration pressure must be avoided as it may induce protein denaturation or remove relevant oligomeric species.
Temperature control during sample preparation directly influences aggregation kinetics. Samples should be equilibrated at measurement temperature for 15-30 minutes prior to analysis to ensure thermal stability. Temperature gradients within samples must be eliminated as they can induce convection currents that interfere with Brownian motion measurements.
Sample handling techniques require standardization to ensure reproducibility. Minimizing mechanical stress through gentle mixing (avoiding vortexing), using low-protein-binding containers, and implementing consistent pipetting techniques all contribute to reliable early aggregation detection. Additionally, time between sample preparation and measurement should be strictly controlled and documented.
Advanced preparation techniques such as dialysis against measurement buffer or size exclusion chromatography immediately prior to DLS analysis can further enhance detection sensitivity for early aggregation events by removing small molecular weight impurities that might interfere with measurements or catalyze aggregation processes.
Protein concentration plays a fundamental role in DLS measurements. For early aggregation detection, concentrations typically between 0.1-2.0 mg/mL provide optimal signal-to-noise ratios while minimizing multiple scattering effects. Higher concentrations may accelerate aggregation processes artificially, while excessively dilute samples might fail to capture early aggregation events due to insufficient signal intensity.
Buffer composition significantly impacts protein stability and aggregation kinetics. Ionic strength, pH, and specific buffer components must be carefully selected to maintain native protein conformation while enabling sensitive detection of aggregation onset. Phosphate buffers at physiological pH (7.2-7.4) often serve as suitable starting points, though optimization for specific proteins remains essential.
Filtration protocols represent another critical aspect of sample preparation. Pre-measurement filtration through 0.02-0.22 μm filters removes dust particles and pre-existing large aggregates that would otherwise dominate scattering signals and mask early aggregation events. However, excessive filtration pressure must be avoided as it may induce protein denaturation or remove relevant oligomeric species.
Temperature control during sample preparation directly influences aggregation kinetics. Samples should be equilibrated at measurement temperature for 15-30 minutes prior to analysis to ensure thermal stability. Temperature gradients within samples must be eliminated as they can induce convection currents that interfere with Brownian motion measurements.
Sample handling techniques require standardization to ensure reproducibility. Minimizing mechanical stress through gentle mixing (avoiding vortexing), using low-protein-binding containers, and implementing consistent pipetting techniques all contribute to reliable early aggregation detection. Additionally, time between sample preparation and measurement should be strictly controlled and documented.
Advanced preparation techniques such as dialysis against measurement buffer or size exclusion chromatography immediately prior to DLS analysis can further enhance detection sensitivity for early aggregation events by removing small molecular weight impurities that might interfere with measurements or catalyze aggregation processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!