How to Optimize Dynamic Light Scattering for Protein Analysis
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DLS Protein Analysis Background and Objectives
Dynamic Light Scattering (DLS) has emerged as a pivotal analytical technique in the field of protein characterization since its introduction in the 1970s. The technology has evolved from rudimentary light scattering instruments to sophisticated systems capable of providing detailed insights into protein behavior in solution. This evolution has been driven by the growing demands of biopharmaceutical development, structural biology research, and protein engineering applications, where understanding protein stability, aggregation, and interaction dynamics is crucial.
The fundamental principle of DLS relies on measuring the Brownian motion of particles in solution and correlating this movement to particle size through the Stokes-Einstein equation. For protein analysis, this translates to the ability to determine hydrodynamic radius, polydispersity, and aggregation state—critical parameters for assessing protein quality and behavior under various conditions.
Current technological trends in DLS for protein analysis include the miniaturization of instruments, integration with other analytical techniques (such as size exclusion chromatography or multi-angle light scattering), and the development of advanced algorithms for data interpretation. These advancements aim to address the inherent limitations of traditional DLS, such as sensitivity to dust contamination, limited resolution for polydisperse samples, and challenges in analyzing complex protein mixtures.
The primary objectives of optimizing DLS for protein analysis encompass several dimensions. First, enhancing measurement sensitivity to detect subtle changes in protein conformation and early-stage aggregation, which is particularly valuable for monitoring protein stability during formulation development. Second, improving resolution to distinguish between closely sized protein species in heterogeneous samples, addressing a significant limitation of conventional DLS systems. Third, developing robust data analysis frameworks that can reliably interpret complex correlation functions from protein samples with multiple components.
Additionally, there is a growing emphasis on establishing standardized protocols for sample preparation, measurement parameters, and data analysis to ensure reproducibility across different laboratories and instrument platforms. This standardization is essential as DLS increasingly becomes a regulatory requirement in biopharmaceutical development and quality control processes.
The optimization of DLS for protein analysis also aims to expand its application scope beyond traditional size measurements to include assessments of protein-protein interactions, thermal stability profiling, and compatibility with increasingly complex biological matrices. These advancements would position DLS as an even more versatile tool in the protein scientist's analytical arsenal, supporting the development of next-generation biotherapeutics and deepening our understanding of protein behavior in biological systems.
The fundamental principle of DLS relies on measuring the Brownian motion of particles in solution and correlating this movement to particle size through the Stokes-Einstein equation. For protein analysis, this translates to the ability to determine hydrodynamic radius, polydispersity, and aggregation state—critical parameters for assessing protein quality and behavior under various conditions.
Current technological trends in DLS for protein analysis include the miniaturization of instruments, integration with other analytical techniques (such as size exclusion chromatography or multi-angle light scattering), and the development of advanced algorithms for data interpretation. These advancements aim to address the inherent limitations of traditional DLS, such as sensitivity to dust contamination, limited resolution for polydisperse samples, and challenges in analyzing complex protein mixtures.
The primary objectives of optimizing DLS for protein analysis encompass several dimensions. First, enhancing measurement sensitivity to detect subtle changes in protein conformation and early-stage aggregation, which is particularly valuable for monitoring protein stability during formulation development. Second, improving resolution to distinguish between closely sized protein species in heterogeneous samples, addressing a significant limitation of conventional DLS systems. Third, developing robust data analysis frameworks that can reliably interpret complex correlation functions from protein samples with multiple components.
Additionally, there is a growing emphasis on establishing standardized protocols for sample preparation, measurement parameters, and data analysis to ensure reproducibility across different laboratories and instrument platforms. This standardization is essential as DLS increasingly becomes a regulatory requirement in biopharmaceutical development and quality control processes.
The optimization of DLS for protein analysis also aims to expand its application scope beyond traditional size measurements to include assessments of protein-protein interactions, thermal stability profiling, and compatibility with increasingly complex biological matrices. These advancements would position DLS as an even more versatile tool in the protein scientist's analytical arsenal, supporting the development of next-generation biotherapeutics and deepening our understanding of protein behavior in biological systems.
Market Demand for Protein Characterization
The protein characterization market has experienced substantial growth in recent years, driven primarily by advancements in proteomics research and biopharmaceutical development. The global market for protein characterization was valued at approximately 1.7 billion USD in 2022 and is projected to reach 3.5 billion USD by 2028, representing a compound annual growth rate of 12.8% during the forecast period.
Biopharmaceutical companies constitute the largest segment of end-users, accounting for nearly 45% of the market share. This dominance stems from the critical need for thorough protein characterization during drug development processes, particularly for biologics and biosimilars. The increasing prevalence of protein-based therapeutics has significantly amplified demand for precise analytical techniques like Dynamic Light Scattering (DLS).
Academic research institutions represent the second-largest market segment, contributing approximately 30% of the demand. The growing focus on structural biology and protein-protein interactions in fundamental research has elevated the importance of DLS and other characterization methods in academic settings.
Regionally, North America leads the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 25%. The Asia-Pacific region, particularly China and India, is experiencing the fastest growth rate due to expanding biotechnology sectors and increasing R&D investments.
Key market drivers include the rising prevalence of protein-based therapeutics, stringent regulatory requirements for biopharmaceutical approval, and technological advancements in analytical instrumentation. The FDA and EMA have established increasingly rigorous standards for protein characterization, making techniques like DLS essential for regulatory compliance.
Industry surveys indicate that over 70% of biopharmaceutical companies consider protein aggregation analysis—a primary application of DLS—as critical to their development processes. The ability to detect submicron particles and provide real-time monitoring of protein stability represents significant value propositions for these organizations.
Emerging trends in the market include the integration of artificial intelligence for data interpretation, miniaturization of instruments for point-of-use applications, and the development of multi-parameter characterization platforms that combine DLS with complementary techniques. The demand for high-throughput screening capabilities has also increased, with over 60% of end-users expressing interest in automated systems that can process multiple samples simultaneously.
Biopharmaceutical companies constitute the largest segment of end-users, accounting for nearly 45% of the market share. This dominance stems from the critical need for thorough protein characterization during drug development processes, particularly for biologics and biosimilars. The increasing prevalence of protein-based therapeutics has significantly amplified demand for precise analytical techniques like Dynamic Light Scattering (DLS).
Academic research institutions represent the second-largest market segment, contributing approximately 30% of the demand. The growing focus on structural biology and protein-protein interactions in fundamental research has elevated the importance of DLS and other characterization methods in academic settings.
Regionally, North America leads the market with approximately 40% share, followed by Europe at 30% and Asia-Pacific at 25%. The Asia-Pacific region, particularly China and India, is experiencing the fastest growth rate due to expanding biotechnology sectors and increasing R&D investments.
Key market drivers include the rising prevalence of protein-based therapeutics, stringent regulatory requirements for biopharmaceutical approval, and technological advancements in analytical instrumentation. The FDA and EMA have established increasingly rigorous standards for protein characterization, making techniques like DLS essential for regulatory compliance.
Industry surveys indicate that over 70% of biopharmaceutical companies consider protein aggregation analysis—a primary application of DLS—as critical to their development processes. The ability to detect submicron particles and provide real-time monitoring of protein stability represents significant value propositions for these organizations.
Emerging trends in the market include the integration of artificial intelligence for data interpretation, miniaturization of instruments for point-of-use applications, and the development of multi-parameter characterization platforms that combine DLS with complementary techniques. The demand for high-throughput screening capabilities has also increased, with over 60% of end-users expressing interest in automated systems that can process multiple samples simultaneously.
Current DLS Technology Limitations
Despite its widespread adoption, Dynamic Light Scattering (DLS) faces several significant limitations when applied to protein analysis. The technique's fundamental resolution constraints make it challenging to distinguish between particles with size differences less than a factor of 3-5, which is particularly problematic for protein aggregation studies where early oligomers may be critical indicators of pathological processes.
Sample preparation introduces another layer of complexity, as DLS measurements are highly sensitive to dust particles and large aggregates that can dominate the scattering signal. This "intensity weighting" bias means that even a small number of large particles can obscure the detection of smaller protein species, potentially leading to misinterpretation of results in heterogeneous samples.
Concentration dependencies present additional challenges, with optimal measurement ranges typically between 0.1-10 mg/mL for proteins. At lower concentrations, signal-to-noise ratios deteriorate significantly, while higher concentrations can induce non-ideal behavior through increased intermolecular interactions, affecting the apparent diffusion coefficient and calculated hydrodynamic radius.
The technique also struggles with polydisperse samples containing multiple protein species or aggregates of varying sizes. In such cases, the autocorrelation function becomes a complex composite of multiple decay processes, making accurate size distribution analysis mathematically challenging and often ambiguous.
Temperature control represents another critical limitation, as even minor fluctuations can significantly alter protein diffusion rates and lead to measurement artifacts. Most commercial instruments maintain temperature within ±0.5°C, which may be insufficient for highly temperature-sensitive proteins or for distinguishing subtle conformational changes.
Data interpretation challenges persist due to the indirect nature of DLS measurements. Converting autocorrelation data to size distributions requires mathematical models and assumptions that may not fully capture the complexity of protein samples. The commonly used cumulants analysis works well for monodisperse samples but becomes increasingly unreliable as sample heterogeneity increases.
Instrument-specific variations further complicate cross-laboratory comparisons, with differences in laser wavelength, detector configuration, and proprietary algorithms affecting the reported results. This lack of standardization makes it difficult to establish universal protocols or reference materials for protein characterization by DLS.
Recent technological advances have addressed some of these limitations through multi-angle DLS, machine learning algorithms for data processing, and hybrid techniques combining DLS with other analytical methods. However, fundamental physical constraints of light scattering phenomena continue to limit the technique's resolution and sensitivity for complex protein systems.
Sample preparation introduces another layer of complexity, as DLS measurements are highly sensitive to dust particles and large aggregates that can dominate the scattering signal. This "intensity weighting" bias means that even a small number of large particles can obscure the detection of smaller protein species, potentially leading to misinterpretation of results in heterogeneous samples.
Concentration dependencies present additional challenges, with optimal measurement ranges typically between 0.1-10 mg/mL for proteins. At lower concentrations, signal-to-noise ratios deteriorate significantly, while higher concentrations can induce non-ideal behavior through increased intermolecular interactions, affecting the apparent diffusion coefficient and calculated hydrodynamic radius.
The technique also struggles with polydisperse samples containing multiple protein species or aggregates of varying sizes. In such cases, the autocorrelation function becomes a complex composite of multiple decay processes, making accurate size distribution analysis mathematically challenging and often ambiguous.
Temperature control represents another critical limitation, as even minor fluctuations can significantly alter protein diffusion rates and lead to measurement artifacts. Most commercial instruments maintain temperature within ±0.5°C, which may be insufficient for highly temperature-sensitive proteins or for distinguishing subtle conformational changes.
Data interpretation challenges persist due to the indirect nature of DLS measurements. Converting autocorrelation data to size distributions requires mathematical models and assumptions that may not fully capture the complexity of protein samples. The commonly used cumulants analysis works well for monodisperse samples but becomes increasingly unreliable as sample heterogeneity increases.
Instrument-specific variations further complicate cross-laboratory comparisons, with differences in laser wavelength, detector configuration, and proprietary algorithms affecting the reported results. This lack of standardization makes it difficult to establish universal protocols or reference materials for protein characterization by DLS.
Recent technological advances have addressed some of these limitations through multi-angle DLS, machine learning algorithms for data processing, and hybrid techniques combining DLS with other analytical methods. However, fundamental physical constraints of light scattering phenomena continue to limit the technique's resolution and sensitivity for complex protein systems.
Current DLS Optimization Approaches
01 Optimization of DLS measurement parameters
Dynamic Light Scattering (DLS) measurements can be optimized by adjusting various parameters such as laser intensity, detector angle, and measurement duration. These optimizations improve the accuracy and reliability of particle size measurements, especially for polydisperse samples. Advanced algorithms can automatically determine optimal measurement conditions based on sample characteristics, reducing noise and increasing signal quality.- Optimization of DLS measurement parameters: Dynamic Light Scattering (DLS) measurements can be optimized by adjusting various parameters such as laser intensity, detector angle, and measurement duration. These optimizations improve the accuracy and reliability of particle size measurements, especially for polydisperse samples. Advanced algorithms can automatically determine optimal measurement conditions based on sample characteristics, reducing noise and improving signal quality.
- Sample preparation techniques for DLS: Proper sample preparation is crucial for accurate DLS measurements. This includes methods for controlling sample concentration, filtering to remove dust and large aggregates, temperature stabilization, and pH adjustment. Specialized preparation protocols can be developed for specific sample types such as proteins, nanoparticles, or polymers to minimize artifacts and ensure reproducible results.
- Advanced data analysis algorithms for DLS: Novel computational approaches can enhance the interpretation of DLS data, particularly for complex or multimodal particle distributions. These include machine learning algorithms, regularization methods, and multi-angle analysis techniques that can extract more information from raw correlation data. Such algorithms improve the resolution of particle size distributions and can distinguish between different populations in heterogeneous samples.
- Hardware innovations for DLS systems: Technological advancements in DLS instrumentation include improved laser sources, more sensitive detectors, and novel optical configurations. These hardware innovations enable measurements of smaller particles, lower concentration samples, and faster acquisition times. Multi-angle detection systems and fiber optic probes allow for more comprehensive characterization of particle dynamics and in-situ measurements in various environments.
- Integration of DLS with complementary techniques: Combining DLS with other analytical methods creates powerful hybrid approaches for comprehensive particle characterization. Integration with techniques such as static light scattering, zeta potential measurements, or spectroscopic methods provides multidimensional information about particle properties. These integrated systems offer simultaneous measurements of size, structure, and surface properties, enhancing the overall analytical capabilities.
02 Sample preparation techniques for DLS
Proper sample preparation is crucial for accurate DLS measurements. This includes methods for controlling sample concentration, filtering to remove dust and large aggregates, and stabilizing temperature to prevent convection currents. Specialized preparation protocols can be developed for specific sample types such as proteins, nanoparticles, or polymers to ensure optimal scattering conditions and prevent unwanted aggregation during measurement.Expand Specific Solutions03 Advanced data analysis algorithms for DLS
Sophisticated algorithms can enhance DLS data interpretation, particularly for complex or multimodal samples. These include regularization methods, machine learning approaches, and multi-angle analysis techniques that can extract more detailed information about particle size distributions. Improved mathematical models account for non-spherical particles and interactions between particles, providing more accurate characterization of diverse sample types.Expand Specific Solutions04 Integration of DLS with complementary techniques
Combining DLS with other analytical methods creates powerful hybrid approaches for comprehensive particle characterization. Integration with techniques such as static light scattering, zeta potential measurements, or microscopy provides multidimensional data that overcomes the limitations of DLS alone. These integrated systems often feature automated measurement sequences and unified data analysis platforms for more complete sample characterization.Expand Specific Solutions05 Hardware innovations for improved DLS performance
Recent hardware developments have significantly enhanced DLS capabilities, including advanced optical components, more sensitive detectors, and temperature control systems. Multi-angle detection systems provide simultaneous measurements at different scattering angles, while fiber optic implementations allow for remote or in-line measurements. Miniaturized DLS systems enable portable applications or integration into larger analytical platforms for high-throughput screening.Expand Specific Solutions
Key Industry Players in Protein Analysis
Dynamic Light Scattering (DLS) for protein analysis is currently in a mature growth phase, with a global market estimated at $350-400 million and expanding at 5-7% annually. The technology has reached significant maturity, with established players like Malvern Panalytical and Wyatt Technology dominating with comprehensive solutions. NanoTemper Technologies and Xtal Concepts represent innovative mid-tier companies advancing specialized applications, while research institutions like National University of Singapore and Agency for Science, Technology & Research drive fundamental improvements. Pharmaceutical giants such as Regeneron increasingly integrate DLS into their protein characterization workflows, indicating the technology's essential role in biopharmaceutical development and quality control processes.
Malvern Panalytical Ltd.
Technical Solution: Malvern Panalytical has developed advanced DLS systems with proprietary Non-Invasive Back Scatter (NIBS) technology that optimizes protein analysis by measuring at a 173° angle, significantly reducing multiple scattering effects and dust interference[1]. Their Zetasizer Ultra platform incorporates Multi-Angle Dynamic Light Scattering (MADLS) technology, enabling size measurements across multiple angles simultaneously to provide more comprehensive characterization of protein samples[2]. The system employs adaptive correlation algorithms that automatically adjust measurement parameters based on sample characteristics, improving resolution for polydisperse protein solutions. Additionally, their instruments feature temperature control systems (15-90°C) with 0.1°C precision to maintain sample stability during measurements and prevent protein aggregation[3]. Malvern's software suite includes specialized protein analysis modules with built-in parameters for common buffer systems and automated data interpretation tools specifically designed for therapeutic protein development workflows.
Strengths: Industry-leading sensitivity for detecting small protein aggregates (down to 0.3nm); comprehensive software with specialized protein analysis algorithms; multi-angle measurement capabilities providing superior resolution for complex protein mixtures. Weaknesses: Higher cost compared to simpler DLS systems; requires more extensive training for optimal use; some advanced features may be unnecessary for routine protein analysis applications.
NanoTemper Technologies GmbH
Technical Solution: NanoTemper Technologies has developed the Prometheus Panta system that integrates DLS with differential scanning fluorimetry (DSF) and static light scattering (SLS) for comprehensive protein characterization. Their approach optimizes DLS for protein analysis through a patented microfluidic sample handling system that requires only 10 μL of sample while preventing contamination and protein adsorption issues[1]. The system employs advanced signal processing algorithms specifically calibrated for protein measurements, enabling accurate size determination even in complex buffer systems containing excipients commonly used in biopharmaceutical formulations[2]. NanoTemper's technology incorporates automated dilution series measurements to detect concentration-dependent aggregation phenomena, with software that can extrapolate behavior to storage conditions. Their DLS implementation features temperature-controlled measurements from 15-95°C with 0.1°C precision, allowing for thermal ramping experiments that reveal protein unfolding and aggregation pathways[3]. The company's analysis software includes specialized tools for calculating colloidal stability parameters (kD, B22) from combined DLS/SLS data, providing critical insights for formulation development of therapeutic proteins.
Strengths: Multimodal analysis combining DLS with complementary techniques provides comprehensive protein characterization; exceptional temperature control for stability studies; intuitive software specifically designed for protein scientists without requiring extensive biophysical expertise. Weaknesses: Relatively new entrant to the DLS market compared to established competitors; more focused on protein therapeutic applications than general protein research; higher cost compared to standalone DLS systems.
Critical Patents in DLS Protein Analysis
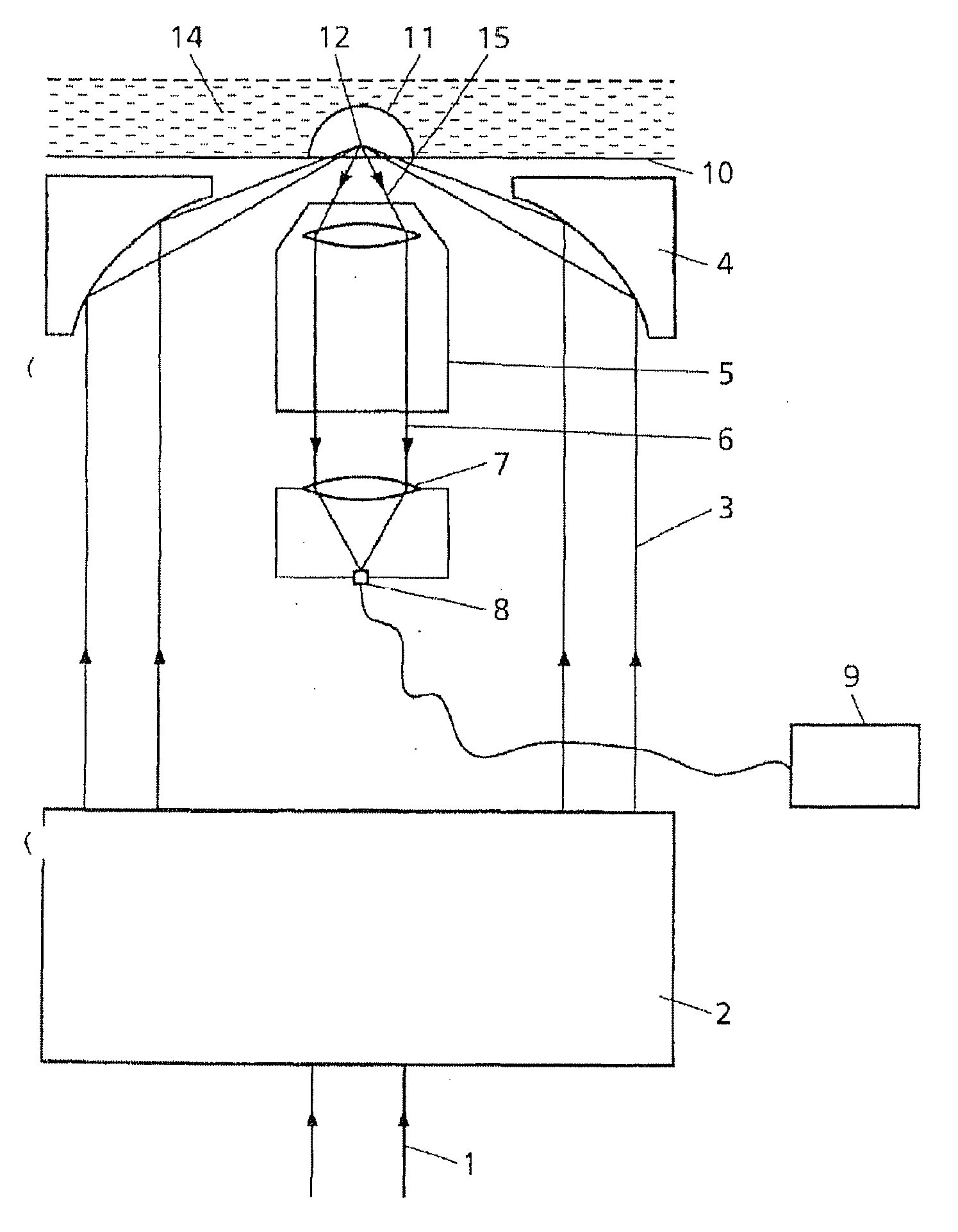
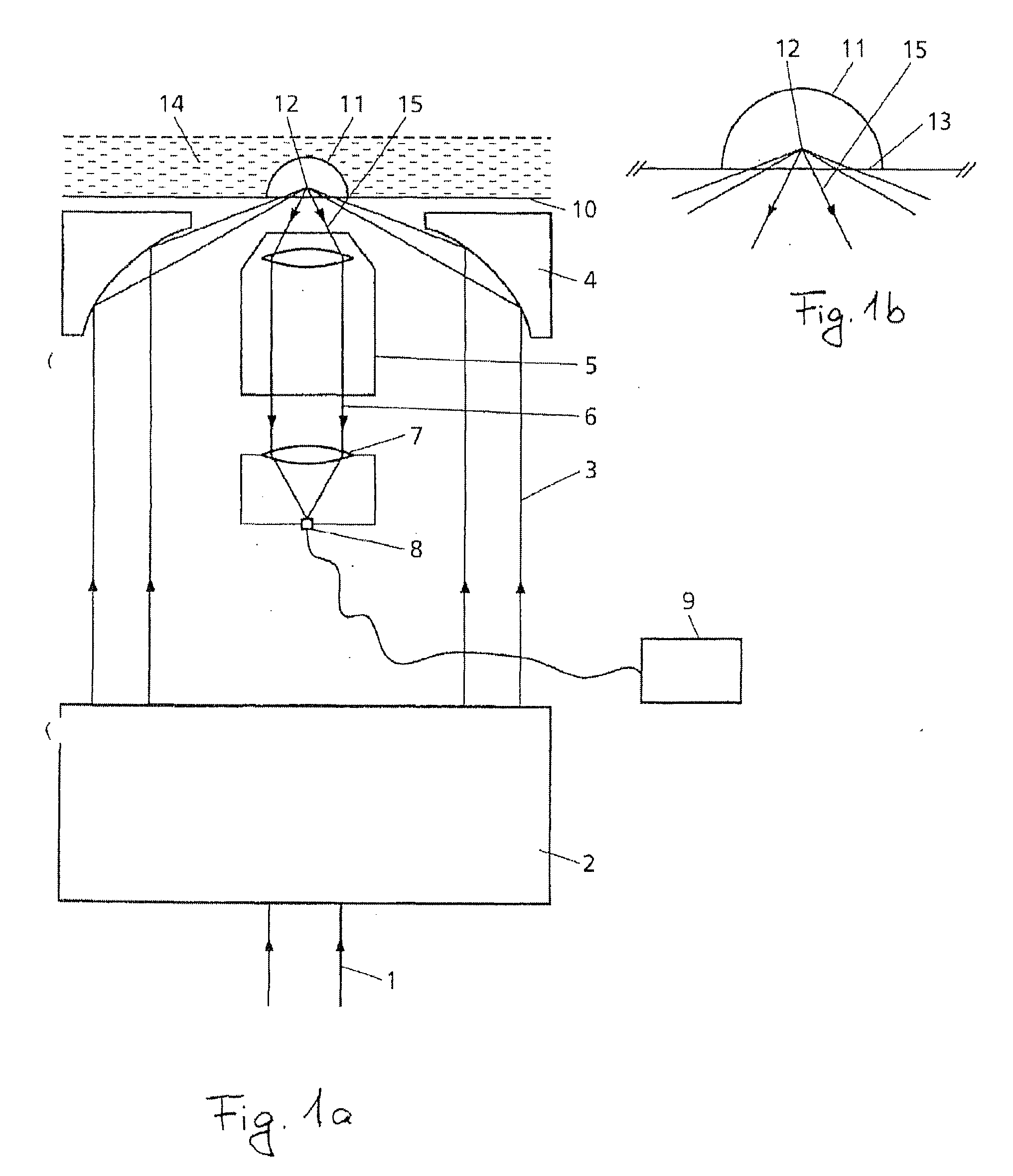
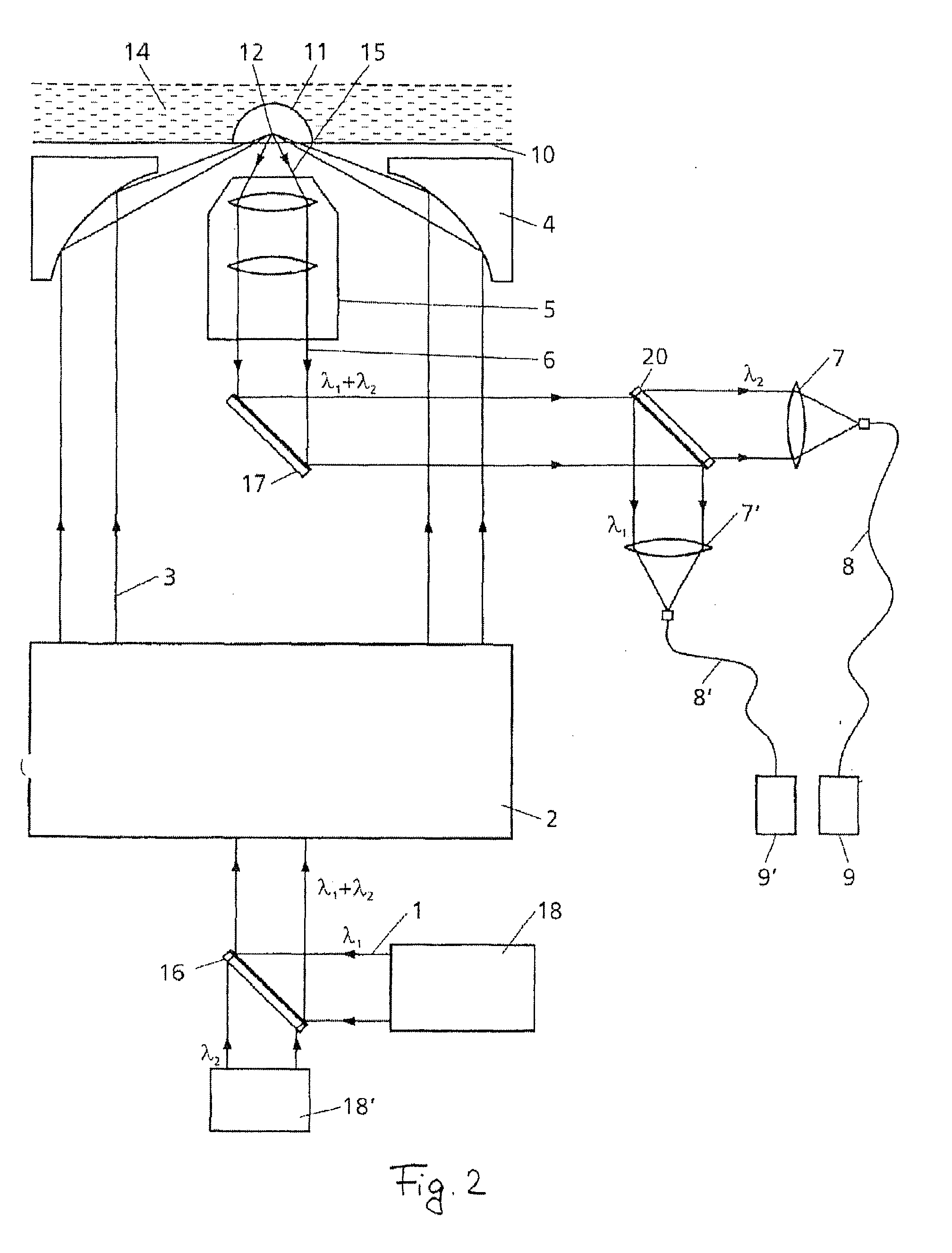
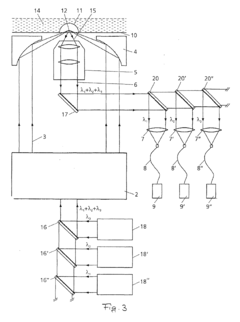
Device and method for measuring static and dynamic scattered light in small volumes
PatentInactiveUS20100315635A1
Innovation
- A device with a confocal optical system generating an annular beam for focused light scattering measurements, allowing for compact and flexible sample examination with strong suppression of undesirable radiation reflections, using microtitration plates and automated pipetting for high-throughput analysis of small sample volumes.
Sample Preparation Protocols
Sample preparation represents a critical foundation for successful Dynamic Light Scattering (DLS) protein analysis. The quality of results directly correlates with proper sample handling techniques. Proteins must be thoroughly filtered using membranes with pore sizes between 0.02-0.45 μm to remove dust particles and large aggregates that can significantly skew measurements. For optimal results, filtration should be performed directly into pre-cleaned measurement cuvettes to minimize contamination risks.
Buffer selection plays a crucial role in maintaining protein stability during DLS analysis. Phosphate-buffered saline (PBS) at physiological pH (7.2-7.4) serves as an excellent starting point for most proteins. However, specific proteins may require customized buffer systems. Buffer components should be filtered to the same standards as protein samples, and all solutions must be prepared using high-purity water (preferably HPLC-grade or equivalent) to minimize background scattering.
Concentration optimization represents another essential aspect of sample preparation. Ideal protein concentrations typically range between 0.1-5.0 mg/mL, depending on the specific protein's molecular weight and scattering properties. Concentrations that are too low may produce insufficient scattering intensity, while excessively high concentrations can lead to multiple scattering effects and particle interactions that complicate data interpretation.
Temperature equilibration cannot be overlooked in the preparation protocol. Samples should be allowed to equilibrate at the measurement temperature for at least 15-20 minutes before analysis to ensure thermal stability and eliminate temperature gradients that could induce convection currents within the sample. This equilibration period also allows any air bubbles introduced during sample handling to dissipate.
Cuvette selection and cleaning procedures significantly impact measurement quality. Quartz or optical-grade glass cuvettes are preferred for their superior optical properties. Cuvettes must undergo rigorous cleaning protocols, typically involving sequential washing with detergent solutions, organic solvents (ethanol or acetone), and finally multiple rinses with filtered water. Dust-free drying in controlled environments prevents recontamination.
For particularly challenging protein samples, additives may be incorporated into preparation protocols. Small amounts (0.01-0.05%) of non-ionic surfactants like Tween-20 can reduce non-specific protein adsorption to surfaces. Similarly, glycerol (5-10%) may enhance protein stability without significantly altering scattering properties. However, these additives must be validated for each specific protein system to ensure they don't interfere with the properties being measured.
Buffer selection plays a crucial role in maintaining protein stability during DLS analysis. Phosphate-buffered saline (PBS) at physiological pH (7.2-7.4) serves as an excellent starting point for most proteins. However, specific proteins may require customized buffer systems. Buffer components should be filtered to the same standards as protein samples, and all solutions must be prepared using high-purity water (preferably HPLC-grade or equivalent) to minimize background scattering.
Concentration optimization represents another essential aspect of sample preparation. Ideal protein concentrations typically range between 0.1-5.0 mg/mL, depending on the specific protein's molecular weight and scattering properties. Concentrations that are too low may produce insufficient scattering intensity, while excessively high concentrations can lead to multiple scattering effects and particle interactions that complicate data interpretation.
Temperature equilibration cannot be overlooked in the preparation protocol. Samples should be allowed to equilibrate at the measurement temperature for at least 15-20 minutes before analysis to ensure thermal stability and eliminate temperature gradients that could induce convection currents within the sample. This equilibration period also allows any air bubbles introduced during sample handling to dissipate.
Cuvette selection and cleaning procedures significantly impact measurement quality. Quartz or optical-grade glass cuvettes are preferred for their superior optical properties. Cuvettes must undergo rigorous cleaning protocols, typically involving sequential washing with detergent solutions, organic solvents (ethanol or acetone), and finally multiple rinses with filtered water. Dust-free drying in controlled environments prevents recontamination.
For particularly challenging protein samples, additives may be incorporated into preparation protocols. Small amounts (0.01-0.05%) of non-ionic surfactants like Tween-20 can reduce non-specific protein adsorption to surfaces. Similarly, glycerol (5-10%) may enhance protein stability without significantly altering scattering properties. However, these additives must be validated for each specific protein system to ensure they don't interfere with the properties being measured.
Data Analysis Algorithms
Data analysis algorithms represent the computational backbone of dynamic light scattering (DLS) for protein analysis. The transformation of raw scattering data into meaningful protein characterization requires sophisticated mathematical processing. Traditional algorithms like cumulants analysis provide basic information about the average size and polydispersity of proteins in solution. This approach fits the autocorrelation function to a single exponential decay, offering simplicity but limited resolution for complex protein mixtures.
Advanced algorithms such as CONTIN and NNLS (Non-Negative Least Squares) have significantly improved the analysis capabilities for heterogeneous protein samples. CONTIN employs regularization techniques to solve the ill-posed inverse Laplace transformation problem, enabling the resolution of multimodal size distributions. NNLS algorithms, meanwhile, constrain solutions to non-negative values, reflecting the physical reality of particle sizes and improving result stability.
Machine learning approaches have recently emerged as powerful tools for DLS data interpretation. Neural networks trained on extensive datasets can identify subtle patterns in scattering data that traditional algorithms might miss. These AI-driven methods show particular promise for complex biological samples where protein aggregates, oligomers, and monomers coexist.
Real-time analysis algorithms have become increasingly important for monitoring protein stability during formulation development. These algorithms process DLS data streams continuously, enabling researchers to observe dynamic changes in protein solutions under varying conditions. Such capabilities are especially valuable for detecting early signs of aggregation or denaturation.
Bayesian statistical methods offer another advancement by incorporating prior knowledge about protein behavior into the analysis framework. These probabilistic approaches provide not just size distributions but also confidence intervals, allowing researchers to make more informed decisions about data reliability.
Cross-correlation algorithms have been developed to address the challenge of multiple scattering effects in concentrated protein solutions. By analyzing scattered light at different angles simultaneously, these algorithms can extract accurate size information even from samples that would be problematic for conventional DLS analysis.
The integration of molecular dynamics simulation data with DLS algorithms represents a frontier in protein analysis. By comparing experimental scattering profiles with theoretical predictions based on protein structures, researchers can gain insights into conformational changes and interaction dynamics that go beyond simple size measurements.
Advanced algorithms such as CONTIN and NNLS (Non-Negative Least Squares) have significantly improved the analysis capabilities for heterogeneous protein samples. CONTIN employs regularization techniques to solve the ill-posed inverse Laplace transformation problem, enabling the resolution of multimodal size distributions. NNLS algorithms, meanwhile, constrain solutions to non-negative values, reflecting the physical reality of particle sizes and improving result stability.
Machine learning approaches have recently emerged as powerful tools for DLS data interpretation. Neural networks trained on extensive datasets can identify subtle patterns in scattering data that traditional algorithms might miss. These AI-driven methods show particular promise for complex biological samples where protein aggregates, oligomers, and monomers coexist.
Real-time analysis algorithms have become increasingly important for monitoring protein stability during formulation development. These algorithms process DLS data streams continuously, enabling researchers to observe dynamic changes in protein solutions under varying conditions. Such capabilities are especially valuable for detecting early signs of aggregation or denaturation.
Bayesian statistical methods offer another advancement by incorporating prior knowledge about protein behavior into the analysis framework. These probabilistic approaches provide not just size distributions but also confidence intervals, allowing researchers to make more informed decisions about data reliability.
Cross-correlation algorithms have been developed to address the challenge of multiple scattering effects in concentrated protein solutions. By analyzing scattered light at different angles simultaneously, these algorithms can extract accurate size information even from samples that would be problematic for conventional DLS analysis.
The integration of molecular dynamics simulation data with DLS algorithms represents a frontier in protein analysis. By comparing experimental scattering profiles with theoretical predictions based on protein structures, researchers can gain insights into conformational changes and interaction dynamics that go beyond simple size measurements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!