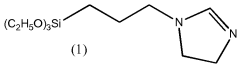
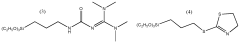
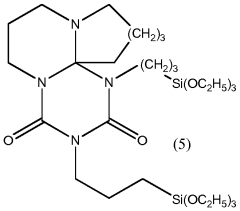
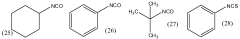
How to Optimize Isocyanate Polymerization Techniques?
JUL 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isocyanate Polymerization Background and Objectives
Isocyanate polymerization has been a cornerstone in polymer chemistry since its discovery in the 1930s. This process, which involves the reaction of isocyanate groups with compounds containing active hydrogen atoms, has revolutionized the production of polyurethanes and other important polymeric materials. The evolution of isocyanate polymerization techniques has been driven by the increasing demand for high-performance materials in various industries, including automotive, construction, and electronics.
The primary objective of optimizing isocyanate polymerization techniques is to enhance the efficiency, control, and versatility of the polymerization process. This optimization aims to address several key challenges that have persisted in the field. These include improving reaction kinetics, reducing side reactions, enhancing product quality, and minimizing environmental impact.
One of the main trends in isocyanate polymerization has been the development of more sophisticated catalysts. These catalysts are designed to provide better control over reaction rates and selectivity, leading to improved product properties and reduced formation of unwanted by-products. Additionally, there has been a growing focus on green chemistry principles, with efforts to develop more environmentally friendly isocyanate polymerization processes.
Another significant trend is the exploration of novel monomers and co-reactants. Researchers are investigating a wide range of isocyanate precursors and polyol structures to create polymers with tailored properties. This includes the development of bio-based isocyanates and polyols, which align with the increasing demand for sustainable materials.
The optimization of isocyanate polymerization also involves advancements in process engineering. This includes the development of more efficient mixing technologies, improved temperature control systems, and innovative reactor designs. These engineering improvements aim to enhance the scalability and reproducibility of isocyanate polymerization processes, making them more suitable for industrial-scale production.
Furthermore, there is a growing interest in combining isocyanate polymerization with other polymerization techniques or processing methods. This includes the exploration of hybrid materials, such as isocyanate-modified epoxies or isocyanate-functionalized nanocomposites. These hybrid approaches offer new possibilities for creating materials with unique property combinations.
As we look to the future, the optimization of isocyanate polymerization techniques is expected to continue evolving. Key areas of focus will likely include the development of smart, stimuli-responsive polyurethanes, the integration of computational modeling for process optimization, and the exploration of novel applications in emerging fields such as 3D printing and biomedical engineering.
The primary objective of optimizing isocyanate polymerization techniques is to enhance the efficiency, control, and versatility of the polymerization process. This optimization aims to address several key challenges that have persisted in the field. These include improving reaction kinetics, reducing side reactions, enhancing product quality, and minimizing environmental impact.
One of the main trends in isocyanate polymerization has been the development of more sophisticated catalysts. These catalysts are designed to provide better control over reaction rates and selectivity, leading to improved product properties and reduced formation of unwanted by-products. Additionally, there has been a growing focus on green chemistry principles, with efforts to develop more environmentally friendly isocyanate polymerization processes.
Another significant trend is the exploration of novel monomers and co-reactants. Researchers are investigating a wide range of isocyanate precursors and polyol structures to create polymers with tailored properties. This includes the development of bio-based isocyanates and polyols, which align with the increasing demand for sustainable materials.
The optimization of isocyanate polymerization also involves advancements in process engineering. This includes the development of more efficient mixing technologies, improved temperature control systems, and innovative reactor designs. These engineering improvements aim to enhance the scalability and reproducibility of isocyanate polymerization processes, making them more suitable for industrial-scale production.
Furthermore, there is a growing interest in combining isocyanate polymerization with other polymerization techniques or processing methods. This includes the exploration of hybrid materials, such as isocyanate-modified epoxies or isocyanate-functionalized nanocomposites. These hybrid approaches offer new possibilities for creating materials with unique property combinations.
As we look to the future, the optimization of isocyanate polymerization techniques is expected to continue evolving. Key areas of focus will likely include the development of smart, stimuli-responsive polyurethanes, the integration of computational modeling for process optimization, and the exploration of novel applications in emerging fields such as 3D printing and biomedical engineering.
Market Analysis for Isocyanate-Based Polymers
The global market for isocyanate-based polymers has experienced significant growth in recent years, driven by increasing demand across various industries. Polyurethanes, formed through the reaction of isocyanates with polyols, represent the largest segment of this market. The versatility of isocyanate-based polymers has led to their widespread adoption in construction, automotive, furniture, and electronics sectors.
In the construction industry, isocyanate-based polymers are extensively used for insulation, sealants, and adhesives. The growing emphasis on energy-efficient buildings has boosted the demand for polyurethane insulation materials. The automotive sector utilizes these polymers for manufacturing seats, dashboards, and other interior components, as well as for lightweight structural parts to improve fuel efficiency.
The furniture industry has embraced isocyanate-based polymers for producing flexible and rigid foams used in mattresses, sofas, and chairs. The electronics sector employs these materials in the production of casings, insulators, and protective coatings for various devices.
Asia-Pacific region leads the market for isocyanate-based polymers, with China being the largest consumer and producer. The rapid industrialization and urbanization in emerging economies have significantly contributed to market growth. North America and Europe follow, with mature markets focusing on innovation and sustainability.
Environmental concerns and regulations have been shaping the market dynamics. There is a growing trend towards bio-based and recyclable isocyanate polymers to address sustainability issues. Manufacturers are investing in research and development to create eco-friendly alternatives and improve production processes to reduce environmental impact.
The market is characterized by intense competition among key players such as BASF, Covestro, Huntsman Corporation, and Dow Chemical Company. These companies are focusing on product innovation, strategic partnerships, and geographical expansion to maintain their market positions.
Despite the positive growth trajectory, the market faces challenges such as volatile raw material prices and health concerns associated with isocyanate exposure. Manufacturers are working on developing safer handling procedures and less hazardous alternatives to mitigate these issues.
Looking ahead, the market for isocyanate-based polymers is expected to continue its growth, driven by technological advancements, increasing applications in emerging industries, and the development of sustainable products. The optimization of isocyanate polymerization techniques will play a crucial role in addressing market demands for improved performance, cost-effectiveness, and environmental sustainability.
In the construction industry, isocyanate-based polymers are extensively used for insulation, sealants, and adhesives. The growing emphasis on energy-efficient buildings has boosted the demand for polyurethane insulation materials. The automotive sector utilizes these polymers for manufacturing seats, dashboards, and other interior components, as well as for lightweight structural parts to improve fuel efficiency.
The furniture industry has embraced isocyanate-based polymers for producing flexible and rigid foams used in mattresses, sofas, and chairs. The electronics sector employs these materials in the production of casings, insulators, and protective coatings for various devices.
Asia-Pacific region leads the market for isocyanate-based polymers, with China being the largest consumer and producer. The rapid industrialization and urbanization in emerging economies have significantly contributed to market growth. North America and Europe follow, with mature markets focusing on innovation and sustainability.
Environmental concerns and regulations have been shaping the market dynamics. There is a growing trend towards bio-based and recyclable isocyanate polymers to address sustainability issues. Manufacturers are investing in research and development to create eco-friendly alternatives and improve production processes to reduce environmental impact.
The market is characterized by intense competition among key players such as BASF, Covestro, Huntsman Corporation, and Dow Chemical Company. These companies are focusing on product innovation, strategic partnerships, and geographical expansion to maintain their market positions.
Despite the positive growth trajectory, the market faces challenges such as volatile raw material prices and health concerns associated with isocyanate exposure. Manufacturers are working on developing safer handling procedures and less hazardous alternatives to mitigate these issues.
Looking ahead, the market for isocyanate-based polymers is expected to continue its growth, driven by technological advancements, increasing applications in emerging industries, and the development of sustainable products. The optimization of isocyanate polymerization techniques will play a crucial role in addressing market demands for improved performance, cost-effectiveness, and environmental sustainability.
Current Challenges in Isocyanate Polymerization
Isocyanate polymerization, a critical process in the production of polyurethanes, faces several significant challenges that hinder its optimization. One of the primary issues is the control of reaction kinetics. The highly exothermic nature of isocyanate reactions makes it difficult to maintain uniform temperature distribution throughout the reaction mixture, leading to potential hot spots and uneven polymerization.
Another challenge lies in the sensitivity of isocyanates to moisture. Even trace amounts of water can lead to side reactions, resulting in the formation of urea linkages and carbon dioxide gas. This not only affects the final polymer properties but also creates processing difficulties due to foam formation. Ensuring a completely anhydrous environment throughout the polymerization process remains a significant hurdle.
The reactivity of isocyanates with various functional groups poses another challenge. While this versatility is advantageous for creating diverse polymer structures, it also complicates the control of reaction selectivity. Achieving the desired balance between different reactions, such as urethane formation, allophanate crosslinking, and biuret formation, requires precise control over reaction conditions and catalyst systems.
Furthermore, the toxicity of isocyanates presents significant safety and environmental concerns. Exposure to isocyanates can cause severe respiratory issues and skin irritation. Implementing robust safety measures and developing less hazardous alternatives without compromising performance remains an ongoing challenge in the industry.
The viscosity increase during polymerization also presents processing difficulties. As the reaction progresses, the increasing molecular weight of the polymer leads to a rapid rise in viscosity. This can result in poor mixing, heat transfer issues, and difficulties in handling the reaction mixture, particularly in large-scale production.
Achieving consistent product quality is another significant challenge. Variations in raw material purity, reaction conditions, and processing parameters can lead to inconsistencies in the final polymer properties. Developing robust quality control measures and predictive models for polymer properties based on reaction conditions is crucial for optimizing isocyanate polymerization techniques.
Lastly, the development of more sustainable and environmentally friendly isocyanate polymerization processes remains a challenge. This includes finding bio-based alternatives to petroleum-derived isocyanates, reducing the use of volatile organic compounds (VOCs) in formulations, and improving the recyclability and end-of-life management of polyurethane products.
Another challenge lies in the sensitivity of isocyanates to moisture. Even trace amounts of water can lead to side reactions, resulting in the formation of urea linkages and carbon dioxide gas. This not only affects the final polymer properties but also creates processing difficulties due to foam formation. Ensuring a completely anhydrous environment throughout the polymerization process remains a significant hurdle.
The reactivity of isocyanates with various functional groups poses another challenge. While this versatility is advantageous for creating diverse polymer structures, it also complicates the control of reaction selectivity. Achieving the desired balance between different reactions, such as urethane formation, allophanate crosslinking, and biuret formation, requires precise control over reaction conditions and catalyst systems.
Furthermore, the toxicity of isocyanates presents significant safety and environmental concerns. Exposure to isocyanates can cause severe respiratory issues and skin irritation. Implementing robust safety measures and developing less hazardous alternatives without compromising performance remains an ongoing challenge in the industry.
The viscosity increase during polymerization also presents processing difficulties. As the reaction progresses, the increasing molecular weight of the polymer leads to a rapid rise in viscosity. This can result in poor mixing, heat transfer issues, and difficulties in handling the reaction mixture, particularly in large-scale production.
Achieving consistent product quality is another significant challenge. Variations in raw material purity, reaction conditions, and processing parameters can lead to inconsistencies in the final polymer properties. Developing robust quality control measures and predictive models for polymer properties based on reaction conditions is crucial for optimizing isocyanate polymerization techniques.
Lastly, the development of more sustainable and environmentally friendly isocyanate polymerization processes remains a challenge. This includes finding bio-based alternatives to petroleum-derived isocyanates, reducing the use of volatile organic compounds (VOCs) in formulations, and improving the recyclability and end-of-life management of polyurethane products.
Existing Optimization Methods for Isocyanate Polymerization
01 Catalyst optimization for isocyanate polymerization
Various catalysts can be used to optimize isocyanate polymerization. These may include metal-based catalysts, organometallic compounds, or organic catalysts. The choice and concentration of catalyst can significantly affect reaction rates, selectivity, and product properties. Optimization involves finding the right balance between reactivity and control over the polymerization process.- Catalyst optimization for isocyanate polymerization: Various catalysts can be used to optimize isocyanate polymerization. These may include metal-based catalysts, organometallic compounds, or organic catalysts. The choice and concentration of catalyst can significantly affect reaction rates, selectivity, and product properties. Optimization involves finding the right balance between reactivity and control over the polymerization process.
- Temperature and pressure control in isocyanate polymerization: Careful control of reaction conditions, particularly temperature and pressure, is crucial for optimizing isocyanate polymerization. Higher temperatures can increase reaction rates but may lead to side reactions or degradation. Pressure control can affect the behavior of reactants and products, especially in the case of foams. Finding the optimal temperature and pressure range is essential for achieving desired product properties.
- Reactant ratio and feed rate optimization: The ratio of isocyanate to other reactants (e.g., polyols) and their feed rates into the reaction system can significantly impact polymerization outcomes. Optimizing these parameters can lead to improved product properties, reduced side reactions, and better control over molecular weight distribution. Careful adjustment of stoichiometry and feed strategies is key to achieving desired polymer characteristics.
- Use of additives and modifiers in isocyanate polymerization: Various additives and modifiers can be incorporated to enhance isocyanate polymerization. These may include chain extenders, crosslinking agents, surfactants, or stabilizers. Such additives can improve reaction control, modify polymer properties, or enhance processing characteristics. Optimizing the type and amount of additives is crucial for tailoring the final product to specific applications.
- Reactor design and mixing optimization: The design of the polymerization reactor and the efficiency of mixing can greatly influence isocyanate polymerization. Factors such as reactor geometry, mixing element design, and flow patterns affect heat and mass transfer, which in turn impact reaction kinetics and product uniformity. Optimizing these aspects can lead to improved process control, product consistency, and overall efficiency of the polymerization process.
02 Temperature and pressure control in isocyanate polymerization
Careful control of reaction temperature and pressure is crucial for optimizing isocyanate polymerization. Higher temperatures generally increase reaction rates but may lead to unwanted side reactions. Pressure control can affect the behavior of reactants, especially for systems involving volatile components. Optimizing these parameters can improve yield, reduce cycle times, and enhance product quality.Expand Specific Solutions03 Reactant ratio and feed rate optimization
The ratio of isocyanate to other reactants (e.g., polyols) and their feed rates into the reaction system play a crucial role in polymerization optimization. Proper balancing of these factors can lead to improved molecular weight distribution, reduced side reactions, and better control over the final polymer properties. Continuous feed systems may offer advantages in maintaining optimal ratios throughout the reaction.Expand Specific Solutions04 Use of additives and modifiers in isocyanate polymerization
Various additives and modifiers can be incorporated to optimize isocyanate polymerization. These may include chain extenders, crosslinking agents, surfactants, or stabilizers. Such additives can help control reaction kinetics, improve polymer morphology, enhance physical properties, or increase the stability of the final product. Careful selection and dosing of additives is key to achieving desired outcomes.Expand Specific Solutions05 Reactor design and mixing optimization
The design of the polymerization reactor and the efficiency of mixing can significantly impact isocyanate polymerization. Factors such as reactor geometry, agitation method, and heat transfer characteristics affect reaction uniformity and product consistency. Advanced reactor designs may incorporate features like static mixers, multi-zone temperature control, or specialized geometries to optimize the polymerization process and product quality.Expand Specific Solutions
Key Players in Isocyanate Polymer Industry
The optimization of isocyanate polymerization techniques is currently in a mature stage of development, with a significant global market size driven by the widespread use of polyurethanes in various industries. The technology's maturity is evident from the involvement of major chemical companies like Covestro, Wanhua Chemical, and BASF, who have established strong positions in this field. These industry leaders, along with other key players such as Mitsui Chemicals and Asahi Kasei, continue to invest in research and development to further enhance efficiency, sustainability, and product performance. The competitive landscape is characterized by ongoing innovation in catalyst systems, process control, and the development of eco-friendly alternatives, reflecting the industry's focus on meeting evolving market demands and environmental regulations.
Covestro Deutschland AG
Technical Solution: Covestro has developed advanced isocyanate polymerization techniques focusing on sustainability and efficiency. Their approach includes the use of catalysts to control reaction rates and improve selectivity[1]. They have implemented continuous flow reactors for better heat management and reaction control[2]. Covestro also utilizes innovative mixing technologies to ensure uniform distribution of reactants, resulting in improved product quality[3]. Additionally, they have developed methods for recycling and reusing unreacted isocyanates, reducing waste and improving overall process efficiency[4].
Strengths: Sustainable processes, efficient catalysis, and advanced reactor designs. Weaknesses: Potentially higher initial investment costs for implementing new technologies.
Wanhua Chemical Group Co., Ltd.
Technical Solution: Wanhua Chemical has pioneered innovative isocyanate polymerization techniques, focusing on process intensification and product customization. They have developed a proprietary micro-reactor technology that allows for precise control of reaction conditions, resulting in higher yields and improved product consistency[1]. Wanhua's approach also includes the use of advanced catalysts that enable lower reaction temperatures, reducing energy consumption[2]. They have implemented in-line monitoring systems for real-time quality control, ensuring consistent product properties[3]. Furthermore, Wanhua has developed a modular production system that allows for quick adaptation to different product specifications, enhancing flexibility in manufacturing[4].
Strengths: High precision control, energy efficiency, and production flexibility. Weaknesses: Potential complexity in scaling up micro-reactor technology for large-scale production.
Innovative Catalysts and Reaction Mechanisms
Pure isocyanuric polyisocyanates and method for their production
PatentInactiveEP0273836A3
Innovation
- A process utilizing catalytic cyclotrimerization of aliphatic, cycloaliphatic, or arylaliphatic isocyanates, followed by extraction with inert gases like carbon dioxide in liquid or supercritical states to achieve polyisocyanates with less than 0.03% isocyanate monomer and 1.0% dimer content, using a countercurrent extraction method and fractional separation to minimize residual impurities.
Polymerisation initiated by bases/isocyanates on oxide surfaces
PatentWO2013117379A1
Innovation
- A novel polymerization method initiated by isocyanates and organic bases with an imine structure, where the isocyanate or carbodiimide is bound to an oxidic surface, allowing for the polymerization of vinylic monomers, resulting in high-purity polymers with controlled molecular weights and improved dispersibility, and enabling easy separation and purification of the polymer from the surface.
Environmental Impact of Isocyanate Production
The environmental impact of isocyanate production is a critical concern in the optimization of isocyanate polymerization techniques. The manufacturing process of isocyanates, particularly toluene diisocyanate (TDI) and methylene diphenyl diisocyanate (MDI), involves several stages that can have significant environmental implications.
One of the primary environmental concerns is the emission of volatile organic compounds (VOCs) during production. These emissions can contribute to air pollution and the formation of ground-level ozone, which has adverse effects on human health and ecosystems. To mitigate this issue, many isocyanate production facilities have implemented advanced air pollution control technologies, such as thermal oxidizers and scrubbers, to reduce VOC emissions.
Water pollution is another significant environmental challenge associated with isocyanate production. The process generates wastewater containing various organic compounds and potentially toxic substances. Proper treatment of this wastewater is essential to prevent contamination of local water bodies and groundwater resources. Advanced wastewater treatment technologies, including biological treatment systems and membrane filtration, are increasingly being employed to address this concern.
Energy consumption is a major factor contributing to the environmental footprint of isocyanate production. The process requires substantial amounts of energy for heating, cooling, and separation operations. Efforts to optimize energy efficiency in isocyanate production have focused on heat integration, improved process control, and the use of more efficient equipment. Some facilities have also explored the use of renewable energy sources to reduce their carbon footprint.
The production of isocyanates also generates solid waste, including spent catalysts and by-products. Proper disposal or recycling of these materials is crucial to minimize environmental impact. Many manufacturers are now implementing waste reduction strategies and exploring opportunities for recycling and reuse of materials within the production process.
Raw material sourcing is another area of environmental concern. The production of isocyanates typically relies on petrochemical feedstocks, which have their own environmental implications related to extraction and processing. Some research efforts are focused on developing bio-based alternatives to traditional petrochemical feedstocks, which could potentially reduce the overall environmental impact of isocyanate production.
In response to these environmental challenges, the isocyanate industry has been working towards more sustainable production methods. This includes the development of new catalysts that enable more efficient reactions, reducing energy consumption and waste generation. Additionally, there is ongoing research into alternative production routes that may have lower environmental impacts, such as non-phosgene processes for isocyanate synthesis.
Regulatory pressures and increasing environmental awareness have driven many isocyanate producers to adopt more stringent environmental management systems and to pursue certifications such as ISO 14001. These efforts aim to continuously improve environmental performance and ensure compliance with evolving environmental regulations.
One of the primary environmental concerns is the emission of volatile organic compounds (VOCs) during production. These emissions can contribute to air pollution and the formation of ground-level ozone, which has adverse effects on human health and ecosystems. To mitigate this issue, many isocyanate production facilities have implemented advanced air pollution control technologies, such as thermal oxidizers and scrubbers, to reduce VOC emissions.
Water pollution is another significant environmental challenge associated with isocyanate production. The process generates wastewater containing various organic compounds and potentially toxic substances. Proper treatment of this wastewater is essential to prevent contamination of local water bodies and groundwater resources. Advanced wastewater treatment technologies, including biological treatment systems and membrane filtration, are increasingly being employed to address this concern.
Energy consumption is a major factor contributing to the environmental footprint of isocyanate production. The process requires substantial amounts of energy for heating, cooling, and separation operations. Efforts to optimize energy efficiency in isocyanate production have focused on heat integration, improved process control, and the use of more efficient equipment. Some facilities have also explored the use of renewable energy sources to reduce their carbon footprint.
The production of isocyanates also generates solid waste, including spent catalysts and by-products. Proper disposal or recycling of these materials is crucial to minimize environmental impact. Many manufacturers are now implementing waste reduction strategies and exploring opportunities for recycling and reuse of materials within the production process.
Raw material sourcing is another area of environmental concern. The production of isocyanates typically relies on petrochemical feedstocks, which have their own environmental implications related to extraction and processing. Some research efforts are focused on developing bio-based alternatives to traditional petrochemical feedstocks, which could potentially reduce the overall environmental impact of isocyanate production.
In response to these environmental challenges, the isocyanate industry has been working towards more sustainable production methods. This includes the development of new catalysts that enable more efficient reactions, reducing energy consumption and waste generation. Additionally, there is ongoing research into alternative production routes that may have lower environmental impacts, such as non-phosgene processes for isocyanate synthesis.
Regulatory pressures and increasing environmental awareness have driven many isocyanate producers to adopt more stringent environmental management systems and to pursue certifications such as ISO 14001. These efforts aim to continuously improve environmental performance and ensure compliance with evolving environmental regulations.
Safety Regulations in Isocyanate Handling
Safety regulations in isocyanate handling are paramount in optimizing isocyanate polymerization techniques. The highly reactive nature of isocyanates necessitates stringent safety measures to protect workers and the environment. Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) in the United States and the European Chemicals Agency (ECHA) have established comprehensive guidelines for the safe handling, storage, and use of isocyanates.
Personal protective equipment (PPE) is a critical component of isocyanate safety protocols. Workers must wear appropriate respiratory protection, such as supplied-air respirators or self-contained breathing apparatus, depending on the concentration levels and exposure duration. Impervious gloves, protective clothing, and eye protection are also mandatory to prevent skin and eye contact with isocyanates.
Proper ventilation systems are essential in facilities handling isocyanates. Local exhaust ventilation should be installed at all points where isocyanates are used or stored to minimize airborne concentrations. Regular air monitoring and exposure assessments are required to ensure that exposure limits are not exceeded and to evaluate the effectiveness of control measures.
Storage and handling procedures for isocyanates must adhere to strict guidelines. Isocyanates should be stored in tightly sealed containers in cool, dry, well-ventilated areas away from direct sunlight and sources of heat or ignition. Segregation from incompatible materials, such as water, acids, and bases, is crucial to prevent hazardous reactions.
Emergency response planning is a vital aspect of isocyanate safety regulations. Facilities must have clearly defined procedures for spill containment, decontamination, and evacuation. Eyewash stations and safety showers should be readily accessible in areas where isocyanates are handled.
Training programs for employees working with isocyanates are mandatory and should cover hazard communication, proper handling techniques, emergency procedures, and the use of PPE. Regular refresher courses and competency assessments ensure that workers maintain up-to-date knowledge of safety protocols.
Waste management and disposal of isocyanate-containing materials must comply with environmental regulations. Proper neutralization and disposal methods should be employed to prevent environmental contamination and ensure worker safety during the disposal process.
Continuous improvement of safety measures is essential in isocyanate handling. Regular safety audits, incident investigations, and implementation of lessons learned contribute to enhancing overall safety performance. Staying informed about evolving regulations and industry best practices is crucial for maintaining compliance and optimizing safety protocols in isocyanate polymerization processes.
Personal protective equipment (PPE) is a critical component of isocyanate safety protocols. Workers must wear appropriate respiratory protection, such as supplied-air respirators or self-contained breathing apparatus, depending on the concentration levels and exposure duration. Impervious gloves, protective clothing, and eye protection are also mandatory to prevent skin and eye contact with isocyanates.
Proper ventilation systems are essential in facilities handling isocyanates. Local exhaust ventilation should be installed at all points where isocyanates are used or stored to minimize airborne concentrations. Regular air monitoring and exposure assessments are required to ensure that exposure limits are not exceeded and to evaluate the effectiveness of control measures.
Storage and handling procedures for isocyanates must adhere to strict guidelines. Isocyanates should be stored in tightly sealed containers in cool, dry, well-ventilated areas away from direct sunlight and sources of heat or ignition. Segregation from incompatible materials, such as water, acids, and bases, is crucial to prevent hazardous reactions.
Emergency response planning is a vital aspect of isocyanate safety regulations. Facilities must have clearly defined procedures for spill containment, decontamination, and evacuation. Eyewash stations and safety showers should be readily accessible in areas where isocyanates are handled.
Training programs for employees working with isocyanates are mandatory and should cover hazard communication, proper handling techniques, emergency procedures, and the use of PPE. Regular refresher courses and competency assessments ensure that workers maintain up-to-date knowledge of safety protocols.
Waste management and disposal of isocyanate-containing materials must comply with environmental regulations. Proper neutralization and disposal methods should be employed to prevent environmental contamination and ensure worker safety during the disposal process.
Continuous improvement of safety measures is essential in isocyanate handling. Regular safety audits, incident investigations, and implementation of lessons learned contribute to enhancing overall safety performance. Staying informed about evolving regulations and industry best practices is crucial for maintaining compliance and optimizing safety protocols in isocyanate polymerization processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!