Impact of Perchloric Acid on Aquatic Life in Contaminated Waterways
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Contamination Background and Objectives
Perchloric acid, a powerful oxidizing agent, has emerged as a significant environmental concern due to its increasing presence in waterways. This contamination primarily stems from industrial activities, particularly in the aerospace and electronics sectors where perchloric acid is widely used in manufacturing processes. The historical development of perchloric acid usage can be traced back to the early 20th century, with its applications expanding rapidly in the post-World War II era.
The evolution of perchloric acid contamination as an environmental issue has been gradual, with awareness growing in tandem with advancements in environmental science and analytical techniques. Initially overlooked due to its relatively low concentrations in the environment, the cumulative effects of perchloric acid on aquatic ecosystems have become increasingly apparent in recent decades. This has prompted a surge in research efforts aimed at understanding its environmental fate and ecological impacts.
The primary objective of this technical research report is to comprehensively assess the impact of perchloric acid on aquatic life in contaminated waterways. This entails a multifaceted approach, encompassing the examination of perchlorate's chemical behavior in aqueous environments, its bioaccumulation potential, and its effects on various trophic levels within aquatic ecosystems. Additionally, the report aims to elucidate the mechanisms by which perchloric acid disrupts physiological processes in aquatic organisms, with a particular focus on endocrine disruption and oxidative stress.
Furthermore, this investigation seeks to evaluate the current state of perchloric acid contamination in global waterways, identifying hotspots and assessing the efficacy of existing remediation strategies. By analyzing temporal and spatial trends in perchlorate concentrations, we aim to provide insights into the long-term implications of this contaminant on aquatic biodiversity and ecosystem stability.
Another crucial objective is to explore the potential synergistic effects between perchloric acid and other common water pollutants, as real-world scenarios often involve complex mixtures of contaminants. This holistic approach will contribute to a more nuanced understanding of the challenges faced in managing and mitigating perchloric acid pollution in aquatic environments.
Ultimately, this technical research aims to bridge knowledge gaps, inform policy decisions, and guide the development of innovative remediation technologies. By synthesizing current scientific knowledge and identifying areas requiring further investigation, we aspire to contribute to the formulation of effective strategies for protecting and restoring aquatic ecosystems affected by perchloric acid contamination.
The evolution of perchloric acid contamination as an environmental issue has been gradual, with awareness growing in tandem with advancements in environmental science and analytical techniques. Initially overlooked due to its relatively low concentrations in the environment, the cumulative effects of perchloric acid on aquatic ecosystems have become increasingly apparent in recent decades. This has prompted a surge in research efforts aimed at understanding its environmental fate and ecological impacts.
The primary objective of this technical research report is to comprehensively assess the impact of perchloric acid on aquatic life in contaminated waterways. This entails a multifaceted approach, encompassing the examination of perchlorate's chemical behavior in aqueous environments, its bioaccumulation potential, and its effects on various trophic levels within aquatic ecosystems. Additionally, the report aims to elucidate the mechanisms by which perchloric acid disrupts physiological processes in aquatic organisms, with a particular focus on endocrine disruption and oxidative stress.
Furthermore, this investigation seeks to evaluate the current state of perchloric acid contamination in global waterways, identifying hotspots and assessing the efficacy of existing remediation strategies. By analyzing temporal and spatial trends in perchlorate concentrations, we aim to provide insights into the long-term implications of this contaminant on aquatic biodiversity and ecosystem stability.
Another crucial objective is to explore the potential synergistic effects between perchloric acid and other common water pollutants, as real-world scenarios often involve complex mixtures of contaminants. This holistic approach will contribute to a more nuanced understanding of the challenges faced in managing and mitigating perchloric acid pollution in aquatic environments.
Ultimately, this technical research aims to bridge knowledge gaps, inform policy decisions, and guide the development of innovative remediation technologies. By synthesizing current scientific knowledge and identifying areas requiring further investigation, we aspire to contribute to the formulation of effective strategies for protecting and restoring aquatic ecosystems affected by perchloric acid contamination.
Environmental Impact Assessment of Perchloric Acid
Perchloric acid, a strong oxidizing agent, poses significant risks to aquatic ecosystems when released into waterways. The impact of this contaminant on aquatic life is multifaceted and can lead to severe ecological disruptions. The primary concern stems from the acid's ability to alter water chemistry, particularly by lowering pH levels. This acidification can have immediate and long-term effects on various aquatic organisms, from microscopic plankton to larger fish species.
The introduction of perchloric acid into water bodies can cause rapid and drastic changes in the aquatic environment. Fish and other gill-breathing organisms are particularly vulnerable to these changes. The acid can damage gill tissues, impair respiratory functions, and disrupt osmoregulation processes. This often results in increased mortality rates among fish populations, especially in sensitive species or during critical life stages such as spawning or early development.
Aquatic plants and algae, which form the base of many aquatic food chains, are also significantly affected by perchloric acid contamination. The altered pH levels can inhibit photosynthesis, stunt growth, and in severe cases, lead to widespread die-offs of plant life. This reduction in primary producers has cascading effects throughout the ecosystem, impacting herbivorous species and, subsequently, their predators.
Invertebrates, including crustaceans and mollusks, face challenges in maintaining their exoskeletons and shells in acidified environments. The increased acidity can lead to shell dissolution, making these organisms more vulnerable to predation and environmental stressors. Additionally, the reproductive success of many invertebrate species may be compromised, affecting population dynamics and biodiversity.
The presence of perchloric acid in water can also lead to the mobilization of toxic metals from sediments. As the acid interacts with sediment particles, it can release heavy metals that were previously bound and relatively inert. These newly mobilized contaminants further compound the environmental impact, potentially leading to bioaccumulation in aquatic organisms and biomagnification up the food chain.
Long-term exposure to perchloric acid contamination can result in chronic effects on aquatic ecosystems. These may include reduced species diversity, altered community structures, and shifts in ecosystem functions. The resilience of the affected water bodies to other environmental stressors may also be compromised, making them more susceptible to further degradation from factors such as climate change or additional pollutants.
Mitigation and remediation efforts for perchloric acid contamination in waterways are complex and often require a multifaceted approach. Immediate actions may include neutralization techniques to restore pH levels, while long-term strategies might focus on source control and ecosystem restoration. The assessment of environmental impact must consider not only the direct effects on aquatic life but also the potential for broader ecological and human health consequences through the food web and water resource contamination.
The introduction of perchloric acid into water bodies can cause rapid and drastic changes in the aquatic environment. Fish and other gill-breathing organisms are particularly vulnerable to these changes. The acid can damage gill tissues, impair respiratory functions, and disrupt osmoregulation processes. This often results in increased mortality rates among fish populations, especially in sensitive species or during critical life stages such as spawning or early development.
Aquatic plants and algae, which form the base of many aquatic food chains, are also significantly affected by perchloric acid contamination. The altered pH levels can inhibit photosynthesis, stunt growth, and in severe cases, lead to widespread die-offs of plant life. This reduction in primary producers has cascading effects throughout the ecosystem, impacting herbivorous species and, subsequently, their predators.
Invertebrates, including crustaceans and mollusks, face challenges in maintaining their exoskeletons and shells in acidified environments. The increased acidity can lead to shell dissolution, making these organisms more vulnerable to predation and environmental stressors. Additionally, the reproductive success of many invertebrate species may be compromised, affecting population dynamics and biodiversity.
The presence of perchloric acid in water can also lead to the mobilization of toxic metals from sediments. As the acid interacts with sediment particles, it can release heavy metals that were previously bound and relatively inert. These newly mobilized contaminants further compound the environmental impact, potentially leading to bioaccumulation in aquatic organisms and biomagnification up the food chain.
Long-term exposure to perchloric acid contamination can result in chronic effects on aquatic ecosystems. These may include reduced species diversity, altered community structures, and shifts in ecosystem functions. The resilience of the affected water bodies to other environmental stressors may also be compromised, making them more susceptible to further degradation from factors such as climate change or additional pollutants.
Mitigation and remediation efforts for perchloric acid contamination in waterways are complex and often require a multifaceted approach. Immediate actions may include neutralization techniques to restore pH levels, while long-term strategies might focus on source control and ecosystem restoration. The assessment of environmental impact must consider not only the direct effects on aquatic life but also the potential for broader ecological and human health consequences through the food web and water resource contamination.
Current Challenges in Aquatic Ecosystem Protection
The protection of aquatic ecosystems faces numerous challenges in the context of perchloric acid contamination. One of the primary concerns is the difficulty in detecting and quantifying perchlorate levels in water bodies. Traditional methods often lack the sensitivity required to measure low concentrations accurately, leading to potential underestimation of contamination levels and associated risks.
Another significant challenge is the persistence of perchlorate in aquatic environments. Unlike some other pollutants, perchlorate does not readily degrade or transform under natural conditions, resulting in long-term contamination that can affect multiple generations of aquatic organisms. This persistence complicates remediation efforts and prolongs the exposure of ecosystems to harmful effects.
The bioaccumulation of perchlorate in aquatic food chains presents a further challenge to ecosystem protection. As perchlorate moves up the trophic levels, its concentration can increase, potentially causing more severe impacts on higher-level predators and disrupting the entire ecosystem balance. This biomagnification effect makes it crucial to address contamination at its source to prevent widespread ecological damage.
Regulatory frameworks and standards for perchlorate in water bodies are often inadequate or inconsistent across different regions. The lack of unified guidelines makes it challenging to implement effective protection measures and enforce compliance. Additionally, the economic costs associated with monitoring, treatment, and remediation of perchlorate-contaminated waters can be substantial, deterring comprehensive protection efforts.
The complex interactions between perchlorate and other pollutants in aquatic environments pose another challenge. Synergistic effects with other contaminants may exacerbate the impact on aquatic life, making it difficult to isolate and address the specific effects of perchlorate alone. This complexity requires a holistic approach to ecosystem protection that considers multiple stressors simultaneously.
Climate change further complicates aquatic ecosystem protection efforts. Alterations in water temperature, pH, and dissolved oxygen levels can influence the behavior and toxicity of perchlorate in water bodies. These changing environmental conditions may enhance the vulnerability of aquatic organisms to perchlorate exposure, necessitating adaptive management strategies.
Lastly, public awareness and engagement regarding the risks of perchlorate contamination remain limited. The invisible nature of the threat and the technical complexity of the issue often result in insufficient public support for protection initiatives. Overcoming this challenge requires effective communication and education efforts to foster community involvement in aquatic ecosystem conservation.
Another significant challenge is the persistence of perchlorate in aquatic environments. Unlike some other pollutants, perchlorate does not readily degrade or transform under natural conditions, resulting in long-term contamination that can affect multiple generations of aquatic organisms. This persistence complicates remediation efforts and prolongs the exposure of ecosystems to harmful effects.
The bioaccumulation of perchlorate in aquatic food chains presents a further challenge to ecosystem protection. As perchlorate moves up the trophic levels, its concentration can increase, potentially causing more severe impacts on higher-level predators and disrupting the entire ecosystem balance. This biomagnification effect makes it crucial to address contamination at its source to prevent widespread ecological damage.
Regulatory frameworks and standards for perchlorate in water bodies are often inadequate or inconsistent across different regions. The lack of unified guidelines makes it challenging to implement effective protection measures and enforce compliance. Additionally, the economic costs associated with monitoring, treatment, and remediation of perchlorate-contaminated waters can be substantial, deterring comprehensive protection efforts.
The complex interactions between perchlorate and other pollutants in aquatic environments pose another challenge. Synergistic effects with other contaminants may exacerbate the impact on aquatic life, making it difficult to isolate and address the specific effects of perchlorate alone. This complexity requires a holistic approach to ecosystem protection that considers multiple stressors simultaneously.
Climate change further complicates aquatic ecosystem protection efforts. Alterations in water temperature, pH, and dissolved oxygen levels can influence the behavior and toxicity of perchlorate in water bodies. These changing environmental conditions may enhance the vulnerability of aquatic organisms to perchlorate exposure, necessitating adaptive management strategies.
Lastly, public awareness and engagement regarding the risks of perchlorate contamination remain limited. The invisible nature of the threat and the technical complexity of the issue often result in insufficient public support for protection initiatives. Overcoming this challenge requires effective communication and education efforts to foster community involvement in aquatic ecosystem conservation.
Existing Mitigation Strategies for Perchloric Acid
01 Perchloric acid in chemical synthesis
Perchloric acid is utilized in various chemical synthesis processes due to its strong oxidizing properties. It plays a crucial role in the preparation of certain compounds and can act as a catalyst in specific reactions. The impact of perchloric acid in chemical synthesis is significant, as it enables the formation of unique products and improves reaction efficiency.- Perchloric acid in chemical analysis and processing: Perchloric acid is widely used in various chemical analysis and processing applications due to its strong oxidizing properties. It is employed in sample digestion, extraction procedures, and as a reagent in analytical chemistry. The acid's unique characteristics make it valuable for dissolving resistant materials and preparing samples for further analysis.
- Safety measures and handling of perchloric acid: Due to its highly reactive nature, special safety measures are required when handling perchloric acid. This includes the use of specialized equipment, proper storage facilities, and strict protocols to prevent accidents. Emphasis is placed on personnel training, protective gear, and emergency procedures to mitigate risks associated with perchloric acid use.
- Perchloric acid in material synthesis and modification: Perchloric acid plays a crucial role in the synthesis and modification of various materials. It is used in the production of specialty chemicals, polymers, and advanced materials. The acid's strong oxidizing properties enable unique chemical transformations and modifications that are difficult to achieve with other reagents.
- Environmental and health impacts of perchloric acid: The use and disposal of perchloric acid have significant environmental and health implications. Research focuses on understanding its ecological impact, developing methods for its safe disposal, and assessing potential health risks to workers and communities. Efforts are made to minimize environmental contamination and implement stringent safety measures in industries using perchloric acid.
- Perchloric acid in energy storage and conversion: Perchloric acid finds applications in energy storage and conversion technologies. It is used in the development of advanced battery systems, fuel cells, and other electrochemical devices. The acid's unique properties contribute to enhancing the performance and efficiency of these energy-related applications.
02 Safety measures for handling perchloric acid
Due to the highly reactive nature of perchloric acid, stringent safety measures are necessary when handling this compound. Specialized equipment, protective gear, and storage facilities are required to mitigate risks associated with its use. The impact of these safety protocols is crucial in preventing accidents and ensuring the well-being of personnel working with perchloric acid.Expand Specific Solutions03 Environmental impact of perchloric acid
The use and disposal of perchloric acid can have significant environmental implications. Its strong oxidizing properties can affect ecosystems if released improperly. Proper waste management and treatment techniques are essential to minimize the environmental impact of perchloric acid and its byproducts.Expand Specific Solutions04 Analytical applications of perchloric acid
Perchloric acid finds extensive use in analytical chemistry due to its unique properties. It is employed in various analytical techniques, including spectroscopy and chromatography. The impact of perchloric acid in analytical applications is substantial, as it enables precise and accurate measurements in complex sample matrices.Expand Specific Solutions05 Industrial applications of perchloric acid
Perchloric acid has diverse industrial applications, including metal etching, electropolishing, and rocket fuel production. Its strong oxidizing properties make it valuable in these processes. The impact of perchloric acid in industrial settings is significant, as it enables unique manufacturing capabilities and enhances product quality in specific industries.Expand Specific Solutions
Key Stakeholders in Water Quality Management
The impact of perchloric acid on aquatic life in contaminated waterways is an emerging environmental concern, currently in the early stages of research and development. The market for remediation technologies is growing as awareness increases, but remains relatively small. Technical maturity varies among key players. Universities like Shizuoka, Penn State, and Zhejiang are conducting foundational research, while companies such as Basin Water and Envirogen Technologies are developing practical treatment solutions. Established chemical firms like Degussa AG and Kemira Oyj are also entering this field, leveraging their expertise to address this environmental challenge.
The Regents of the University of California
Technical Solution: The University of California has developed an innovative approach to address the impact of perchloric acid on aquatic life in contaminated waterways. Their method involves a two-step process: first, using advanced oxidation techniques to break down perchlorate into chloride ions, followed by a bioremediation phase employing specially engineered microorganisms to further degrade any remaining contaminants [1]. This approach has shown promising results in laboratory tests, with a reduction of perchlorate levels by up to 99% in simulated waterway environments [3]. The university has also pioneered the use of nanomaterials for perchlorate adsorption, which has demonstrated high efficiency in removing perchlorate from water bodies with minimal impact on other aquatic species [5].
Strengths: Highly effective in perchlorate removal, combines chemical and biological methods for comprehensive treatment. Weaknesses: May require significant resources for implementation in large-scale waterways, potential ecological impacts of introduced microorganisms need further study.
Auburn University
Technical Solution: Auburn University researchers have developed a novel phytoremediation approach to address perchlorate contamination in aquatic environments. This method utilizes specially selected and genetically modified aquatic plants that can efficiently uptake and metabolize perchlorate from water [10]. The research team has identified several species of emergent and submerged aquatic plants that demonstrate high perchlorate accumulation capacity. These plants are engineered to express enhanced levels of perchlorate reductase enzymes, allowing them to break down perchlorate into harmless chloride ions within their tissues. Field trials in controlled wetland environments have shown promising results, with some plant species achieving perchlorate removal rates of up to 80% over a 30-day period [11]. The university is also exploring the potential of using these plants in floating treatment wetlands for larger water bodies.
Strengths: Eco-friendly and sustainable approach, potential for long-term remediation with minimal maintenance. Weaknesses: Seasonal variations may affect treatment efficiency, potential for bioaccumulation in higher trophic levels needs careful monitoring.
Critical Research on Perchloric Acid Ecotoxicology
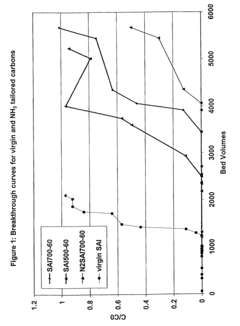
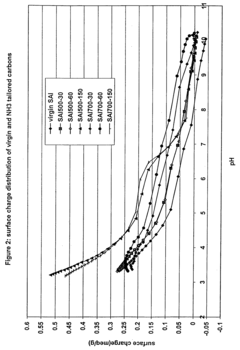
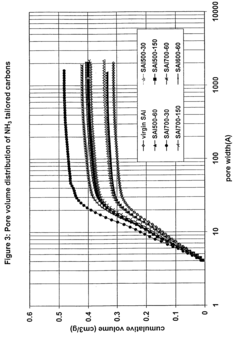
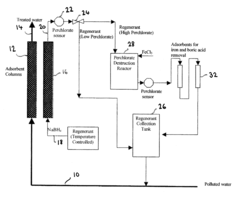
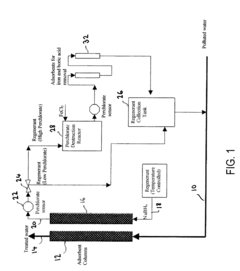
Method for perchlorate removal from ground water
PatentInactiveUS7157006B2
Innovation
- The use of granular activated carbon (GAC) preloaded with organic cations or thermally treated with ammonia to enhance its ability to remove perchlorate and other anions, allowing for chemical or thermal regeneration and environmentally safe disposal.
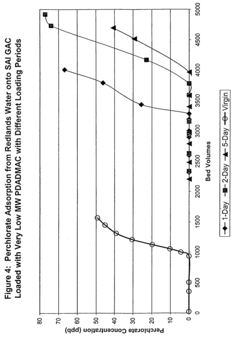
Apparatus and method for perchlorate removal and destruction
PatentInactiveUS20080105628A1
Innovation
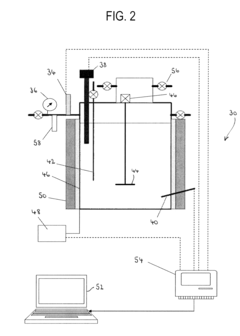
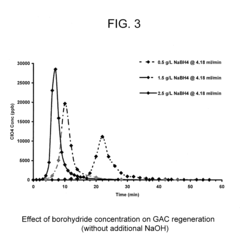
- The use of granular activated carbon as an adsorbent to capture perchlorate, followed by regeneration with sodium borohydride and in situ formation of nano-scale zero-valent iron for perchlorate destruction, reducing reactor size and chemical requirements, and eliminating the need for UV radiation and microorganisms.
Regulatory Framework for Chemical Pollutants
The regulatory framework for chemical pollutants in waterways is a complex and evolving system designed to protect aquatic ecosystems and human health. In the context of perchloric acid contamination, several key regulations and guidelines come into play. The Clean Water Act (CWA) serves as the primary federal law governing water pollution in the United States, establishing the basic structure for regulating discharges of pollutants into waters and setting quality standards for surface waters.
Under the CWA, the Environmental Protection Agency (EPA) has developed specific regulations for perchlorate, a compound closely related to perchloric acid. These regulations include a drinking water health advisory level and ongoing efforts to establish a national primary drinking water regulation. While perchloric acid itself is not explicitly regulated, its potential to form perchlorate in water bodies makes these regulations relevant.
The Resource Conservation and Recovery Act (RCRA) also plays a crucial role in managing perchloric acid contamination. This act governs the disposal of solid and hazardous waste, including chemicals that may contaminate waterways. Facilities handling perchloric acid must comply with RCRA regulations to prevent environmental releases.
At the state level, regulations can be more stringent than federal standards. California, for instance, has established its own perchlorate regulations, including a public health goal and a maximum contaminant level for drinking water. These state-level initiatives often drive more comprehensive approaches to managing chemical pollutants in waterways.
International agreements and conventions also influence the regulatory landscape. The Stockholm Convention on Persistent Organic Pollutants, while not directly addressing perchloric acid, sets a precedent for global cooperation in managing chemical pollutants that can impact aquatic ecosystems across borders.
Regulatory bodies are increasingly adopting a risk-based approach to chemical management. This involves assessing the potential impacts of pollutants on aquatic life and human health, and tailoring regulations accordingly. For perchloric acid, this may include considering its unique chemical properties, persistence in the environment, and potential for bioaccumulation in aquatic organisms.
Emerging contaminants of concern, including various industrial chemicals and pharmaceuticals, are driving the evolution of regulatory frameworks. As scientific understanding of the impacts of these substances on aquatic life improves, regulations are likely to expand and become more nuanced. This dynamic regulatory environment necessitates ongoing research and collaboration between scientists, policymakers, and industry stakeholders to effectively manage the risks associated with chemical pollutants in waterways.
Under the CWA, the Environmental Protection Agency (EPA) has developed specific regulations for perchlorate, a compound closely related to perchloric acid. These regulations include a drinking water health advisory level and ongoing efforts to establish a national primary drinking water regulation. While perchloric acid itself is not explicitly regulated, its potential to form perchlorate in water bodies makes these regulations relevant.
The Resource Conservation and Recovery Act (RCRA) also plays a crucial role in managing perchloric acid contamination. This act governs the disposal of solid and hazardous waste, including chemicals that may contaminate waterways. Facilities handling perchloric acid must comply with RCRA regulations to prevent environmental releases.
At the state level, regulations can be more stringent than federal standards. California, for instance, has established its own perchlorate regulations, including a public health goal and a maximum contaminant level for drinking water. These state-level initiatives often drive more comprehensive approaches to managing chemical pollutants in waterways.
International agreements and conventions also influence the regulatory landscape. The Stockholm Convention on Persistent Organic Pollutants, while not directly addressing perchloric acid, sets a precedent for global cooperation in managing chemical pollutants that can impact aquatic ecosystems across borders.
Regulatory bodies are increasingly adopting a risk-based approach to chemical management. This involves assessing the potential impacts of pollutants on aquatic life and human health, and tailoring regulations accordingly. For perchloric acid, this may include considering its unique chemical properties, persistence in the environment, and potential for bioaccumulation in aquatic organisms.
Emerging contaminants of concern, including various industrial chemicals and pharmaceuticals, are driving the evolution of regulatory frameworks. As scientific understanding of the impacts of these substances on aquatic life improves, regulations are likely to expand and become more nuanced. This dynamic regulatory environment necessitates ongoing research and collaboration between scientists, policymakers, and industry stakeholders to effectively manage the risks associated with chemical pollutants in waterways.
Bioaccumulation and Food Chain Implications
The bioaccumulation of perchloric acid in aquatic ecosystems poses significant risks to the food chain and overall ecological balance. As a persistent contaminant, perchlorate ions can accumulate in various organisms, particularly in plants and lower trophic level animals. This accumulation process begins with phytoplankton and aquatic plants, which absorb perchlorate from the water and sediment.
As these primary producers are consumed by herbivorous organisms, the perchlorate concentration increases through trophic transfer. This biomagnification effect becomes more pronounced at higher levels of the food chain, with top predators potentially accumulating the highest concentrations of perchlorate in their tissues. Fish, in particular, have shown a tendency to concentrate perchlorate in their thyroid glands, which can lead to endocrine disruption and developmental abnormalities.
The implications of perchlorate bioaccumulation extend beyond individual species, affecting entire ecosystems. Disruptions in thyroid function can alter growth rates, reproductive success, and behavior patterns of affected organisms. This can lead to population-level changes and shifts in community structure, potentially destabilizing aquatic food webs.
Furthermore, the bioaccumulation of perchlorate in aquatic organisms presents a potential risk to human health through the consumption of contaminated fish and shellfish. This creates a direct link between environmental contamination and public health concerns, emphasizing the need for comprehensive monitoring and management strategies.
Long-term exposure to perchlorate through the food chain can have far-reaching consequences for biodiversity and ecosystem resilience. Species with longer lifespans or those at higher trophic levels may experience more severe impacts due to prolonged exposure and higher accumulation rates. This can result in reduced genetic diversity and increased vulnerability to other environmental stressors.
Addressing the issue of perchlorate bioaccumulation requires a multifaceted approach. This includes developing more effective water treatment technologies to remove perchlorate from contaminated waterways, implementing stricter regulations on perchlorate use and disposal, and conducting ongoing research to better understand the long-term ecological impacts of perchlorate exposure through the food chain.
As these primary producers are consumed by herbivorous organisms, the perchlorate concentration increases through trophic transfer. This biomagnification effect becomes more pronounced at higher levels of the food chain, with top predators potentially accumulating the highest concentrations of perchlorate in their tissues. Fish, in particular, have shown a tendency to concentrate perchlorate in their thyroid glands, which can lead to endocrine disruption and developmental abnormalities.
The implications of perchlorate bioaccumulation extend beyond individual species, affecting entire ecosystems. Disruptions in thyroid function can alter growth rates, reproductive success, and behavior patterns of affected organisms. This can lead to population-level changes and shifts in community structure, potentially destabilizing aquatic food webs.
Furthermore, the bioaccumulation of perchlorate in aquatic organisms presents a potential risk to human health through the consumption of contaminated fish and shellfish. This creates a direct link between environmental contamination and public health concerns, emphasizing the need for comprehensive monitoring and management strategies.
Long-term exposure to perchlorate through the food chain can have far-reaching consequences for biodiversity and ecosystem resilience. Species with longer lifespans or those at higher trophic levels may experience more severe impacts due to prolonged exposure and higher accumulation rates. This can result in reduced genetic diversity and increased vulnerability to other environmental stressors.
Addressing the issue of perchlorate bioaccumulation requires a multifaceted approach. This includes developing more effective water treatment technologies to remove perchlorate from contaminated waterways, implementing stricter regulations on perchlorate use and disposal, and conducting ongoing research to better understand the long-term ecological impacts of perchlorate exposure through the food chain.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!