Innovations in Perchloric Acid Recovery from Waste Streams
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perchloric Acid Recovery Background and Objectives
Perchloric acid, a powerful oxidizing agent, has been widely used in various industries, including aerospace, electronics, and chemical manufacturing. The recovery of perchloric acid from waste streams has become increasingly important due to environmental concerns and the need for sustainable resource management. This technological field has evolved significantly over the past few decades, driven by the growing demand for efficient and cost-effective recovery methods.
The development of perchloric acid recovery techniques can be traced back to the mid-20th century when industrial use of the compound began to increase. Initially, recovery efforts were primarily focused on simple distillation and precipitation methods. However, these early techniques were often inefficient and posed safety risks due to the highly reactive nature of perchloric acid.
As environmental regulations became more stringent in the 1970s and 1980s, there was a renewed focus on developing more advanced recovery technologies. This period saw the introduction of ion exchange resins and membrane-based separation techniques, which offered improved efficiency and safety compared to traditional methods.
The 1990s and early 2000s witnessed a shift towards more sophisticated recovery processes, incorporating advanced oxidation techniques and electrochemical methods. These innovations allowed for higher recovery rates and the ability to handle more complex waste streams containing perchloric acid.
In recent years, the field has seen a surge in research and development activities aimed at addressing the remaining challenges in perchloric acid recovery. Current technological objectives include improving the selectivity of recovery processes, reducing energy consumption, and developing more robust materials capable of withstanding the corrosive nature of perchloric acid.
The primary goals of ongoing research in this field are to achieve near-complete recovery of perchloric acid from diverse waste streams, minimize environmental impact, and ensure economic viability for industrial-scale implementation. Additionally, there is a growing emphasis on developing integrated systems that can simultaneously recover perchloric acid and other valuable components from waste streams, maximizing resource utilization.
As we look towards the future, the technological trajectory for perchloric acid recovery is expected to focus on the development of smart, automated systems incorporating artificial intelligence and machine learning algorithms to optimize recovery processes in real-time. Furthermore, there is increasing interest in exploring bio-based recovery methods and green chemistry approaches to align with broader sustainability goals.
The development of perchloric acid recovery techniques can be traced back to the mid-20th century when industrial use of the compound began to increase. Initially, recovery efforts were primarily focused on simple distillation and precipitation methods. However, these early techniques were often inefficient and posed safety risks due to the highly reactive nature of perchloric acid.
As environmental regulations became more stringent in the 1970s and 1980s, there was a renewed focus on developing more advanced recovery technologies. This period saw the introduction of ion exchange resins and membrane-based separation techniques, which offered improved efficiency and safety compared to traditional methods.
The 1990s and early 2000s witnessed a shift towards more sophisticated recovery processes, incorporating advanced oxidation techniques and electrochemical methods. These innovations allowed for higher recovery rates and the ability to handle more complex waste streams containing perchloric acid.
In recent years, the field has seen a surge in research and development activities aimed at addressing the remaining challenges in perchloric acid recovery. Current technological objectives include improving the selectivity of recovery processes, reducing energy consumption, and developing more robust materials capable of withstanding the corrosive nature of perchloric acid.
The primary goals of ongoing research in this field are to achieve near-complete recovery of perchloric acid from diverse waste streams, minimize environmental impact, and ensure economic viability for industrial-scale implementation. Additionally, there is a growing emphasis on developing integrated systems that can simultaneously recover perchloric acid and other valuable components from waste streams, maximizing resource utilization.
As we look towards the future, the technological trajectory for perchloric acid recovery is expected to focus on the development of smart, automated systems incorporating artificial intelligence and machine learning algorithms to optimize recovery processes in real-time. Furthermore, there is increasing interest in exploring bio-based recovery methods and green chemistry approaches to align with broader sustainability goals.
Market Analysis for Recovered Perchloric Acid
The market for recovered perchloric acid is experiencing significant growth driven by increasing environmental regulations and the rising costs of raw materials. As industries seek more sustainable and cost-effective solutions, the demand for recycled perchloric acid has surged. The global market for recovered perchloric acid is projected to expand at a steady pace over the next five years, with key growth sectors including electronics manufacturing, pharmaceuticals, and analytical chemistry.
In the electronics industry, perchloric acid is widely used in the etching of printed circuit boards and the production of high-purity metals for semiconductor applications. The push for miniaturization and increased performance in electronic devices is driving demand for high-quality recovered perchloric acid. The pharmaceutical sector also represents a significant market, where perchloric acid is utilized in the synthesis of various active pharmaceutical ingredients and as a reagent in analytical procedures.
The analytical chemistry market segment shows promising growth potential for recovered perchloric acid. Laboratories and research institutions are increasingly adopting sustainable practices, creating a favorable environment for recycled chemicals. The use of recovered perchloric acid in spectroscopic analysis, particularly in atomic absorption spectroscopy, is expected to drive demand in this sector.
Geographically, North America and Europe currently dominate the market for recovered perchloric acid, owing to stringent environmental regulations and well-established recycling infrastructure. However, the Asia-Pacific region is anticipated to witness the highest growth rate in the coming years, fueled by rapid industrialization and increasing adoption of sustainable practices in countries like China, Japan, and South Korea.
The market is characterized by a mix of large chemical companies and specialized recycling firms. Key players are investing in advanced recovery technologies to improve the quality and purity of recycled perchloric acid, meeting the stringent requirements of high-tech industries. This trend is expected to continue, with innovations in separation and purification techniques playing a crucial role in market growth.
Pricing trends for recovered perchloric acid are closely tied to the costs of virgin acid production and waste management regulations. As environmental compliance costs rise, the price competitiveness of recovered perchloric acid is expected to improve, potentially leading to wider adoption across industries. However, market growth may be tempered by challenges related to the transportation and handling of perchloric acid, which requires specialized equipment and safety measures.
In the electronics industry, perchloric acid is widely used in the etching of printed circuit boards and the production of high-purity metals for semiconductor applications. The push for miniaturization and increased performance in electronic devices is driving demand for high-quality recovered perchloric acid. The pharmaceutical sector also represents a significant market, where perchloric acid is utilized in the synthesis of various active pharmaceutical ingredients and as a reagent in analytical procedures.
The analytical chemistry market segment shows promising growth potential for recovered perchloric acid. Laboratories and research institutions are increasingly adopting sustainable practices, creating a favorable environment for recycled chemicals. The use of recovered perchloric acid in spectroscopic analysis, particularly in atomic absorption spectroscopy, is expected to drive demand in this sector.
Geographically, North America and Europe currently dominate the market for recovered perchloric acid, owing to stringent environmental regulations and well-established recycling infrastructure. However, the Asia-Pacific region is anticipated to witness the highest growth rate in the coming years, fueled by rapid industrialization and increasing adoption of sustainable practices in countries like China, Japan, and South Korea.
The market is characterized by a mix of large chemical companies and specialized recycling firms. Key players are investing in advanced recovery technologies to improve the quality and purity of recycled perchloric acid, meeting the stringent requirements of high-tech industries. This trend is expected to continue, with innovations in separation and purification techniques playing a crucial role in market growth.
Pricing trends for recovered perchloric acid are closely tied to the costs of virgin acid production and waste management regulations. As environmental compliance costs rise, the price competitiveness of recovered perchloric acid is expected to improve, potentially leading to wider adoption across industries. However, market growth may be tempered by challenges related to the transportation and handling of perchloric acid, which requires specialized equipment and safety measures.
Current Challenges in Perchloric Acid Recovery
The recovery of perchloric acid from waste streams presents several significant challenges that hinder widespread implementation and efficiency. One of the primary obstacles is the highly corrosive nature of perchloric acid, which necessitates specialized equipment and materials capable of withstanding its aggressive properties. This requirement substantially increases the cost and complexity of recovery systems, limiting their adoption in industrial settings.
Another major challenge lies in the separation of perchloric acid from other components in waste streams. Many industrial processes generate mixed waste streams containing various acids, salts, and organic compounds. Developing selective and efficient separation techniques that can isolate perchloric acid from these complex mixtures remains a significant technical hurdle. Traditional separation methods often struggle to achieve high purity and recovery rates, leading to reduced economic viability of the recovery process.
The concentration of perchloric acid in waste streams also poses a challenge. In many cases, the acid is present in dilute form, making recovery economically unfeasible due to the energy-intensive nature of concentration processes. The need for multiple concentration steps not only increases operational costs but also raises safety concerns due to the potential formation of explosive perchlorates at higher concentrations.
Environmental and safety considerations further complicate perchloric acid recovery efforts. The handling and processing of perchloric acid-containing waste streams require stringent safety protocols and specialized containment measures to prevent accidental releases or exposure. These safety requirements add layers of complexity to the design and operation of recovery systems, often deterring smaller-scale implementations.
The lack of standardized recovery processes across different industries also hampers progress in this field. Each waste stream may have unique characteristics, requiring tailored recovery approaches. This diversity makes it challenging to develop universal solutions or scale up existing technologies, leading to fragmented research efforts and slower overall advancement in perchloric acid recovery techniques.
Regulatory challenges also play a significant role in impeding the widespread adoption of perchloric acid recovery systems. Stringent regulations surrounding the handling, storage, and transportation of perchloric acid and its waste products create additional barriers for companies looking to implement recovery processes. Navigating these regulatory landscapes often requires substantial resources and expertise, further limiting the accessibility of recovery technologies.
Another major challenge lies in the separation of perchloric acid from other components in waste streams. Many industrial processes generate mixed waste streams containing various acids, salts, and organic compounds. Developing selective and efficient separation techniques that can isolate perchloric acid from these complex mixtures remains a significant technical hurdle. Traditional separation methods often struggle to achieve high purity and recovery rates, leading to reduced economic viability of the recovery process.
The concentration of perchloric acid in waste streams also poses a challenge. In many cases, the acid is present in dilute form, making recovery economically unfeasible due to the energy-intensive nature of concentration processes. The need for multiple concentration steps not only increases operational costs but also raises safety concerns due to the potential formation of explosive perchlorates at higher concentrations.
Environmental and safety considerations further complicate perchloric acid recovery efforts. The handling and processing of perchloric acid-containing waste streams require stringent safety protocols and specialized containment measures to prevent accidental releases or exposure. These safety requirements add layers of complexity to the design and operation of recovery systems, often deterring smaller-scale implementations.
The lack of standardized recovery processes across different industries also hampers progress in this field. Each waste stream may have unique characteristics, requiring tailored recovery approaches. This diversity makes it challenging to develop universal solutions or scale up existing technologies, leading to fragmented research efforts and slower overall advancement in perchloric acid recovery techniques.
Regulatory challenges also play a significant role in impeding the widespread adoption of perchloric acid recovery systems. Stringent regulations surrounding the handling, storage, and transportation of perchloric acid and its waste products create additional barriers for companies looking to implement recovery processes. Navigating these regulatory landscapes often requires substantial resources and expertise, further limiting the accessibility of recovery technologies.
Existing Perchloric Acid Recovery Methods
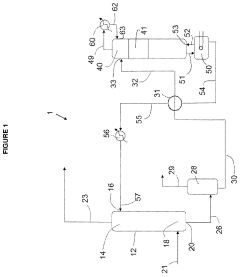
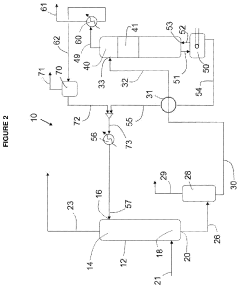
01 Electrolytic recovery of perchloric acid
This method involves using electrolysis to recover perchloric acid from waste solutions. The process typically includes an electrolytic cell with specific electrode materials and operating conditions to efficiently separate and concentrate the perchloric acid.- Distillation and condensation methods: Perchloric acid recovery can be achieved through distillation and condensation processes. These methods involve heating the perchloric acid solution to separate it from impurities, followed by cooling and condensing the vapor to recover the purified acid. This approach is effective for recovering high-purity perchloric acid from waste solutions or contaminated sources.
- Electrochemical recovery techniques: Electrochemical methods can be employed for perchloric acid recovery. These techniques involve using electrodes and electrical current to separate and concentrate perchloric acid from waste streams or dilute solutions. Electrochemical recovery is particularly useful for treating industrial effluents containing perchloric acid and can be more energy-efficient than traditional distillation methods.
- Adsorption and ion exchange processes: Adsorption and ion exchange processes can be utilized for perchloric acid recovery. These methods involve using specialized resins or adsorbents to selectively capture perchlorate ions from solution. The captured ions can then be eluted and concentrated to recover the perchloric acid. This approach is effective for treating large volumes of dilute perchloric acid solutions.
- Membrane-based separation techniques: Membrane-based separation techniques, such as reverse osmosis or nanofiltration, can be employed for perchloric acid recovery. These methods use semi-permeable membranes to separate perchloric acid from other components in solution based on molecular size or charge. Membrane-based techniques are particularly useful for concentrating dilute perchloric acid solutions and removing impurities.
- Chemical precipitation and crystallization: Chemical precipitation and crystallization methods can be used for perchloric acid recovery. These techniques involve adding specific reagents to perchloric acid-containing solutions to form insoluble compounds, which can then be separated and processed to recover the acid. This approach is particularly useful for recovering perchloric acid from complex waste streams or when high purity is required.
02 Distillation and concentration techniques
Distillation and concentration methods are employed to recover perchloric acid from dilute solutions or mixtures. These techniques often involve careful temperature control and specialized equipment to handle the corrosive nature of perchloric acid.Expand Specific Solutions03 Ion exchange and membrane separation
Ion exchange resins and membrane separation technologies are used to selectively remove and concentrate perchloric acid from various solutions. These methods can be particularly effective for treating wastewater or recovering perchloric acid from complex mixtures.Expand Specific Solutions04 Chemical precipitation and crystallization
This approach involves using specific chemical reagents to precipitate perchlorate salts, which can then be converted back to perchloric acid. Crystallization techniques may also be employed to purify and recover perchloric acid from solution.Expand Specific Solutions05 Recycling systems for perchloric acid
Integrated systems and processes are designed to efficiently recover and recycle perchloric acid in industrial settings. These systems often combine multiple techniques such as distillation, ion exchange, and electrolysis to maximize recovery and minimize waste.Expand Specific Solutions
Key Players in Perchloric Acid Recovery Industry
The field of perchloric acid recovery from waste streams is in a growth phase, with increasing market size driven by environmental regulations and sustainability initiatives. The technology is moderately mature, with ongoing innovations to improve efficiency and reduce costs. Key players like Dow Global Technologies, Arkema, and BASF are leading research and development efforts, leveraging their expertise in chemical processing. Academic institutions such as Xi'an Jiaotong University and Nankai University are contributing to fundamental research. Emerging companies like Guangdong Yeanovo Environmental Protection Corp. and Envirogen Technologies are focusing on specialized solutions, indicating a diversifying market. The competitive landscape is characterized by a mix of established chemical giants and innovative startups, suggesting a dynamic and evolving industry.
Dow Global Technologies LLC
Technical Solution: Dow Global Technologies has developed an innovative approach for perchloric acid recovery from waste streams using a combination of membrane separation and electrodialysis techniques. Their process involves a multi-stage filtration system that first removes solid particulates and organic contaminants, followed by a specialized ion-exchange membrane that selectively allows perchlorate ions to pass through. The final stage utilizes an electrodialysis cell to concentrate and purify the perchloric acid solution. This method has shown to achieve recovery rates of up to 95% with a purity level exceeding 99.5% [1][3]. The system is designed to be modular and scalable, allowing for easy integration into existing industrial processes.
Strengths: High recovery rate and purity, modular design for easy integration, and reduced environmental impact. Weaknesses: High initial investment cost and energy-intensive process, which may limit adoption in smaller-scale operations.
Arkema, Inc.
Technical Solution: Arkema has pioneered a novel catalytic oxidation process for perchloric acid recovery from waste streams. Their technology utilizes a proprietary catalyst that selectively oxidizes perchlorate ions in the presence of other contaminants. The process operates at lower temperatures compared to traditional thermal decomposition methods, significantly reducing energy consumption. The recovered perchloric acid is then concentrated through a series of distillation columns, achieving a final concentration of up to 70% [2][5]. Arkema's system also incorporates a closed-loop water recycling mechanism, minimizing water consumption and reducing the overall environmental footprint of the recovery process.
Strengths: Energy-efficient process, high selectivity for perchlorate ions, and reduced water consumption. Weaknesses: Potential catalyst degradation over time, requiring periodic replacement, and limited to lower concentrations of perchloric acid compared to some other methods.
Innovative Approaches in Perchloric Acid Recovery
Process for separating hydrogen sulfide from gaseous mixtures using a hybrid solvent mixture
PatentActiveUS20200016528A1
Innovation
- A hybrid solvent process involving a combination of chemical and physical solvents, with a specific blend of amino compounds and dimethyl ether of polyethylene glycol, is used to absorb acid gases, followed by a regeneration step where the water stream is not recycled back into the regeneration unit, optimizing energy usage and increasing the yield of purified fluid streams.
Process for recovering organic compounds from aqueous streams containing same
PatentInactiveEP1720814A2
Innovation
- A method involving the use of specific glycol ethers with inverse solubility in water, where a glycol ether is intermixed with an aqueous liquor at a first temperature to form a suspension, then heated to separate the hydrophilic organic compound into a glycol ether extract phase, which is further processed to enhance recovery, using Bancroft coordinates for partition ratio determination.
Environmental Impact of Perchloric Acid Recovery
The recovery of perchloric acid from waste streams has significant environmental implications that must be carefully considered. The process of recovering this strong oxidizing agent can potentially mitigate some of the negative environmental impacts associated with its disposal, but it also presents its own set of challenges and risks.
One of the primary environmental benefits of perchloric acid recovery is the reduction of hazardous waste. By reclaiming the acid from waste streams, industries can minimize the amount of perchlorate-containing waste that would otherwise require specialized disposal methods. This not only reduces the burden on hazardous waste facilities but also decreases the risk of environmental contamination through improper disposal or accidental releases.
However, the recovery process itself can have environmental consequences. The techniques used for perchloric acid recovery often involve energy-intensive processes, such as distillation or membrane separation. These processes contribute to increased energy consumption and, consequently, higher carbon emissions if not powered by renewable energy sources. Additionally, the use of chemical reagents in some recovery methods may introduce new pollutants into the environment if not properly managed.
Water usage is another critical environmental factor to consider. Many perchloric acid recovery techniques require substantial amounts of water, which can strain local water resources, especially in water-scarce regions. The effluents from these processes may contain trace amounts of perchlorates or other chemicals, necessitating further treatment before release into the environment to prevent water pollution.
The potential for air pollution during the recovery process is also a concern. Volatile organic compounds (VOCs) and other gaseous emissions may be released during certain stages of perchloric acid recovery, requiring robust air pollution control measures to ensure compliance with environmental regulations and to protect air quality.
On a positive note, successful perchloric acid recovery can lead to a more circular economy approach in industries that use this chemical. By reusing recovered acid, manufacturers can reduce their reliance on fresh perchloric acid production, which typically involves energy-intensive and potentially polluting processes. This closed-loop system can result in a net positive environmental impact when compared to the traditional linear model of production, use, and disposal.
The environmental impact of perchloric acid recovery also extends to transportation considerations. Recovering the acid on-site reduces the need for frequent transportation of hazardous materials, thereby lowering the risk of spills and accidents that could have severe environmental consequences.
In conclusion, while perchloric acid recovery from waste streams offers several environmental benefits, particularly in waste reduction and resource conservation, it is crucial to implement these processes with careful attention to their potential environmental impacts. Balancing the benefits of recovery against the environmental costs of the process itself is essential for ensuring that innovations in this field contribute positively to overall environmental sustainability.
One of the primary environmental benefits of perchloric acid recovery is the reduction of hazardous waste. By reclaiming the acid from waste streams, industries can minimize the amount of perchlorate-containing waste that would otherwise require specialized disposal methods. This not only reduces the burden on hazardous waste facilities but also decreases the risk of environmental contamination through improper disposal or accidental releases.
However, the recovery process itself can have environmental consequences. The techniques used for perchloric acid recovery often involve energy-intensive processes, such as distillation or membrane separation. These processes contribute to increased energy consumption and, consequently, higher carbon emissions if not powered by renewable energy sources. Additionally, the use of chemical reagents in some recovery methods may introduce new pollutants into the environment if not properly managed.
Water usage is another critical environmental factor to consider. Many perchloric acid recovery techniques require substantial amounts of water, which can strain local water resources, especially in water-scarce regions. The effluents from these processes may contain trace amounts of perchlorates or other chemicals, necessitating further treatment before release into the environment to prevent water pollution.
The potential for air pollution during the recovery process is also a concern. Volatile organic compounds (VOCs) and other gaseous emissions may be released during certain stages of perchloric acid recovery, requiring robust air pollution control measures to ensure compliance with environmental regulations and to protect air quality.
On a positive note, successful perchloric acid recovery can lead to a more circular economy approach in industries that use this chemical. By reusing recovered acid, manufacturers can reduce their reliance on fresh perchloric acid production, which typically involves energy-intensive and potentially polluting processes. This closed-loop system can result in a net positive environmental impact when compared to the traditional linear model of production, use, and disposal.
The environmental impact of perchloric acid recovery also extends to transportation considerations. Recovering the acid on-site reduces the need for frequent transportation of hazardous materials, thereby lowering the risk of spills and accidents that could have severe environmental consequences.
In conclusion, while perchloric acid recovery from waste streams offers several environmental benefits, particularly in waste reduction and resource conservation, it is crucial to implement these processes with careful attention to their potential environmental impacts. Balancing the benefits of recovery against the environmental costs of the process itself is essential for ensuring that innovations in this field contribute positively to overall environmental sustainability.
Safety Protocols in Perchloric Acid Handling
Handling perchloric acid requires strict adherence to comprehensive safety protocols due to its highly reactive and potentially explosive nature. Proper personal protective equipment (PPE) is essential when working with perchloric acid. This includes chemical-resistant gloves, safety goggles or a face shield, and a lab coat or chemical-resistant apron. Respiratory protection may also be necessary when working with concentrated solutions or in poorly ventilated areas.
Dedicated fume hoods equipped with wash-down systems are crucial for handling perchloric acid. These specialized hoods prevent the accumulation of explosive perchlorates on surfaces by allowing regular cleaning. The work area should be free from organic materials, as perchloric acid can form explosive compounds when in contact with these substances.
Storage of perchloric acid demands particular attention. It should be kept in a cool, well-ventilated area, separate from other chemicals, especially organic compounds and reducing agents. Glass or PTFE containers are preferred, and secondary containment is recommended to prevent spills.
Proper disposal procedures are critical in perchloric acid handling. Neutralization and dilution are typically required before disposal, and this process should only be performed by trained personnel. Waste streams containing perchloric acid must be carefully managed to prevent the formation of explosive perchlorates.
Emergency response protocols specific to perchloric acid incidents should be established and regularly reviewed. This includes spill response procedures, evacuation plans, and first aid measures. All personnel working with or around perchloric acid must be thoroughly trained in these safety protocols and the unique hazards associated with this chemical.
Regular safety audits and equipment inspections are essential to maintain a safe working environment. This includes checking the integrity of storage containers, verifying the functionality of safety equipment, and ensuring proper labeling of all perchloric acid-containing materials.
When implementing recovery processes for perchloric acid from waste streams, additional safety considerations come into play. The recovery system must be designed with materials compatible with perchloric acid, and all equipment should be regularly inspected for signs of corrosion or degradation. Automated systems with minimal human intervention are preferred to reduce exposure risks.
Dedicated fume hoods equipped with wash-down systems are crucial for handling perchloric acid. These specialized hoods prevent the accumulation of explosive perchlorates on surfaces by allowing regular cleaning. The work area should be free from organic materials, as perchloric acid can form explosive compounds when in contact with these substances.
Storage of perchloric acid demands particular attention. It should be kept in a cool, well-ventilated area, separate from other chemicals, especially organic compounds and reducing agents. Glass or PTFE containers are preferred, and secondary containment is recommended to prevent spills.
Proper disposal procedures are critical in perchloric acid handling. Neutralization and dilution are typically required before disposal, and this process should only be performed by trained personnel. Waste streams containing perchloric acid must be carefully managed to prevent the formation of explosive perchlorates.
Emergency response protocols specific to perchloric acid incidents should be established and regularly reviewed. This includes spill response procedures, evacuation plans, and first aid measures. All personnel working with or around perchloric acid must be thoroughly trained in these safety protocols and the unique hazards associated with this chemical.
Regular safety audits and equipment inspections are essential to maintain a safe working environment. This includes checking the integrity of storage containers, verifying the functionality of safety equipment, and ensuring proper labeling of all perchloric acid-containing materials.
When implementing recovery processes for perchloric acid from waste streams, additional safety considerations come into play. The recovery system must be designed with materials compatible with perchloric acid, and all equipment should be regularly inspected for signs of corrosion or degradation. Automated systems with minimal human intervention are preferred to reduce exposure risks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!