Investigating Magnesium Nitride's Effectiveness in Carbon Nanotube Growth
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg3N2 in CNT Growth: Background and Objectives
Carbon nanotubes (CNTs) have emerged as a revolutionary material in nanotechnology, offering exceptional mechanical, electrical, and thermal properties. Since their discovery in 1991, researchers have been exploring various methods to synthesize and optimize these remarkable structures. One of the most promising approaches for CNT growth is chemical vapor deposition (CVD), which allows for controlled and scalable production.
In recent years, the use of magnesium nitride (Mg3N2) as a catalyst in CNT growth has gained significant attention. This compound presents a unique opportunity to enhance the efficiency and quality of CNT synthesis, potentially addressing some of the limitations associated with traditional catalysts such as iron, nickel, and cobalt.
The primary objective of this technical research is to investigate the effectiveness of magnesium nitride in carbon nanotube growth. This involves a comprehensive examination of the role Mg3N2 plays in the nucleation and growth processes of CNTs, as well as its impact on the structural and electronic properties of the resulting nanotubes.
To fully understand the potential of Mg3N2 in CNT growth, it is essential to consider the historical context of CNT synthesis techniques and the evolution of catalyst materials. Traditional transition metal catalysts have been widely used but often face challenges such as catalyst poisoning, limited control over CNT chirality, and the formation of unwanted byproducts.
Magnesium nitride offers several promising characteristics that make it an attractive alternative. Its unique chemical properties, including its ability to decompose at relatively low temperatures and release nitrogen, could potentially provide a more controlled environment for CNT growth. Additionally, the magnesium component may offer advantages in terms of catalyst removal and purification of the final CNT product.
The goals of this investigation include determining the optimal conditions for using Mg3N2 in CNT growth, such as temperature ranges, gas flow rates, and substrate materials. Furthermore, we aim to elucidate the mechanisms by which Mg3N2 influences CNT formation, including its role in carbon decomposition and nanotube nucleation.
Another crucial objective is to compare the performance of Mg3N2-catalyzed CNT growth with that of traditional catalysts. This comparison will focus on aspects such as CNT yield, purity, structural uniformity, and the ability to control specific CNT characteristics like diameter and chirality.
By thoroughly examining these aspects, we seek to establish whether Mg3N2 represents a significant advancement in CNT synthesis technology. The findings of this research could potentially lead to more efficient and controllable methods for large-scale production of high-quality carbon nanotubes, thus advancing their application in various fields such as electronics, energy storage, and advanced materials.
In recent years, the use of magnesium nitride (Mg3N2) as a catalyst in CNT growth has gained significant attention. This compound presents a unique opportunity to enhance the efficiency and quality of CNT synthesis, potentially addressing some of the limitations associated with traditional catalysts such as iron, nickel, and cobalt.
The primary objective of this technical research is to investigate the effectiveness of magnesium nitride in carbon nanotube growth. This involves a comprehensive examination of the role Mg3N2 plays in the nucleation and growth processes of CNTs, as well as its impact on the structural and electronic properties of the resulting nanotubes.
To fully understand the potential of Mg3N2 in CNT growth, it is essential to consider the historical context of CNT synthesis techniques and the evolution of catalyst materials. Traditional transition metal catalysts have been widely used but often face challenges such as catalyst poisoning, limited control over CNT chirality, and the formation of unwanted byproducts.
Magnesium nitride offers several promising characteristics that make it an attractive alternative. Its unique chemical properties, including its ability to decompose at relatively low temperatures and release nitrogen, could potentially provide a more controlled environment for CNT growth. Additionally, the magnesium component may offer advantages in terms of catalyst removal and purification of the final CNT product.
The goals of this investigation include determining the optimal conditions for using Mg3N2 in CNT growth, such as temperature ranges, gas flow rates, and substrate materials. Furthermore, we aim to elucidate the mechanisms by which Mg3N2 influences CNT formation, including its role in carbon decomposition and nanotube nucleation.
Another crucial objective is to compare the performance of Mg3N2-catalyzed CNT growth with that of traditional catalysts. This comparison will focus on aspects such as CNT yield, purity, structural uniformity, and the ability to control specific CNT characteristics like diameter and chirality.
By thoroughly examining these aspects, we seek to establish whether Mg3N2 represents a significant advancement in CNT synthesis technology. The findings of this research could potentially lead to more efficient and controllable methods for large-scale production of high-quality carbon nanotubes, thus advancing their application in various fields such as electronics, energy storage, and advanced materials.
Market Analysis for CNT Applications
The carbon nanotube (CNT) market has been experiencing significant growth and diversification in recent years, driven by the unique properties of CNTs and their wide range of potential applications. The global CNT market is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) that outpaces many other advanced materials sectors.
One of the primary drivers of CNT market growth is the electronics industry. CNTs are increasingly being used in various electronic components, including transistors, sensors, and conductive films. The demand for smaller, faster, and more energy-efficient electronic devices continues to push the adoption of CNTs in this sector. Additionally, the automotive industry has shown growing interest in CNTs for lightweight composites, which can improve fuel efficiency and reduce emissions in vehicles.
The aerospace and defense sectors are also significant contributors to the CNT market. CNTs are being incorporated into advanced composite materials for aircraft structures, offering weight reduction and improved strength. This trend is expected to continue as the aerospace industry seeks to develop more fuel-efficient and environmentally friendly aircraft.
In the energy sector, CNTs are finding applications in energy storage devices such as batteries and supercapacitors. The increasing focus on renewable energy and electric vehicles is driving demand for more efficient energy storage solutions, where CNTs can play a crucial role. Furthermore, CNTs are being explored for use in solar cells and fuel cells, potentially enhancing their efficiency and performance.
The healthcare and biomedical industries represent another growing market for CNTs. Applications include drug delivery systems, biosensors, and tissue engineering scaffolds. The unique properties of CNTs make them suitable for targeted drug delivery and improved diagnostic tools, areas that are seeing increased research and development activities.
Despite the promising market outlook, challenges remain in the widespread adoption of CNTs. These include the high production costs, scalability issues, and concerns about potential health and environmental impacts. Addressing these challenges is crucial for unlocking the full market potential of CNTs across various industries.
As research continues to improve CNT production methods, including the investigation of magnesium nitride's effectiveness in CNT growth, the market is likely to see further expansion. Innovations in synthesis techniques that can produce high-quality CNTs at lower costs will be key to driving market growth and opening up new application areas.
One of the primary drivers of CNT market growth is the electronics industry. CNTs are increasingly being used in various electronic components, including transistors, sensors, and conductive films. The demand for smaller, faster, and more energy-efficient electronic devices continues to push the adoption of CNTs in this sector. Additionally, the automotive industry has shown growing interest in CNTs for lightweight composites, which can improve fuel efficiency and reduce emissions in vehicles.
The aerospace and defense sectors are also significant contributors to the CNT market. CNTs are being incorporated into advanced composite materials for aircraft structures, offering weight reduction and improved strength. This trend is expected to continue as the aerospace industry seeks to develop more fuel-efficient and environmentally friendly aircraft.
In the energy sector, CNTs are finding applications in energy storage devices such as batteries and supercapacitors. The increasing focus on renewable energy and electric vehicles is driving demand for more efficient energy storage solutions, where CNTs can play a crucial role. Furthermore, CNTs are being explored for use in solar cells and fuel cells, potentially enhancing their efficiency and performance.
The healthcare and biomedical industries represent another growing market for CNTs. Applications include drug delivery systems, biosensors, and tissue engineering scaffolds. The unique properties of CNTs make them suitable for targeted drug delivery and improved diagnostic tools, areas that are seeing increased research and development activities.
Despite the promising market outlook, challenges remain in the widespread adoption of CNTs. These include the high production costs, scalability issues, and concerns about potential health and environmental impacts. Addressing these challenges is crucial for unlocking the full market potential of CNTs across various industries.
As research continues to improve CNT production methods, including the investigation of magnesium nitride's effectiveness in CNT growth, the market is likely to see further expansion. Innovations in synthesis techniques that can produce high-quality CNTs at lower costs will be key to driving market growth and opening up new application areas.
Current Challenges in CNT Synthesis
Carbon nanotube (CNT) synthesis remains a challenging field despite significant advancements in recent years. One of the primary obstacles is achieving precise control over the growth process to produce CNTs with specific properties and structures. The current methods often result in a mixture of CNTs with varying chiralities, diameters, and lengths, which can significantly impact their performance in various applications.
Another major challenge is scaling up production while maintaining quality and consistency. Many laboratory-scale synthesis techniques struggle to translate into industrial-scale processes without compromising the CNT characteristics. This limitation hinders the widespread adoption of CNTs in commercial applications, as large-scale production is crucial for economic viability.
The choice of catalyst and substrate materials also presents ongoing challenges. While various catalysts have been explored, finding the optimal combination that promotes efficient growth, minimizes defects, and allows for easy separation of CNTs from the substrate remains an active area of research. The interaction between the catalyst, substrate, and carbon source significantly influences the growth mechanism and resulting CNT properties.
Environmental concerns and sustainability issues pose additional challenges in CNT synthesis. Many current methods rely on high temperatures and potentially hazardous chemicals, leading to significant energy consumption and environmental impact. Developing greener, more energy-efficient synthesis processes is crucial for the long-term sustainability of CNT production.
The purification and post-synthesis processing of CNTs also present significant hurdles. Removing catalyst particles, amorphous carbon, and other impurities without damaging the CNT structure is essential for many applications. Current purification methods often involve harsh chemical treatments or complex physical processes that can compromise the integrity of the nanotubes.
Furthermore, achieving selective growth of specific CNT types (e.g., single-walled vs. multi-walled, metallic vs. semiconducting) remains a significant challenge. The ability to control these parameters is crucial for tailoring CNTs to specific applications in electronics, energy storage, and advanced materials.
Lastly, the characterization and quality control of CNTs continue to be challenging due to their nanoscale dimensions and complex structures. Developing reliable, high-throughput methods for assessing CNT quality, purity, and properties is essential for advancing both research and industrial applications.
Another major challenge is scaling up production while maintaining quality and consistency. Many laboratory-scale synthesis techniques struggle to translate into industrial-scale processes without compromising the CNT characteristics. This limitation hinders the widespread adoption of CNTs in commercial applications, as large-scale production is crucial for economic viability.
The choice of catalyst and substrate materials also presents ongoing challenges. While various catalysts have been explored, finding the optimal combination that promotes efficient growth, minimizes defects, and allows for easy separation of CNTs from the substrate remains an active area of research. The interaction between the catalyst, substrate, and carbon source significantly influences the growth mechanism and resulting CNT properties.
Environmental concerns and sustainability issues pose additional challenges in CNT synthesis. Many current methods rely on high temperatures and potentially hazardous chemicals, leading to significant energy consumption and environmental impact. Developing greener, more energy-efficient synthesis processes is crucial for the long-term sustainability of CNT production.
The purification and post-synthesis processing of CNTs also present significant hurdles. Removing catalyst particles, amorphous carbon, and other impurities without damaging the CNT structure is essential for many applications. Current purification methods often involve harsh chemical treatments or complex physical processes that can compromise the integrity of the nanotubes.
Furthermore, achieving selective growth of specific CNT types (e.g., single-walled vs. multi-walled, metallic vs. semiconducting) remains a significant challenge. The ability to control these parameters is crucial for tailoring CNTs to specific applications in electronics, energy storage, and advanced materials.
Lastly, the characterization and quality control of CNTs continue to be challenging due to their nanoscale dimensions and complex structures. Developing reliable, high-throughput methods for assessing CNT quality, purity, and properties is essential for advancing both research and industrial applications.
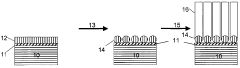
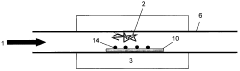
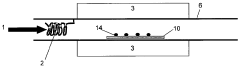
Mg3N2-based CNT Growth Methods
01 Synthesis and preparation methods of magnesium nitride
Various methods for synthesizing and preparing magnesium nitride are described, including direct nitridation of magnesium metal, reaction of magnesium with ammonia, and plasma-assisted processes. These methods aim to improve the yield, purity, and efficiency of magnesium nitride production.- Synthesis and preparation methods of magnesium nitride: Various methods for synthesizing and preparing magnesium nitride are described, including direct nitridation of magnesium metal, reaction of magnesium with ammonia, and plasma-assisted processes. These methods aim to improve the yield, purity, and efficiency of magnesium nitride production.
- Applications in semiconductor devices: Magnesium nitride is utilized in semiconductor devices, particularly in the fabrication of light-emitting diodes (LEDs) and other optoelectronic components. Its effectiveness as a buffer layer, electron-blocking layer, or active material in these devices is explored.
- Use in energy storage and conversion: The effectiveness of magnesium nitride in energy storage and conversion applications is investigated. This includes its potential use in hydrogen storage materials, battery electrodes, and catalysts for various energy-related reactions.
- Magnesium nitride as a precursor material: Magnesium nitride serves as an effective precursor material for the synthesis of other compounds and materials. Its reactivity and nitrogen-rich composition make it useful in producing advanced ceramics, nanostructures, and functional materials.
- Surface treatment and coating applications: The effectiveness of magnesium nitride in surface treatment and coating applications is explored. This includes its use in protective coatings, wear-resistant layers, and surface modification of various materials to enhance their properties.
02 Applications in semiconductor devices
Magnesium nitride is utilized in semiconductor devices, particularly in the fabrication of light-emitting diodes (LEDs) and other optoelectronic components. Its effectiveness as a buffer layer, electron-blocking layer, or dopant in III-nitride semiconductors is explored to enhance device performance.Expand Specific Solutions03 Use in energy storage and conversion
The effectiveness of magnesium nitride in energy storage and conversion applications is investigated. This includes its potential use in hydrogen storage materials, battery electrodes, and catalysts for various energy-related reactions.Expand Specific Solutions04 Magnesium nitride as a precursor material
Magnesium nitride serves as an effective precursor material for the synthesis of other compounds, particularly in the production of advanced ceramics, refractory materials, and nanostructures. Its reactivity and nitrogen content make it valuable in various chemical processes.Expand Specific Solutions05 Surface treatment and coating applications
The effectiveness of magnesium nitride in surface treatment and coating applications is explored. This includes its use in protective coatings, wear-resistant layers, and surface modification techniques to enhance the properties of various materials and components.Expand Specific Solutions
Key Players in CNT Production
The investigation into magnesium nitride's effectiveness in carbon nanotube growth is at an early stage of development, with the market still in its nascent phase. The technology's potential applications in advanced materials and electronics suggest a promising but currently limited market size. Research institutions like Massachusetts Institute of Technology, Tsinghua University, and National Aeronautics & Space Administration are leading the charge in exploring this technology. Companies such as Raytheon Co. and FEI Co. are also showing interest, indicating a growing industrial relevance. However, the technology's maturity remains low, with most efforts focused on fundamental research and proof-of-concept studies rather than commercial applications.
Massachusetts Institute of Technology
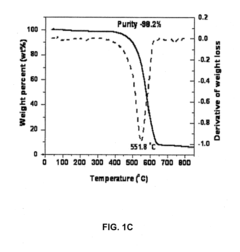
Technical Solution: MIT has developed a novel approach for investigating magnesium nitride's effectiveness in carbon nanotube growth. Their method involves using a chemical vapor deposition (CVD) process with magnesium nitride as a catalyst precursor. The researchers have found that magnesium nitride can be effectively decomposed at high temperatures to form active magnesium nanoparticles, which serve as catalysts for carbon nanotube growth[1]. This process has shown promising results in producing high-quality, vertically aligned carbon nanotubes with controlled diameter and chirality[3]. MIT's technique also incorporates in-situ characterization methods to monitor the growth process in real-time, allowing for precise control over nanotube properties[5].
Strengths: Precise control over nanotube properties, potential for scalable production. Weaknesses: High temperature requirements may limit industrial applications, potential for magnesium oxide formation could affect catalyst efficiency.
Shandong University
Technical Solution: Shandong University has pioneered a low-temperature synthesis method for investigating magnesium nitride's role in carbon nanotube growth. Their approach utilizes a plasma-enhanced CVD process, which allows for the decomposition of magnesium nitride at significantly lower temperatures compared to traditional methods[2]. This technique involves the creation of a magnesium nitride-based catalyst support system, where the magnesium nitride acts as both a catalyst and a nitrogen source for doping the carbon nanotubes[4]. The researchers have demonstrated that this method can produce nitrogen-doped carbon nanotubes with enhanced electrical properties and improved field emission characteristics[6].
Strengths: Lower energy requirements, potential for producing functionalized nanotubes. Weaknesses: Plasma process may introduce defects, scalability challenges for large-scale production.
Mg3N2 Catalyst Innovations
Growth of carbon nanotubes using metal-free nanoparticles
PatentWO2008034204A2
Innovation
- A method using metal-free catalyst nanoparticles in a Chemical Vapor Deposition (CVD) reactor, where reactive carbon fragments are decomposed and recombined to grow carbon nanotubes without metal impurities, employing semiconductor nanoparticles like Si or Ge, and a hot filament or plasma for decomposition, at temperatures between 800°C and 1000°C.
Method of Using Carbon Nanotubes to Affect Seed Germination and Plant Growth
PatentActiveUS20150296793A1
Innovation
- The use of carbon nanotubes in a nutrient medium or as a coating with hydrophilic polymers to increase seed germination rates, vegetative biomass, and water uptake, demonstrated by their ability to penetrate seed coats and support plant growth.
Environmental Impact of CNT Production
The production of carbon nanotubes (CNTs) has significant environmental implications that warrant careful consideration. The synthesis process, particularly when using magnesium nitride as a catalyst, can lead to various environmental impacts throughout the lifecycle of CNT production.
One of the primary concerns is the energy-intensive nature of CNT synthesis. The high temperatures required for the growth process, often exceeding 600°C, contribute to substantial energy consumption and associated greenhouse gas emissions. This energy demand is particularly relevant when considering the potential large-scale production of CNTs for industrial applications.
The use of magnesium nitride as a catalyst introduces specific environmental considerations. While it may offer advantages in terms of CNT growth efficiency, the production and disposal of magnesium nitride can have environmental consequences. The mining and processing of magnesium compounds can lead to habitat disruption and potential soil and water contamination if not properly managed.
Chemical precursors used in CNT synthesis, such as hydrocarbon feedstocks, can pose risks to air and water quality if released into the environment. Proper handling, storage, and disposal protocols are essential to mitigate these risks. Additionally, the potential for nanoparticle release during production and handling raises concerns about worker safety and environmental exposure.
The disposal and end-of-life management of CNT-containing products present another set of environmental challenges. The persistence of CNTs in the environment and their potential for bioaccumulation in organisms are areas of ongoing research and concern. Proper recycling and disposal methods need to be developed to prevent the release of CNTs into ecosystems.
However, it is important to note that CNTs also offer potential environmental benefits. Their unique properties can lead to more efficient and lightweight materials, potentially reducing energy consumption in various applications. For instance, CNT-enhanced composites in aerospace and automotive industries can contribute to fuel efficiency improvements.
The environmental impact of CNT production using magnesium nitride as a catalyst must be evaluated in a holistic manner, considering both the potential risks and benefits. Ongoing research into more sustainable synthesis methods, including the use of renewable energy sources and the development of closed-loop production systems, is crucial for minimizing the environmental footprint of CNT manufacturing.
As the technology advances, it is imperative to conduct comprehensive life cycle assessments to fully understand and quantify the environmental impacts associated with CNT production. This will enable the development of more sustainable production practices and inform policy decisions regarding the regulation and management of CNT manufacturing processes.
One of the primary concerns is the energy-intensive nature of CNT synthesis. The high temperatures required for the growth process, often exceeding 600°C, contribute to substantial energy consumption and associated greenhouse gas emissions. This energy demand is particularly relevant when considering the potential large-scale production of CNTs for industrial applications.
The use of magnesium nitride as a catalyst introduces specific environmental considerations. While it may offer advantages in terms of CNT growth efficiency, the production and disposal of magnesium nitride can have environmental consequences. The mining and processing of magnesium compounds can lead to habitat disruption and potential soil and water contamination if not properly managed.
Chemical precursors used in CNT synthesis, such as hydrocarbon feedstocks, can pose risks to air and water quality if released into the environment. Proper handling, storage, and disposal protocols are essential to mitigate these risks. Additionally, the potential for nanoparticle release during production and handling raises concerns about worker safety and environmental exposure.
The disposal and end-of-life management of CNT-containing products present another set of environmental challenges. The persistence of CNTs in the environment and their potential for bioaccumulation in organisms are areas of ongoing research and concern. Proper recycling and disposal methods need to be developed to prevent the release of CNTs into ecosystems.
However, it is important to note that CNTs also offer potential environmental benefits. Their unique properties can lead to more efficient and lightweight materials, potentially reducing energy consumption in various applications. For instance, CNT-enhanced composites in aerospace and automotive industries can contribute to fuel efficiency improvements.
The environmental impact of CNT production using magnesium nitride as a catalyst must be evaluated in a holistic manner, considering both the potential risks and benefits. Ongoing research into more sustainable synthesis methods, including the use of renewable energy sources and the development of closed-loop production systems, is crucial for minimizing the environmental footprint of CNT manufacturing.
As the technology advances, it is imperative to conduct comprehensive life cycle assessments to fully understand and quantify the environmental impacts associated with CNT production. This will enable the development of more sustainable production practices and inform policy decisions regarding the regulation and management of CNT manufacturing processes.
Scalability of Mg3N2-CNT Synthesis
The scalability of Mg3N2-CNT synthesis is a critical factor in determining the potential for large-scale production and industrial applications of carbon nanotubes grown using magnesium nitride as a catalyst. Current research indicates promising results in laboratory settings, but several challenges must be addressed to achieve commercial viability.
One of the primary advantages of using Mg3N2 as a catalyst is its relatively low cost compared to traditional metal catalysts like iron or nickel. This cost-effectiveness could potentially translate into more economical large-scale production processes. Additionally, magnesium nitride's ability to produce high-quality, well-aligned carbon nanotubes with controlled diameters suggests that it may be suitable for applications requiring precise nanotube properties.
However, scaling up the synthesis process presents several technical hurdles. The reaction conditions, including temperature and pressure, must be carefully controlled to maintain consistent nanotube quality across larger production volumes. This may require the development of specialized equipment capable of maintaining uniform conditions over larger reaction areas.
Another challenge lies in the preparation and handling of magnesium nitride. As a moisture-sensitive compound, it requires careful storage and processing to prevent degradation. Scaling up production would necessitate the implementation of robust handling protocols and potentially the design of specialized equipment to maintain an inert atmosphere during the entire synthesis process.
The yield and efficiency of the Mg3N2-CNT synthesis process at larger scales also require further investigation. While laboratory-scale experiments have shown promising results, it remains to be seen whether these can be replicated in industrial settings without compromising nanotube quality or increasing production costs significantly.
Environmental considerations must also be taken into account when scaling up the synthesis process. The potential release of nitrogen-containing compounds during the reaction may require the implementation of additional safety measures and emission control systems in large-scale production facilities.
Despite these challenges, the potential benefits of scaling up Mg3N2-CNT synthesis are significant. If successful, it could lead to more cost-effective production of high-quality carbon nanotubes, opening up new possibilities for their application in various industries, including electronics, energy storage, and advanced materials.
To fully assess the scalability of this synthesis method, further research and development efforts are needed. These should focus on optimizing reaction conditions for larger-scale production, developing specialized equipment for handling magnesium nitride, and conducting comprehensive cost-benefit analyses of industrial-scale implementation.
One of the primary advantages of using Mg3N2 as a catalyst is its relatively low cost compared to traditional metal catalysts like iron or nickel. This cost-effectiveness could potentially translate into more economical large-scale production processes. Additionally, magnesium nitride's ability to produce high-quality, well-aligned carbon nanotubes with controlled diameters suggests that it may be suitable for applications requiring precise nanotube properties.
However, scaling up the synthesis process presents several technical hurdles. The reaction conditions, including temperature and pressure, must be carefully controlled to maintain consistent nanotube quality across larger production volumes. This may require the development of specialized equipment capable of maintaining uniform conditions over larger reaction areas.
Another challenge lies in the preparation and handling of magnesium nitride. As a moisture-sensitive compound, it requires careful storage and processing to prevent degradation. Scaling up production would necessitate the implementation of robust handling protocols and potentially the design of specialized equipment to maintain an inert atmosphere during the entire synthesis process.
The yield and efficiency of the Mg3N2-CNT synthesis process at larger scales also require further investigation. While laboratory-scale experiments have shown promising results, it remains to be seen whether these can be replicated in industrial settings without compromising nanotube quality or increasing production costs significantly.
Environmental considerations must also be taken into account when scaling up the synthesis process. The potential release of nitrogen-containing compounds during the reaction may require the implementation of additional safety measures and emission control systems in large-scale production facilities.
Despite these challenges, the potential benefits of scaling up Mg3N2-CNT synthesis are significant. If successful, it could lead to more cost-effective production of high-quality carbon nanotubes, opening up new possibilities for their application in various industries, including electronics, energy storage, and advanced materials.
To fully assess the scalability of this synthesis method, further research and development efforts are needed. These should focus on optimizing reaction conditions for larger-scale production, developing specialized equipment for handling magnesium nitride, and conducting comprehensive cost-benefit analyses of industrial-scale implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!