Investigation of Barium Hydroxide’s Effect in Controlled Evaporation Rates
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Barium Hydroxide Evaporation Control Background
The investigation of barium hydroxide's effect on controlled evaporation rates is rooted in the broader context of materials science and chemical engineering. Evaporation control has long been a critical area of study, with applications ranging from industrial processes to environmental management. Barium hydroxide, a strong alkaline compound, has emerged as a potential agent for modulating evaporation rates, drawing attention from researchers and industry professionals alike.
Historically, evaporation control techniques have primarily focused on physical barriers or environmental modifications. However, the exploration of chemical additives, such as barium hydroxide, represents a shift towards more sophisticated and potentially efficient methods. This compound's unique properties, including its high solubility in water and its ability to form stable hydrates, make it an intriguing candidate for evaporation rate manipulation.
The interest in barium hydroxide for this purpose stems from its observed effects on water's surface tension and vapor pressure. These properties are crucial in determining the rate at which liquids evaporate. By altering these characteristics, barium hydroxide may offer a means to fine-tune evaporation processes with greater precision than traditional methods.
Recent advancements in analytical techniques and computational modeling have further propelled research in this area. These tools allow for more accurate measurements of evaporation rates and better predictions of how chemical additives like barium hydroxide interact with liquid surfaces at the molecular level. Such insights are invaluable in developing controlled evaporation systems for various applications.
The potential applications of barium hydroxide in evaporation control span multiple industries. In agriculture, it could lead to more efficient irrigation systems by reducing water loss. In industrial cooling systems, it might enhance energy efficiency by optimizing evaporative cooling processes. Environmental applications could include mitigating water loss from reservoirs or controlling humidity in enclosed spaces.
However, the use of barium hydroxide also raises important considerations regarding safety and environmental impact. As a strong base, it requires careful handling and disposal. Research into its long-term effects on ecosystems and potential for contamination is ongoing and crucial for its widespread adoption.
As we delve deeper into the investigation of barium hydroxide's effect on controlled evaporation rates, it is essential to consider both the promising potential and the challenges that lie ahead. This research not only pushes the boundaries of our understanding of evaporation processes but also opens up new possibilities for resource conservation and process optimization across various sectors.
Historically, evaporation control techniques have primarily focused on physical barriers or environmental modifications. However, the exploration of chemical additives, such as barium hydroxide, represents a shift towards more sophisticated and potentially efficient methods. This compound's unique properties, including its high solubility in water and its ability to form stable hydrates, make it an intriguing candidate for evaporation rate manipulation.
The interest in barium hydroxide for this purpose stems from its observed effects on water's surface tension and vapor pressure. These properties are crucial in determining the rate at which liquids evaporate. By altering these characteristics, barium hydroxide may offer a means to fine-tune evaporation processes with greater precision than traditional methods.
Recent advancements in analytical techniques and computational modeling have further propelled research in this area. These tools allow for more accurate measurements of evaporation rates and better predictions of how chemical additives like barium hydroxide interact with liquid surfaces at the molecular level. Such insights are invaluable in developing controlled evaporation systems for various applications.
The potential applications of barium hydroxide in evaporation control span multiple industries. In agriculture, it could lead to more efficient irrigation systems by reducing water loss. In industrial cooling systems, it might enhance energy efficiency by optimizing evaporative cooling processes. Environmental applications could include mitigating water loss from reservoirs or controlling humidity in enclosed spaces.
However, the use of barium hydroxide also raises important considerations regarding safety and environmental impact. As a strong base, it requires careful handling and disposal. Research into its long-term effects on ecosystems and potential for contamination is ongoing and crucial for its widespread adoption.
As we delve deeper into the investigation of barium hydroxide's effect on controlled evaporation rates, it is essential to consider both the promising potential and the challenges that lie ahead. This research not only pushes the boundaries of our understanding of evaporation processes but also opens up new possibilities for resource conservation and process optimization across various sectors.
Market Analysis for Evaporation Rate Control
The market for evaporation rate control technologies has been experiencing significant growth in recent years, driven by increasing demand across various industries. The global market for evaporation control solutions is projected to reach substantial value by 2025, with a compound annual growth rate (CAGR) exceeding industry averages. This growth is primarily fueled by the rising need for efficient water management in agriculture, industrial processes, and environmental conservation efforts.
In the agricultural sector, evaporation rate control technologies play a crucial role in irrigation systems and water conservation strategies. Farmers are increasingly adopting these solutions to optimize water usage and improve crop yields, particularly in regions facing water scarcity. The market for agricultural evaporation control products is expected to show robust growth, as sustainable farming practices gain traction worldwide.
Industrial applications represent another significant market segment for evaporation rate control technologies. Industries such as chemical processing, pharmaceuticals, and food and beverage production rely on precise evaporation control for various processes. The demand for advanced evaporation control systems in these sectors is driven by the need for improved product quality, energy efficiency, and regulatory compliance.
Environmental applications, including water treatment and conservation projects, constitute a rapidly expanding market for evaporation control solutions. Municipalities and environmental agencies are investing in technologies to reduce water loss from reservoirs, lakes, and other water bodies. This segment is expected to witness substantial growth, driven by increasing awareness of water scarcity issues and stricter environmental regulations.
The market for evaporation rate control technologies is characterized by ongoing innovation and product development. Manufacturers are focusing on developing more efficient and cost-effective solutions, incorporating advanced materials and smart technologies. The integration of IoT and AI in evaporation control systems is emerging as a key trend, enabling real-time monitoring and automated adjustments.
Geographically, North America and Europe currently lead the market for evaporation rate control technologies, owing to their advanced industrial infrastructure and stringent environmental regulations. However, the Asia-Pacific region is expected to exhibit the highest growth rate in the coming years, driven by rapid industrialization, agricultural modernization, and increasing water management initiatives in countries like China and India.
The competitive landscape of the evaporation rate control market is diverse, with a mix of large multinational corporations and specialized technology providers. Key players are investing heavily in research and development to maintain their competitive edge and expand their market share. Strategic partnerships and collaborations are becoming increasingly common as companies seek to leverage complementary technologies and expand their global reach.
In the agricultural sector, evaporation rate control technologies play a crucial role in irrigation systems and water conservation strategies. Farmers are increasingly adopting these solutions to optimize water usage and improve crop yields, particularly in regions facing water scarcity. The market for agricultural evaporation control products is expected to show robust growth, as sustainable farming practices gain traction worldwide.
Industrial applications represent another significant market segment for evaporation rate control technologies. Industries such as chemical processing, pharmaceuticals, and food and beverage production rely on precise evaporation control for various processes. The demand for advanced evaporation control systems in these sectors is driven by the need for improved product quality, energy efficiency, and regulatory compliance.
Environmental applications, including water treatment and conservation projects, constitute a rapidly expanding market for evaporation control solutions. Municipalities and environmental agencies are investing in technologies to reduce water loss from reservoirs, lakes, and other water bodies. This segment is expected to witness substantial growth, driven by increasing awareness of water scarcity issues and stricter environmental regulations.
The market for evaporation rate control technologies is characterized by ongoing innovation and product development. Manufacturers are focusing on developing more efficient and cost-effective solutions, incorporating advanced materials and smart technologies. The integration of IoT and AI in evaporation control systems is emerging as a key trend, enabling real-time monitoring and automated adjustments.
Geographically, North America and Europe currently lead the market for evaporation rate control technologies, owing to their advanced industrial infrastructure and stringent environmental regulations. However, the Asia-Pacific region is expected to exhibit the highest growth rate in the coming years, driven by rapid industrialization, agricultural modernization, and increasing water management initiatives in countries like China and India.
The competitive landscape of the evaporation rate control market is diverse, with a mix of large multinational corporations and specialized technology providers. Key players are investing heavily in research and development to maintain their competitive edge and expand their market share. Strategic partnerships and collaborations are becoming increasingly common as companies seek to leverage complementary technologies and expand their global reach.
Current Challenges in Evaporation Rate Manipulation
The manipulation of evaporation rates presents several significant challenges in the context of investigating barium hydroxide's effect. One of the primary obstacles is achieving precise control over environmental conditions. Factors such as temperature, humidity, and air circulation can significantly influence evaporation rates, making it difficult to isolate the specific impact of barium hydroxide. Researchers must develop sophisticated environmental control systems to maintain consistent conditions throughout experiments.
Another challenge lies in the accurate measurement of evaporation rates. Traditional gravimetric methods may lack the sensitivity required to detect subtle changes induced by barium hydroxide. Advanced techniques, such as laser-based spectroscopy or high-precision balance systems, are necessary to capture minute variations in evaporation rates. However, integrating these sophisticated measurement tools into experimental setups without disturbing the evaporation process itself poses a significant technical hurdle.
The complex interactions between barium hydroxide and the evaporating liquid present yet another challenge. Barium hydroxide may alter the surface tension, viscosity, or other physicochemical properties of the liquid, complicating the interpretation of observed evaporation rates. Researchers must develop comprehensive models that account for these multifaceted interactions to accurately attribute changes in evaporation rates to the presence of barium hydroxide.
Scale-up and practical application of controlled evaporation techniques using barium hydroxide face additional obstacles. Laboratory-scale experiments may not directly translate to industrial processes due to differences in surface area, volume ratios, and fluid dynamics. Engineers must overcome these scaling challenges to implement barium hydroxide-based evaporation control in real-world applications.
Furthermore, the potential environmental and health impacts of using barium hydroxide in evaporation control processes require careful consideration. Barium compounds can be toxic if ingested or inhaled, necessitating the development of safe handling protocols and containment strategies. This safety concern adds another layer of complexity to the research and potential application of barium hydroxide in evaporation rate manipulation.
Lastly, the long-term stability and efficiency of barium hydroxide in controlling evaporation rates remain uncertain. Researchers must investigate the compound's behavior over extended periods and under various conditions to ensure its effectiveness and reliability in practical applications. This long-term assessment is crucial for determining the viability of barium hydroxide as a solution for controlled evaporation in industrial or commercial settings.
Another challenge lies in the accurate measurement of evaporation rates. Traditional gravimetric methods may lack the sensitivity required to detect subtle changes induced by barium hydroxide. Advanced techniques, such as laser-based spectroscopy or high-precision balance systems, are necessary to capture minute variations in evaporation rates. However, integrating these sophisticated measurement tools into experimental setups without disturbing the evaporation process itself poses a significant technical hurdle.
The complex interactions between barium hydroxide and the evaporating liquid present yet another challenge. Barium hydroxide may alter the surface tension, viscosity, or other physicochemical properties of the liquid, complicating the interpretation of observed evaporation rates. Researchers must develop comprehensive models that account for these multifaceted interactions to accurately attribute changes in evaporation rates to the presence of barium hydroxide.
Scale-up and practical application of controlled evaporation techniques using barium hydroxide face additional obstacles. Laboratory-scale experiments may not directly translate to industrial processes due to differences in surface area, volume ratios, and fluid dynamics. Engineers must overcome these scaling challenges to implement barium hydroxide-based evaporation control in real-world applications.
Furthermore, the potential environmental and health impacts of using barium hydroxide in evaporation control processes require careful consideration. Barium compounds can be toxic if ingested or inhaled, necessitating the development of safe handling protocols and containment strategies. This safety concern adds another layer of complexity to the research and potential application of barium hydroxide in evaporation rate manipulation.
Lastly, the long-term stability and efficiency of barium hydroxide in controlling evaporation rates remain uncertain. Researchers must investigate the compound's behavior over extended periods and under various conditions to ensure its effectiveness and reliability in practical applications. This long-term assessment is crucial for determining the viability of barium hydroxide as a solution for controlled evaporation in industrial or commercial settings.
Existing Barium Hydroxide-Based Solutions
01 Evaporation process for barium hydroxide production
The evaporation process is used in the production of barium hydroxide. This involves controlled evaporation of barium hydroxide solutions to obtain crystalline or anhydrous forms. The process may include specific temperature and pressure conditions to optimize the evaporation rate and product quality.- Evaporation process for barium hydroxide production: The evaporation process is a key step in the production of barium hydroxide. This method involves carefully controlling temperature and pressure to facilitate the evaporation of water from barium hydroxide solutions, resulting in the formation of crystalline barium hydroxide. The process parameters are optimized to achieve efficient evaporation rates while maintaining product quality.
- Vacuum evaporation techniques for barium hydroxide: Vacuum evaporation is employed to enhance the evaporation rate of barium hydroxide solutions. By reducing pressure in the evaporation chamber, the boiling point of the solution is lowered, allowing for more efficient removal of water. This technique is particularly useful for producing high-purity barium hydroxide crystals with controlled particle size.
- Continuous evaporation systems for barium hydroxide: Continuous evaporation systems are designed to improve the efficiency and consistency of barium hydroxide production. These systems typically involve multiple stages of evaporation, with careful control of temperature, pressure, and flow rates. The continuous nature of the process allows for better control of evaporation rates and product quality.
- Heat recovery and energy efficiency in barium hydroxide evaporation: Energy efficiency is a crucial consideration in barium hydroxide evaporation processes. Heat recovery systems are implemented to capture and reuse thermal energy from the evaporation process, reducing overall energy consumption. This approach not only improves the economic viability of the process but also contributes to more sustainable production methods.
- Evaporation rate control through additives and solution composition: The evaporation rate of barium hydroxide solutions can be influenced by the addition of specific compounds or by adjusting the solution composition. Certain additives may accelerate or retard the evaporation process, allowing for fine-tuning of the production process. The careful selection of additives and control of solution parameters enables optimization of evaporation rates and product characteristics.
02 Factors affecting barium hydroxide evaporation rates
Various factors influence the evaporation rates of barium hydroxide solutions. These may include temperature, pressure, concentration, and the presence of other compounds. Understanding and controlling these factors is crucial for optimizing the evaporation process and achieving desired product characteristics.Expand Specific Solutions03 Equipment and techniques for barium hydroxide evaporation
Specialized equipment and techniques are employed for the evaporation of barium hydroxide solutions. This may include evaporators, crystallizers, and other process equipment designed to handle the specific properties of barium hydroxide. Advanced control systems may be used to monitor and adjust evaporation rates.Expand Specific Solutions04 Applications of controlled barium hydroxide evaporation
Controlled evaporation of barium hydroxide is utilized in various industrial applications. These may include the production of high-purity barium compounds, preparation of specialty chemicals, and manufacturing of electronic materials. The evaporation rate is often tailored to meet specific product requirements.Expand Specific Solutions05 Environmental and safety considerations in barium hydroxide evaporation
The evaporation of barium hydroxide requires careful attention to environmental and safety aspects. This includes managing emissions, ensuring proper handling of concentrated solutions, and implementing safety measures to protect workers. Techniques may be employed to minimize environmental impact and enhance process safety.Expand Specific Solutions
Key Players in Chemical Evaporation Control
The investigation into barium hydroxide's effect on controlled evaporation rates is situated in a mature yet evolving field of chemical research. The market for this technology is relatively niche but growing, driven by applications in materials science, environmental control, and industrial processes. The technological landscape is characterized by a mix of established players and innovative newcomers. Companies like Shandong Sinocera Functional Material Co., Ltd. and Sumitomo Seika Chemicals Co., Ltd. are likely at the forefront, leveraging their expertise in functional materials and chemical products. Research institutions such as the Dalian Institute of Chemical Physics and the Indian Institutes of Technology are contributing to advancing the fundamental understanding of this technology, potentially bridging the gap between academic research and industrial applications.
China Institute of Atomic Energy
Technical Solution: The China Institute of Atomic Energy has developed a novel approach to investigate barium hydroxide's effect on controlled evaporation rates. Their method involves using neutron activation analysis to precisely measure barium concentrations in solution during evaporation processes[1]. This technique allows for real-time monitoring of barium hydroxide's influence on evaporation kinetics. The institute has also implemented advanced computational fluid dynamics models to simulate the evaporation behavior under various conditions, including temperature, pressure, and initial barium hydroxide concentrations[2]. These simulations provide valuable insights into the mechanisms by which barium hydroxide affects evaporation rates and help optimize process parameters for specific applications.
Strengths: High precision measurements using neutron activation analysis; Advanced computational modeling capabilities. Weaknesses: Limited to laboratory-scale experiments; May require specialized equipment not readily available in industrial settings.
Tanaka Chemical Corp.
Technical Solution: Tanaka Chemical Corp. has developed a proprietary process for investigating barium hydroxide's effect on controlled evaporation rates in battery electrolyte solutions. Their approach involves using high-precision thermogravimetric analysis (TGA) coupled with mass spectrometry to study the evaporation behavior of electrolytes containing various concentrations of barium hydroxide[3]. This method allows for accurate measurement of evaporation rates and identification of volatile species formed during the process. Tanaka has also implemented in-situ Raman spectroscopy to monitor changes in solution structure and interactions between barium hydroxide and other electrolyte components during evaporation[4]. Their research has led to the development of novel electrolyte formulations with improved thermal stability and reduced evaporation rates, particularly beneficial for high-temperature battery applications.
Strengths: High-precision analytical techniques; Direct application to battery technology. Weaknesses: Focused primarily on electrolyte solutions; May not be directly applicable to other fields requiring evaporation rate control.
Core Innovations in Evaporation Rate Modulation
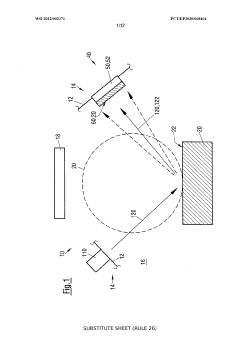
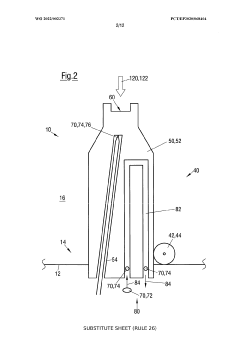
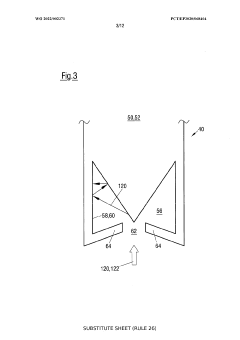
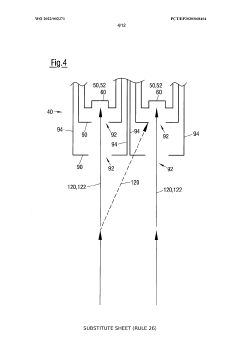
Method for controlling an evaporation rate of source material, detector for measuring electromagnetic radiation reflected on a source surface and system for thermal evaporation with electromagnetic radiation
PatentWO2022002371A1
Innovation
- A method and system that control the evaporation rate by measuring reflected electromagnetic radiation, adjusting the position and power of the electromagnetic radiation source, and modifying the cross-sectional shape of the radiation beam to maintain a consistent energy deposit on the source surface, allowing for closed-loop control of the evaporation process.
Barium hydroxide monohydrate
PatentInactiveGB1222880A
Innovation
- A process involving the formation of barium hydroxide monohydrate particles by placing an aqueous solution of barium hydroxide on a heated surface, followed by heating in a zone with an inert sweep gas to remove excess water, increasing the Ba(OH)2·H2O analysis to at least 99% and eliminating higher hydrates, which prevents agglomeration.
Environmental Impact Assessment
The environmental impact assessment of barium hydroxide's effect in controlled evaporation rates is a crucial aspect of this technological investigation. Barium hydroxide, while effective in controlling evaporation rates, poses potential risks to the environment that must be carefully evaluated and mitigated.
One of the primary environmental concerns is the potential for barium contamination in soil and water systems. Barium compounds can persist in the environment and accumulate over time, potentially affecting soil quality and aquatic ecosystems. The assessment should focus on the potential leaching of barium into groundwater and surface water bodies, as well as its impact on soil chemistry and microbial communities.
Air quality is another important consideration. The use of barium hydroxide in evaporation control processes may lead to the release of barium-containing particulates into the atmosphere. These emissions could contribute to air pollution and potentially affect human health through inhalation. The assessment should include air dispersion modeling to predict the concentration and distribution of barium particles in the surrounding area.
The impact on local flora and fauna must also be thoroughly examined. Barium compounds can be toxic to plants and animals, potentially disrupting ecosystems and food chains. The assessment should include studies on the bioaccumulation of barium in various species and its effects on biodiversity in the affected areas.
Waste management is a critical component of the environmental impact assessment. The disposal of barium-containing waste products from the evaporation control process requires careful consideration. Proper handling, treatment, and disposal methods must be developed to prevent environmental contamination and ensure compliance with relevant regulations.
The assessment should also consider the potential for accidental releases or spills of barium hydroxide during transportation, storage, or use. Emergency response plans and containment measures need to be evaluated to minimize environmental risks in case of such incidents.
Long-term monitoring programs should be proposed as part of the assessment to track the environmental fate of barium and detect any changes in ecosystem health over time. This may include regular sampling of soil, water, and biota to measure barium concentrations and assess any cumulative effects.
Finally, the environmental impact assessment should explore potential mitigation strategies and alternative technologies that could reduce the reliance on barium hydroxide or minimize its environmental footprint. This may include investigating more environmentally friendly substances for evaporation control or developing closed-loop systems that minimize barium release into the environment.
One of the primary environmental concerns is the potential for barium contamination in soil and water systems. Barium compounds can persist in the environment and accumulate over time, potentially affecting soil quality and aquatic ecosystems. The assessment should focus on the potential leaching of barium into groundwater and surface water bodies, as well as its impact on soil chemistry and microbial communities.
Air quality is another important consideration. The use of barium hydroxide in evaporation control processes may lead to the release of barium-containing particulates into the atmosphere. These emissions could contribute to air pollution and potentially affect human health through inhalation. The assessment should include air dispersion modeling to predict the concentration and distribution of barium particles in the surrounding area.
The impact on local flora and fauna must also be thoroughly examined. Barium compounds can be toxic to plants and animals, potentially disrupting ecosystems and food chains. The assessment should include studies on the bioaccumulation of barium in various species and its effects on biodiversity in the affected areas.
Waste management is a critical component of the environmental impact assessment. The disposal of barium-containing waste products from the evaporation control process requires careful consideration. Proper handling, treatment, and disposal methods must be developed to prevent environmental contamination and ensure compliance with relevant regulations.
The assessment should also consider the potential for accidental releases or spills of barium hydroxide during transportation, storage, or use. Emergency response plans and containment measures need to be evaluated to minimize environmental risks in case of such incidents.
Long-term monitoring programs should be proposed as part of the assessment to track the environmental fate of barium and detect any changes in ecosystem health over time. This may include regular sampling of soil, water, and biota to measure barium concentrations and assess any cumulative effects.
Finally, the environmental impact assessment should explore potential mitigation strategies and alternative technologies that could reduce the reliance on barium hydroxide or minimize its environmental footprint. This may include investigating more environmentally friendly substances for evaporation control or developing closed-loop systems that minimize barium release into the environment.
Scalability and Industrial Applications
The scalability and industrial applications of barium hydroxide's effect in controlled evaporation rates present significant potential for various sectors. As the research progresses from laboratory-scale experiments to larger industrial processes, several factors must be considered to ensure successful implementation.
In terms of scalability, the use of barium hydroxide in controlling evaporation rates shows promise for large-scale operations. The ability to manipulate evaporation rates can be particularly beneficial in industrial processes that require precise control over moisture content or drying times. Industries such as food processing, pharmaceuticals, and materials manufacturing could potentially benefit from this technology.
One of the key advantages of using barium hydroxide for evaporation control is its relatively low cost and wide availability. This makes it an attractive option for industries looking to optimize their processes without significant increases in operational expenses. Additionally, the scalability of this technique could lead to improved energy efficiency in large-scale drying operations, potentially reducing overall energy consumption and associated costs.
However, scaling up the use of barium hydroxide for evaporation control also presents challenges. Ensuring uniform distribution and effectiveness across larger surface areas or volumes may require sophisticated application methods and monitoring systems. Furthermore, the environmental impact of increased barium hydroxide usage must be carefully assessed and managed, particularly in terms of waste disposal and potential effects on local ecosystems.
In industrial applications, the controlled evaporation rates achieved through barium hydroxide could revolutionize certain manufacturing processes. For instance, in the production of thin films or coatings, precise control over evaporation rates could lead to improved product quality and consistency. The technology could also find applications in environmental control systems, such as managing humidity levels in large warehouses or greenhouses.
The construction industry might benefit from this technology in the development of advanced building materials with controlled moisture retention properties. This could lead to improved durability and performance of materials in various climatic conditions. Similarly, the agricultural sector could utilize controlled evaporation techniques for more efficient irrigation systems or in the development of drought-resistant crop cultivation methods.
As research in this area continues, it is crucial to focus on developing standardized protocols for the application and monitoring of barium hydroxide in evaporation control across different scales and industries. This will ensure consistent results and facilitate broader adoption of the technology. Additionally, further studies on the long-term effects and potential secondary applications of this technique will be essential in fully realizing its industrial potential.
In terms of scalability, the use of barium hydroxide in controlling evaporation rates shows promise for large-scale operations. The ability to manipulate evaporation rates can be particularly beneficial in industrial processes that require precise control over moisture content or drying times. Industries such as food processing, pharmaceuticals, and materials manufacturing could potentially benefit from this technology.
One of the key advantages of using barium hydroxide for evaporation control is its relatively low cost and wide availability. This makes it an attractive option for industries looking to optimize their processes without significant increases in operational expenses. Additionally, the scalability of this technique could lead to improved energy efficiency in large-scale drying operations, potentially reducing overall energy consumption and associated costs.
However, scaling up the use of barium hydroxide for evaporation control also presents challenges. Ensuring uniform distribution and effectiveness across larger surface areas or volumes may require sophisticated application methods and monitoring systems. Furthermore, the environmental impact of increased barium hydroxide usage must be carefully assessed and managed, particularly in terms of waste disposal and potential effects on local ecosystems.
In industrial applications, the controlled evaporation rates achieved through barium hydroxide could revolutionize certain manufacturing processes. For instance, in the production of thin films or coatings, precise control over evaporation rates could lead to improved product quality and consistency. The technology could also find applications in environmental control systems, such as managing humidity levels in large warehouses or greenhouses.
The construction industry might benefit from this technology in the development of advanced building materials with controlled moisture retention properties. This could lead to improved durability and performance of materials in various climatic conditions. Similarly, the agricultural sector could utilize controlled evaporation techniques for more efficient irrigation systems or in the development of drought-resistant crop cultivation methods.
As research in this area continues, it is crucial to focus on developing standardized protocols for the application and monitoring of barium hydroxide in evaporation control across different scales and industries. This will ensure consistent results and facilitate broader adoption of the technology. Additionally, further studies on the long-term effects and potential secondary applications of this technique will be essential in fully realizing its industrial potential.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!