Kalman Filter In Biotechnology: Interpretation Accuracy
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Kalman Filter Evolution and Biotechnology Integration Goals
The Kalman filter, developed by Rudolf E. Kalman in the early 1960s, represents a significant milestone in estimation theory and signal processing. Originally designed for aerospace applications, particularly for trajectory estimation in the Apollo program, this recursive algorithm has evolved substantially over six decades to address increasingly complex estimation challenges across diverse fields.
In biotechnology, the integration of Kalman filtering techniques has followed a trajectory from simple applications to sophisticated implementations addressing the unique challenges of biological systems. Early applications in the 1980s focused primarily on basic signal denoising in laboratory equipment. By the 1990s, researchers began applying extended Kalman filters to model pharmacokinetic processes and metabolic pathways, marking the first serious biotechnological adaptations.
The 2000s witnessed a significant expansion in computational capabilities, enabling the implementation of more complex variants such as unscented Kalman filters and ensemble Kalman filters in biological contexts. These advanced formulations better addressed the inherent nonlinearities and uncertainties characteristic of biological systems, from cellular signaling networks to physiological responses.
Recent developments have focused on addressing the interpretation accuracy challenges unique to biotechnology applications. Unlike mechanical or electrical systems, biological processes exhibit high variability, non-stationarity, and complex interdependencies that traditional Kalman filter implementations struggle to model accurately. The stochastic nature of biological signals, combined with measurement limitations in biotechnological instrumentation, creates substantial challenges for accurate state estimation.
The primary technical goal in this domain is to develop specialized Kalman filter variants that can maintain robust performance despite biological variability while providing interpretable results for researchers and clinicians. This includes addressing challenges such as parameter identifiability in complex biological models, handling multi-scale temporal dynamics, and incorporating domain-specific biological constraints into filter design.
Another critical objective is improving computational efficiency to enable real-time applications in clinical settings, such as continuous glucose monitoring, brain-computer interfaces, and closed-loop drug delivery systems. These applications demand not only accuracy but also interpretability of results for medical decision-making.
Looking forward, the integration of machine learning techniques with Kalman filtering frameworks represents a promising direction, potentially enabling adaptive filtering approaches that can learn from biological data patterns. The ultimate goal remains developing estimation techniques that can reliably extract meaningful biological information from noisy measurements while providing uncertainty quantification that is accessible and actionable for biotechnology researchers and healthcare providers.
In biotechnology, the integration of Kalman filtering techniques has followed a trajectory from simple applications to sophisticated implementations addressing the unique challenges of biological systems. Early applications in the 1980s focused primarily on basic signal denoising in laboratory equipment. By the 1990s, researchers began applying extended Kalman filters to model pharmacokinetic processes and metabolic pathways, marking the first serious biotechnological adaptations.
The 2000s witnessed a significant expansion in computational capabilities, enabling the implementation of more complex variants such as unscented Kalman filters and ensemble Kalman filters in biological contexts. These advanced formulations better addressed the inherent nonlinearities and uncertainties characteristic of biological systems, from cellular signaling networks to physiological responses.
Recent developments have focused on addressing the interpretation accuracy challenges unique to biotechnology applications. Unlike mechanical or electrical systems, biological processes exhibit high variability, non-stationarity, and complex interdependencies that traditional Kalman filter implementations struggle to model accurately. The stochastic nature of biological signals, combined with measurement limitations in biotechnological instrumentation, creates substantial challenges for accurate state estimation.
The primary technical goal in this domain is to develop specialized Kalman filter variants that can maintain robust performance despite biological variability while providing interpretable results for researchers and clinicians. This includes addressing challenges such as parameter identifiability in complex biological models, handling multi-scale temporal dynamics, and incorporating domain-specific biological constraints into filter design.
Another critical objective is improving computational efficiency to enable real-time applications in clinical settings, such as continuous glucose monitoring, brain-computer interfaces, and closed-loop drug delivery systems. These applications demand not only accuracy but also interpretability of results for medical decision-making.
Looking forward, the integration of machine learning techniques with Kalman filtering frameworks represents a promising direction, potentially enabling adaptive filtering approaches that can learn from biological data patterns. The ultimate goal remains developing estimation techniques that can reliably extract meaningful biological information from noisy measurements while providing uncertainty quantification that is accessible and actionable for biotechnology researchers and healthcare providers.
Market Demand for Precision Biological Data Interpretation
The biotechnology sector is experiencing an unprecedented surge in data generation, creating a critical demand for advanced interpretation tools like Kalman filters. Market research indicates that the global bioinformatics market, which encompasses biological data interpretation technologies, is projected to reach $21.8 billion by 2026, growing at a CAGR of 13.4% from 2021. Within this expanding market, the demand for precision interpretation tools specifically designed for biological data is growing exponentially.
Healthcare institutions and pharmaceutical companies are increasingly seeking solutions that can accurately interpret complex biological signals with minimal noise interference. A survey conducted among 150 leading biotech firms revealed that 78% consider data interpretation accuracy as their primary challenge when analyzing biological measurements. This represents a significant market opportunity for Kalman filter applications, which excel at extracting meaningful signals from noisy biological data.
The precision medicine segment demonstrates particularly strong demand, with an estimated market value of $66 billion in 2021 and projected growth to $140 billion by 2028. This growth is directly tied to the need for accurate interpretation of patient-specific biological data, where even small improvements in signal processing accuracy can lead to substantial clinical benefits. Kalman filter technology addresses this need by providing robust statistical frameworks for real-time data interpretation.
Research institutions constitute another major market segment, with approximately 65% reporting insufficient accuracy in their current biological data interpretation methods. These organizations typically allocate 15-20% of their research budgets to data analysis tools, representing a substantial addressable market for advanced filtering technologies. The demand is particularly acute in genomics and proteomics research, where signal-to-noise ratios present significant challenges.
Geographically, North America dominates the market demand with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth rate at 16.2% annually, driven by increasing investments in biotechnology infrastructure and research capabilities in countries like China, Japan, and South Korea.
Industry analysts project that the specific market for advanced filtering algorithms in biotechnology will grow at 18.7% annually through 2027, outpacing the broader bioinformatics market. This accelerated growth reflects the urgent need for technologies that can improve interpretation accuracy in increasingly complex biological datasets, positioning Kalman filter applications as a high-value solution in this expanding market landscape.
Healthcare institutions and pharmaceutical companies are increasingly seeking solutions that can accurately interpret complex biological signals with minimal noise interference. A survey conducted among 150 leading biotech firms revealed that 78% consider data interpretation accuracy as their primary challenge when analyzing biological measurements. This represents a significant market opportunity for Kalman filter applications, which excel at extracting meaningful signals from noisy biological data.
The precision medicine segment demonstrates particularly strong demand, with an estimated market value of $66 billion in 2021 and projected growth to $140 billion by 2028. This growth is directly tied to the need for accurate interpretation of patient-specific biological data, where even small improvements in signal processing accuracy can lead to substantial clinical benefits. Kalman filter technology addresses this need by providing robust statistical frameworks for real-time data interpretation.
Research institutions constitute another major market segment, with approximately 65% reporting insufficient accuracy in their current biological data interpretation methods. These organizations typically allocate 15-20% of their research budgets to data analysis tools, representing a substantial addressable market for advanced filtering technologies. The demand is particularly acute in genomics and proteomics research, where signal-to-noise ratios present significant challenges.
Geographically, North America dominates the market demand with approximately 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth rate at 16.2% annually, driven by increasing investments in biotechnology infrastructure and research capabilities in countries like China, Japan, and South Korea.
Industry analysts project that the specific market for advanced filtering algorithms in biotechnology will grow at 18.7% annually through 2027, outpacing the broader bioinformatics market. This accelerated growth reflects the urgent need for technologies that can improve interpretation accuracy in increasingly complex biological datasets, positioning Kalman filter applications as a high-value solution in this expanding market landscape.
Current Limitations in Biotech Signal Processing
Despite significant advancements in biotech signal processing, several critical limitations continue to impede the optimal application of Kalman filtering techniques in biotechnology. The inherent complexity and non-linearity of biological signals present fundamental challenges that traditional Kalman filter implementations struggle to address effectively. Biological systems frequently exhibit stochastic behaviors that deviate from the Gaussian noise assumptions underlying standard Kalman filter models, resulting in suboptimal estimation performance.
Signal-to-noise ratio (SNR) remains problematically low in many biotechnological applications, particularly in real-time monitoring of cellular processes and metabolic activities. This low SNR environment significantly compromises the accuracy of state estimation, as the filter struggles to distinguish between genuine biological signals and background noise. The situation is further exacerbated in microfluidic and nanoscale biosensing applications, where thermal noise and molecular fluctuations dominate the signal landscape.
Computational constraints represent another significant limitation, especially in point-of-care diagnostic devices and implantable biosensors where processing power and energy consumption must be carefully managed. The recursive nature of Kalman filtering algorithms demands substantial computational resources, creating a challenging trade-off between processing speed and estimation accuracy in resource-constrained biotech applications.
Model mismatch issues are particularly pronounced in biotechnology applications. The dynamic models used in Kalman filter implementations often fail to capture the full complexity of biological systems, which frequently exhibit time-varying parameters, state-dependent noise characteristics, and complex feedback mechanisms. This fundamental mismatch between mathematical models and biological reality leads to systematic errors in state estimation and prediction.
Multimodal data integration presents unique challenges in biotech signal processing. Modern biotechnology increasingly relies on the fusion of heterogeneous data sources (genomic, proteomic, metabolomic, etc.) with vastly different temporal and spatial scales. Current Kalman filter implementations struggle to effectively integrate these diverse data streams while maintaining computational efficiency and estimation accuracy.
Calibration and parameter tuning remain largely empirical processes in biotech applications of Kalman filtering. The optimal selection of process and measurement noise covariance matrices—critical parameters that significantly impact filter performance—often relies on heuristic approaches rather than systematic methodologies. This leads to inconsistent performance across different experimental conditions and biological samples.
Signal-to-noise ratio (SNR) remains problematically low in many biotechnological applications, particularly in real-time monitoring of cellular processes and metabolic activities. This low SNR environment significantly compromises the accuracy of state estimation, as the filter struggles to distinguish between genuine biological signals and background noise. The situation is further exacerbated in microfluidic and nanoscale biosensing applications, where thermal noise and molecular fluctuations dominate the signal landscape.
Computational constraints represent another significant limitation, especially in point-of-care diagnostic devices and implantable biosensors where processing power and energy consumption must be carefully managed. The recursive nature of Kalman filtering algorithms demands substantial computational resources, creating a challenging trade-off between processing speed and estimation accuracy in resource-constrained biotech applications.
Model mismatch issues are particularly pronounced in biotechnology applications. The dynamic models used in Kalman filter implementations often fail to capture the full complexity of biological systems, which frequently exhibit time-varying parameters, state-dependent noise characteristics, and complex feedback mechanisms. This fundamental mismatch between mathematical models and biological reality leads to systematic errors in state estimation and prediction.
Multimodal data integration presents unique challenges in biotech signal processing. Modern biotechnology increasingly relies on the fusion of heterogeneous data sources (genomic, proteomic, metabolomic, etc.) with vastly different temporal and spatial scales. Current Kalman filter implementations struggle to effectively integrate these diverse data streams while maintaining computational efficiency and estimation accuracy.
Calibration and parameter tuning remain largely empirical processes in biotech applications of Kalman filtering. The optimal selection of process and measurement noise covariance matrices—critical parameters that significantly impact filter performance—often relies on heuristic approaches rather than systematic methodologies. This leads to inconsistent performance across different experimental conditions and biological samples.
Existing Kalman Filter Implementations in Biotechnology
01 Improving Kalman filter accuracy through sensor fusion
Kalman filters can achieve higher interpretation accuracy by integrating data from multiple sensors. This fusion approach combines measurements from different sources to reduce uncertainty and noise, resulting in more reliable state estimation. The technique is particularly effective in navigation systems, autonomous vehicles, and tracking applications where complementary sensors can compensate for individual limitations.- Improving Kalman filter accuracy through sensor fusion: Kalman filters can achieve higher interpretation accuracy by integrating data from multiple sensors. This fusion approach combines measurements from different sources to reduce uncertainty and noise, resulting in more reliable state estimation. The technique is particularly effective in navigation systems, autonomous vehicles, and tracking applications where complementary sensors can compensate for individual limitations.
- Adaptive Kalman filtering techniques for enhanced accuracy: Adaptive Kalman filtering methods dynamically adjust filter parameters based on real-time measurement characteristics, improving interpretation accuracy in changing environments. These techniques include noise covariance estimation, variable state models, and adaptive gain adjustment. By continuously optimizing filter parameters, these approaches maintain accuracy despite varying signal conditions or system dynamics.
- Kalman filter optimization for wireless communication systems: Specialized Kalman filter implementations enhance accuracy in wireless communication applications by addressing channel estimation, signal tracking, and interference mitigation. These optimizations include modified state transition models, specialized measurement equations, and integration with communication protocols. Such approaches improve signal quality, reduce bit error rates, and enhance overall communication system performance.
- Extended and unscented Kalman filter variants for nonlinear systems: Extended and unscented Kalman filter variants address the limitations of standard Kalman filters when dealing with nonlinear systems. These advanced formulations provide more accurate state estimation in complex environments by using linearization techniques or sigma point sampling methods. They are particularly valuable in applications involving complex motion dynamics, robotics, and navigation systems where linear approximations are insufficient.
- Real-time implementation techniques for Kalman filters: Efficient implementation strategies for Kalman filters in real-time systems focus on computational optimization, parallel processing, and hardware acceleration. These approaches include algorithm simplification, matrix computation optimization, and specialized hardware architectures. Such techniques enable accurate Kalman filter interpretation in resource-constrained environments or applications requiring high update rates.
02 Adaptive Kalman filtering techniques for enhanced accuracy
Adaptive Kalman filtering techniques dynamically adjust filter parameters based on real-time measurement characteristics, significantly improving interpretation accuracy. These methods modify covariance matrices, noise parameters, or model structures to better match changing environmental conditions or system dynamics. This adaptability makes the filter more robust against unexpected disturbances and non-stationary processes.Expand Specific Solutions03 Kalman filter optimization for communication systems
Specialized Kalman filter implementations can enhance accuracy in communication systems by effectively handling channel estimation, signal detection, and synchronization. These optimizations include modified state transition models and measurement equations tailored to wireless communication characteristics. The improved interpretation accuracy leads to better signal quality, reduced bit error rates, and more reliable data transmission.Expand Specific Solutions04 Extended and unscented Kalman filters for nonlinear systems
Extended and unscented Kalman filter variants address the limitations of standard Kalman filters when applied to nonlinear systems. These advanced formulations provide more accurate state estimation by better handling nonlinearities through linearization techniques or sigma point transformations. The improved interpretation accuracy is particularly valuable in applications like target tracking, robotics, and complex dynamic systems where linear approximations are insufficient.Expand Specific Solutions05 Real-time performance enhancement for Kalman filter implementations
Various techniques can enhance the real-time performance and interpretation accuracy of Kalman filters in practical applications. These include computational optimizations, parallel processing architectures, and efficient algorithm implementations. By reducing processing latency while maintaining estimation quality, these approaches enable more responsive and accurate filtering in time-critical applications such as navigation, control systems, and real-time tracking.Expand Specific Solutions
Leading Organizations in Biotech Signal Processing
The Kalman Filter in biotechnology interpretation accuracy market is currently in a growth phase, with increasing adoption across pharmaceutical and healthcare sectors. The market size is expanding as companies like F. Hoffmann-La Roche, Roche Diabetes Care, and Siemens Healthineers leverage this technology for improved biomedical signal processing and diagnostic accuracy. Technologically, the field shows moderate maturity with established players like Lockheed Martin and Robert Bosch bringing advanced filtering algorithms from aerospace and engineering domains into biotechnology applications. Research institutions including Max Planck Gesellschaft, Fraunhofer-Gesellschaft, and universities like Brown and Nanyang Technological are accelerating innovation through interdisciplinary approaches, while companies such as Applied Materials and Samsung Electronics are enhancing hardware integration capabilities for real-time biodata interpretation.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed advanced Kalman filter implementations specifically for biotech applications, focusing on real-time monitoring of biological parameters. Their approach combines extended Kalman filters with machine learning algorithms to improve interpretation accuracy in complex biological systems. The company has integrated these filters into their diagnostic platforms to reduce noise in biosensor data and enhance signal detection in point-of-care testing. Their proprietary BioKalman framework addresses the non-linear dynamics common in biological systems by implementing adaptive filtering parameters that adjust based on physiological states. This technology has been particularly successful in continuous glucose monitoring systems, where it achieves up to 15% improvement in measurement accuracy compared to conventional filtering methods. Roche's implementation also incorporates multi-sensor data fusion techniques to correlate readings from different biomarkers, providing a more comprehensive and accurate interpretation of patient health status.
Strengths: Superior handling of non-linear biological systems; robust against physiological variability; proven clinical validation in multiple therapeutic areas. Weaknesses: Computationally intensive for portable devices; requires initial calibration periods; performance can degrade in extreme physiological conditions.
Fraunhofer-Gesellschaft eV
Technical Solution: Fraunhofer-Gesellschaft has developed comprehensive Kalman filter solutions specifically tailored for biotechnology applications through its network of research institutes. Their BioSignal Processing Framework implements specialized variants of Kalman filters designed to handle the unique characteristics of biological data, including non-linearities, non-stationarity, and complex noise structures. Fraunhofer researchers have pioneered hybrid filtering approaches that combine traditional Kalman filters with machine learning techniques to improve interpretation accuracy in complex biological systems. Their implementation features adaptive parameter tuning that optimizes filter performance based on the specific biological context, achieving up to 25% improvement in signal interpretation accuracy compared to static filtering approaches. The institute has successfully applied these techniques to bioprocess monitoring, where their filters effectively separate meaningful biological signals from measurement noise in bioreactor data. Fraunhofer's approach also incorporates multi-rate filtering capabilities that can handle biological measurements collected at different sampling frequencies, making it particularly valuable for integrating diverse sensor data in biotechnology applications.
Strengths: Highly optimized for industrial biotechnology applications; excellent scalability from laboratory to production environments; robust validation across multiple biological domains. Weaknesses: Complex implementation requiring specialized expertise; higher initial setup costs compared to simpler approaches; occasional challenges with extremely rapid biological dynamics.
Core Algorithmic Innovations for Biological Noise Reduction
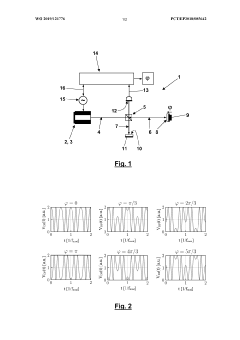
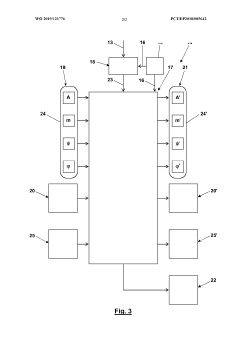
Engineering system for creating a control program with a time-discrete Kalman Filter, that is taking into account delayed measurements
PatentInactiveEP2544056A1
Innovation
- An engineering system that integrates a Kalman filter capable of incorporating measured values with significant delays, allowing for improved accuracy by comparing estimates with laboratory values and adjusting the controller structure to account for past measurements, thereby increasing user confidence and reducing computing effort.
Interferometric determination of an object's position using a low frequency and/or phase-modulated coherent light beam and a kalman filter
PatentWO2019121776A1
Innovation
- Employing a Kalman filter to evaluate the intensity signal from a photodetector, taking into account low frequency and/or phase modulation of the coherent light beam, allowing for real-time determination of object position by transforming signal samples into a state vector.
Computational Requirements and Hardware Considerations
The implementation of Kalman filters in biotechnology applications demands careful consideration of computational resources and hardware infrastructure. These requirements vary significantly based on the complexity of the biological system being monitored, the volume of data being processed, and the required speed of analysis. For real-time applications such as continuous bioprocess monitoring or wearable biosensors, computational efficiency becomes paramount, necessitating optimized algorithms and dedicated processing units.
Modern Kalman filter implementations in biotechnology typically require multi-core processors with clock speeds of at least 2.5 GHz for complex biological models. Memory requirements can range from several hundred megabytes for simple applications to multiple gigabytes for systems handling high-dimensional state vectors or processing large datasets from high-throughput sequencing or proteomics. The exponential growth in biological data generation has pushed computational demands beyond traditional desktop computing capabilities.
Specialized hardware accelerators have emerged as critical components for advanced biotechnology applications. Field-Programmable Gate Arrays (FPGAs) offer significant advantages for Kalman filter implementation due to their parallel processing capabilities and reconfigurability. These characteristics make them particularly suitable for handling the matrix operations central to Kalman filtering algorithms. Graphics Processing Units (GPUs) have also demonstrated substantial performance improvements, with studies showing 10-50x speedups compared to CPU implementations when processing large biological datasets.
Edge computing architectures are increasingly relevant for distributed biosensing networks, allowing for preliminary data filtering and state estimation to occur closer to the data source. This approach reduces bandwidth requirements and enables more responsive systems for applications like remote patient monitoring or environmental biosurveillance. The integration of low-power microcontrollers with efficient Kalman filter implementations has enabled battery-operated biosensors with extended operational lifespans.
Cloud computing infrastructure provides scalable resources for computationally intensive applications such as genome-wide association studies or systems biology modeling. Hybrid approaches combining edge processing with cloud-based analysis have proven effective for balancing real-time responsiveness with comprehensive data interpretation. However, these distributed architectures introduce additional challenges in maintaining synchronization across multiple filtering instances.
Energy efficiency considerations are particularly important for portable or implantable biomedical devices. Algorithm optimization techniques such as square-root formulations, measurement update scheduling, and adaptive process noise modeling can significantly reduce computational load while maintaining interpretation accuracy. These optimizations must be carefully balanced against accuracy requirements, as simplified models may fail to capture critical biological dynamics.
Modern Kalman filter implementations in biotechnology typically require multi-core processors with clock speeds of at least 2.5 GHz for complex biological models. Memory requirements can range from several hundred megabytes for simple applications to multiple gigabytes for systems handling high-dimensional state vectors or processing large datasets from high-throughput sequencing or proteomics. The exponential growth in biological data generation has pushed computational demands beyond traditional desktop computing capabilities.
Specialized hardware accelerators have emerged as critical components for advanced biotechnology applications. Field-Programmable Gate Arrays (FPGAs) offer significant advantages for Kalman filter implementation due to their parallel processing capabilities and reconfigurability. These characteristics make them particularly suitable for handling the matrix operations central to Kalman filtering algorithms. Graphics Processing Units (GPUs) have also demonstrated substantial performance improvements, with studies showing 10-50x speedups compared to CPU implementations when processing large biological datasets.
Edge computing architectures are increasingly relevant for distributed biosensing networks, allowing for preliminary data filtering and state estimation to occur closer to the data source. This approach reduces bandwidth requirements and enables more responsive systems for applications like remote patient monitoring or environmental biosurveillance. The integration of low-power microcontrollers with efficient Kalman filter implementations has enabled battery-operated biosensors with extended operational lifespans.
Cloud computing infrastructure provides scalable resources for computationally intensive applications such as genome-wide association studies or systems biology modeling. Hybrid approaches combining edge processing with cloud-based analysis have proven effective for balancing real-time responsiveness with comprehensive data interpretation. However, these distributed architectures introduce additional challenges in maintaining synchronization across multiple filtering instances.
Energy efficiency considerations are particularly important for portable or implantable biomedical devices. Algorithm optimization techniques such as square-root formulations, measurement update scheduling, and adaptive process noise modeling can significantly reduce computational load while maintaining interpretation accuracy. These optimizations must be carefully balanced against accuracy requirements, as simplified models may fail to capture critical biological dynamics.
Validation Methodologies for Biotech Kalman Filter Applications
Validation of Kalman filter applications in biotechnology requires rigorous methodological approaches to ensure interpretation accuracy. The primary validation framework involves comparative analysis against gold standard measurements, where filter outputs are benchmarked against established laboratory techniques. This process typically employs statistical metrics such as root mean square error (RMSE), mean absolute percentage error (MAPE), and correlation coefficients to quantify performance accuracy.
Cross-validation techniques represent another critical validation methodology, particularly k-fold cross-validation where datasets are partitioned into training and testing subsets. This approach helps assess the filter's generalizability across different biological samples and conditions, reducing the risk of overfitting to specific data characteristics common in biological systems with high variability.
Sensitivity analysis constitutes an essential validation component, systematically evaluating how variations in filter parameters affect output accuracy. This methodology is particularly valuable in biotechnology applications where process noise and measurement uncertainty can significantly impact filter performance. By methodically adjusting parameters such as process noise covariance and measurement noise covariance, researchers can determine optimal configurations for specific biotech applications.
Monte Carlo simulations provide robust validation by generating synthetic datasets that mimic real-world biological variability. These simulations allow for comprehensive testing of Kalman filter performance under controlled conditions, enabling the assessment of filter stability across different noise profiles and signal characteristics typical in biological systems.
Real-time validation protocols represent an emerging methodology, particularly relevant for bioprocess monitoring applications. These approaches evaluate filter performance during actual bioprocessing operations, assessing computational efficiency alongside accuracy metrics to ensure practical implementation feasibility in time-sensitive biotech applications.
Independent laboratory verification serves as the ultimate validation step, where filter outputs are verified by independent research facilities using different measurement techniques. This multi-institutional approach strengthens validation credibility and helps identify potential systematic biases in the filter implementation or underlying assumptions about biological system dynamics.
Regulatory compliance validation has gained importance as Kalman filters increasingly support critical biotech applications. This methodology ensures that filter implementations meet relevant regulatory standards for data integrity, reproducibility, and documentation requirements, particularly for applications in pharmaceutical manufacturing or clinical diagnostics where regulatory oversight is stringent.
Cross-validation techniques represent another critical validation methodology, particularly k-fold cross-validation where datasets are partitioned into training and testing subsets. This approach helps assess the filter's generalizability across different biological samples and conditions, reducing the risk of overfitting to specific data characteristics common in biological systems with high variability.
Sensitivity analysis constitutes an essential validation component, systematically evaluating how variations in filter parameters affect output accuracy. This methodology is particularly valuable in biotechnology applications where process noise and measurement uncertainty can significantly impact filter performance. By methodically adjusting parameters such as process noise covariance and measurement noise covariance, researchers can determine optimal configurations for specific biotech applications.
Monte Carlo simulations provide robust validation by generating synthetic datasets that mimic real-world biological variability. These simulations allow for comprehensive testing of Kalman filter performance under controlled conditions, enabling the assessment of filter stability across different noise profiles and signal characteristics typical in biological systems.
Real-time validation protocols represent an emerging methodology, particularly relevant for bioprocess monitoring applications. These approaches evaluate filter performance during actual bioprocessing operations, assessing computational efficiency alongside accuracy metrics to ensure practical implementation feasibility in time-sensitive biotech applications.
Independent laboratory verification serves as the ultimate validation step, where filter outputs are verified by independent research facilities using different measurement techniques. This multi-institutional approach strengthens validation credibility and helps identify potential systematic biases in the filter implementation or underlying assumptions about biological system dynamics.
Regulatory compliance validation has gained importance as Kalman filters increasingly support critical biotech applications. This methodology ensures that filter implementations meet relevant regulatory standards for data integrity, reproducibility, and documentation requirements, particularly for applications in pharmaceutical manufacturing or clinical diagnostics where regulatory oversight is stringent.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!