Key Material Interactions in Advanced Antimicrobial Surface Coatings
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Antimicrobial Coating Technology Background and Objectives
Antimicrobial surface coatings have evolved significantly over the past several decades, transitioning from simple biocidal treatments to sophisticated engineered surfaces with targeted antimicrobial properties. The field emerged in the 1940s with the development of quaternary ammonium compounds for surface disinfection, but has since expanded to encompass a diverse array of technologies including silver nanoparticles, copper-based materials, photocatalytic titanium dioxide, and more recently, advanced polymer matrices with controlled release mechanisms.
The evolution of these technologies has been driven by increasing concerns about healthcare-associated infections, antibiotic resistance, and the need for passive infection control strategies in both medical and consumer environments. The COVID-19 pandemic has further accelerated interest in antimicrobial surfaces as part of comprehensive infection prevention strategies, expanding potential applications beyond traditional healthcare settings into public spaces, transportation, and consumer products.
Current technological trajectories indicate a shift from conventional biocide-releasing systems toward more sustainable, long-lasting approaches that minimize environmental impact while maintaining efficacy. This includes the development of contact-killing surfaces, anti-adhesion technologies, and biomimetic approaches inspired by naturally antimicrobial surfaces found in certain plants and animals.
The primary technical objective in this field is to develop antimicrobial coatings that demonstrate broad-spectrum efficacy against bacteria, viruses, and fungi while maintaining long-term stability under real-world conditions. These coatings must balance antimicrobial performance with mechanical durability, chemical stability, and compatibility with underlying substrates. Additionally, they must address growing concerns about potential toxicity, environmental persistence, and the development of microbial resistance.
Material interactions represent the cornerstone of advanced antimicrobial coating development, as they determine not only the antimicrobial mechanisms but also the coating's physical properties, durability, and long-term performance. Understanding the complex interplay between coating matrices, active antimicrobial agents, substrate materials, and the target microorganisms is essential for designing next-generation solutions.
The field is increasingly moving toward multifunctional coatings that combine antimicrobial properties with other desirable characteristics such as self-cleaning, anti-fouling, or sensing capabilities. This convergence of technologies presents both opportunities and challenges, requiring interdisciplinary approaches that span materials science, microbiology, surface chemistry, and engineering.
The evolution of these technologies has been driven by increasing concerns about healthcare-associated infections, antibiotic resistance, and the need for passive infection control strategies in both medical and consumer environments. The COVID-19 pandemic has further accelerated interest in antimicrobial surfaces as part of comprehensive infection prevention strategies, expanding potential applications beyond traditional healthcare settings into public spaces, transportation, and consumer products.
Current technological trajectories indicate a shift from conventional biocide-releasing systems toward more sustainable, long-lasting approaches that minimize environmental impact while maintaining efficacy. This includes the development of contact-killing surfaces, anti-adhesion technologies, and biomimetic approaches inspired by naturally antimicrobial surfaces found in certain plants and animals.
The primary technical objective in this field is to develop antimicrobial coatings that demonstrate broad-spectrum efficacy against bacteria, viruses, and fungi while maintaining long-term stability under real-world conditions. These coatings must balance antimicrobial performance with mechanical durability, chemical stability, and compatibility with underlying substrates. Additionally, they must address growing concerns about potential toxicity, environmental persistence, and the development of microbial resistance.
Material interactions represent the cornerstone of advanced antimicrobial coating development, as they determine not only the antimicrobial mechanisms but also the coating's physical properties, durability, and long-term performance. Understanding the complex interplay between coating matrices, active antimicrobial agents, substrate materials, and the target microorganisms is essential for designing next-generation solutions.
The field is increasingly moving toward multifunctional coatings that combine antimicrobial properties with other desirable characteristics such as self-cleaning, anti-fouling, or sensing capabilities. This convergence of technologies presents both opportunities and challenges, requiring interdisciplinary approaches that span materials science, microbiology, surface chemistry, and engineering.
Market Analysis for Advanced Antimicrobial Surface Solutions
The global market for advanced antimicrobial surface coatings has experienced significant growth in recent years, driven primarily by increasing healthcare-associated infections and growing awareness of hygiene across multiple sectors. The current market value stands at approximately 3.6 billion USD with projections indicating growth to reach 6.3 billion USD by 2027, representing a compound annual growth rate of 11.8% during the forecast period.
Healthcare remains the dominant application sector, accounting for nearly 40% of the total market share. Hospitals, clinics, and medical device manufacturers are increasingly adopting antimicrobial coatings to reduce nosocomial infections and comply with stringent regulatory requirements. The COVID-19 pandemic has further accelerated this trend, with heightened focus on surface transmission of pathogens.
Food processing and packaging represents the second-largest market segment at 22% market share, where antimicrobial coatings help extend shelf life and ensure food safety. The construction industry follows at 15%, incorporating these technologies in high-touch surfaces in public buildings, schools, and commercial spaces.
Regional analysis reveals North America currently leads the market with 38% share, followed by Europe at 30% and Asia-Pacific at 24%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 13.5% annually, driven by rapid industrialization, increasing healthcare expenditure, and growing awareness about infection control in countries like China, India, and Japan.
Consumer preferences are shifting toward environmentally sustainable antimicrobial solutions. Market research indicates 67% of institutional buyers now prioritize non-toxic, eco-friendly antimicrobial coatings over traditional biocide-based products. This has created a significant opportunity for bio-based antimicrobial materials, which currently represent only 18% of the market but are growing at twice the rate of conventional solutions.
Key market restraints include high implementation costs, with advanced antimicrobial coatings typically commanding a 30-45% price premium over standard coatings. Regulatory hurdles also present challenges, particularly in regions with strict chemical substance regulations like the European Union, where approval processes can take 18-24 months.
The competitive landscape features both established chemical companies and innovative startups. Market consolidation is evident with 15 significant mergers and acquisitions recorded in the past three years, as larger corporations seek to acquire specialized antimicrobial coating technologies to expand their product portfolios.
Healthcare remains the dominant application sector, accounting for nearly 40% of the total market share. Hospitals, clinics, and medical device manufacturers are increasingly adopting antimicrobial coatings to reduce nosocomial infections and comply with stringent regulatory requirements. The COVID-19 pandemic has further accelerated this trend, with heightened focus on surface transmission of pathogens.
Food processing and packaging represents the second-largest market segment at 22% market share, where antimicrobial coatings help extend shelf life and ensure food safety. The construction industry follows at 15%, incorporating these technologies in high-touch surfaces in public buildings, schools, and commercial spaces.
Regional analysis reveals North America currently leads the market with 38% share, followed by Europe at 30% and Asia-Pacific at 24%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 13.5% annually, driven by rapid industrialization, increasing healthcare expenditure, and growing awareness about infection control in countries like China, India, and Japan.
Consumer preferences are shifting toward environmentally sustainable antimicrobial solutions. Market research indicates 67% of institutional buyers now prioritize non-toxic, eco-friendly antimicrobial coatings over traditional biocide-based products. This has created a significant opportunity for bio-based antimicrobial materials, which currently represent only 18% of the market but are growing at twice the rate of conventional solutions.
Key market restraints include high implementation costs, with advanced antimicrobial coatings typically commanding a 30-45% price premium over standard coatings. Regulatory hurdles also present challenges, particularly in regions with strict chemical substance regulations like the European Union, where approval processes can take 18-24 months.
The competitive landscape features both established chemical companies and innovative startups. Market consolidation is evident with 15 significant mergers and acquisitions recorded in the past three years, as larger corporations seek to acquire specialized antimicrobial coating technologies to expand their product portfolios.
Current Challenges in Antimicrobial Surface Technology
Despite significant advancements in antimicrobial surface technology, several critical challenges continue to impede widespread implementation and optimal performance. One fundamental obstacle is the durability of antimicrobial coatings, particularly in high-touch or high-wear environments. Many current solutions demonstrate excellent initial efficacy but experience rapid degradation under mechanical stress, chemical exposure, or routine cleaning protocols, significantly reducing their functional lifespan and cost-effectiveness.
The development of resistance mechanisms by microorganisms presents another formidable challenge. As antimicrobial surfaces become more prevalent, selective pressure drives the evolution of resistant strains. This evolutionary arms race necessitates continuous innovation in coating technologies to maintain efficacy against adapting pathogens, creating a complex development cycle that increases research costs and time-to-market.
Biocompatibility concerns remain paramount, especially for applications in healthcare settings or food processing environments. Many highly effective antimicrobial agents exhibit cytotoxicity or trigger inflammatory responses in mammalian cells, limiting their application scope. The delicate balance between antimicrobial potency and biocompatibility represents a significant technical hurdle that researchers continue to navigate.
Environmental persistence and unintended ecological impacts constitute growing concerns. Some antimicrobial compounds, particularly those containing heavy metals or persistent organic molecules, can leach into the environment and accumulate in ecosystems. This raises regulatory challenges and sustainability questions that must be addressed through green chemistry approaches and lifecycle assessments.
Scalability and manufacturing complexity further complicate commercial implementation. Many promising laboratory-scale antimicrobial technologies encounter significant barriers during scale-up, including process inconsistencies, quality control challenges, and prohibitive production costs. The integration of advanced antimicrobial properties often requires specialized equipment or processing conditions that may not be readily available in existing manufacturing infrastructure.
Regulatory frameworks worldwide present a fragmented landscape of requirements and approval processes. The classification of antimicrobial surfaces often falls into ambiguous categories between medical devices, biocides, and consumer products, creating compliance uncertainties and market entry barriers. This regulatory complexity is particularly challenging for innovative technologies that employ novel mechanisms of action or materials not previously evaluated by regulatory bodies.
The cost-benefit equation remains problematic for many potential applications, with premium pricing for advanced antimicrobial surfaces limiting market penetration despite their potential public health benefits. This economic challenge is exacerbated by difficulties in quantifying the return on investment, particularly for preventative applications where the benefit is the absence of adverse events.
The development of resistance mechanisms by microorganisms presents another formidable challenge. As antimicrobial surfaces become more prevalent, selective pressure drives the evolution of resistant strains. This evolutionary arms race necessitates continuous innovation in coating technologies to maintain efficacy against adapting pathogens, creating a complex development cycle that increases research costs and time-to-market.
Biocompatibility concerns remain paramount, especially for applications in healthcare settings or food processing environments. Many highly effective antimicrobial agents exhibit cytotoxicity or trigger inflammatory responses in mammalian cells, limiting their application scope. The delicate balance between antimicrobial potency and biocompatibility represents a significant technical hurdle that researchers continue to navigate.
Environmental persistence and unintended ecological impacts constitute growing concerns. Some antimicrobial compounds, particularly those containing heavy metals or persistent organic molecules, can leach into the environment and accumulate in ecosystems. This raises regulatory challenges and sustainability questions that must be addressed through green chemistry approaches and lifecycle assessments.
Scalability and manufacturing complexity further complicate commercial implementation. Many promising laboratory-scale antimicrobial technologies encounter significant barriers during scale-up, including process inconsistencies, quality control challenges, and prohibitive production costs. The integration of advanced antimicrobial properties often requires specialized equipment or processing conditions that may not be readily available in existing manufacturing infrastructure.
Regulatory frameworks worldwide present a fragmented landscape of requirements and approval processes. The classification of antimicrobial surfaces often falls into ambiguous categories between medical devices, biocides, and consumer products, creating compliance uncertainties and market entry barriers. This regulatory complexity is particularly challenging for innovative technologies that employ novel mechanisms of action or materials not previously evaluated by regulatory bodies.
The cost-benefit equation remains problematic for many potential applications, with premium pricing for advanced antimicrobial surfaces limiting market penetration despite their potential public health benefits. This economic challenge is exacerbated by difficulties in quantifying the return on investment, particularly for preventative applications where the benefit is the absence of adverse events.
Current Material Interaction Mechanisms in Antimicrobial Surfaces
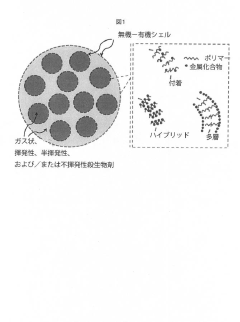
01 Metal-based antimicrobial coatings
Metal-based antimicrobial coatings utilize silver, copper, zinc, and other metals to provide effective surface protection against microorganisms. These metals release ions that disrupt bacterial cell membranes and interfere with cellular processes. The coatings can be applied to various substrates including medical devices, textiles, and industrial surfaces. The interaction between the metal ions and the substrate material is crucial for ensuring proper adhesion, durability, and controlled release of the antimicrobial agents.- Metal-based antimicrobial coatings: Metal-based antimicrobial coatings utilize silver, copper, zinc, and other metals as active ingredients to inhibit microbial growth on surfaces. These metals can be incorporated as nanoparticles, ions, or compounds within polymer matrices to provide sustained antimicrobial activity. The interaction between the metal ions and the substrate material affects both durability and efficacy, with controlled release mechanisms ensuring long-term protection while minimizing environmental impact.
- Polymer-based antimicrobial surface technologies: Polymer matrices serve as carriers for antimicrobial agents, providing controlled release and enhanced surface adhesion. These systems include quaternary ammonium compounds embedded in polymers, antimicrobial peptides bonded to polymer chains, and hydrophobic polymer surfaces that resist bacterial attachment. The interaction between the polymer and the substrate material significantly impacts coating durability, while the polymer chemistry affects antimicrobial agent release kinetics and long-term efficacy.
- Natural and bio-based antimicrobial coating materials: Bio-based antimicrobial coatings utilize natural compounds such as essential oils, plant extracts, and antimicrobial peptides as alternatives to synthetic chemicals. These materials interact with surfaces through various binding mechanisms including covalent attachment, physical adsorption, and entrapment within matrices. The compatibility between natural antimicrobials and substrate materials affects coating stability, with specialized formulations required to maintain antimicrobial efficacy while ensuring adhesion to different surface types.
- Surface modification techniques for antimicrobial properties: Surface modification techniques alter material surface properties to enhance antimicrobial performance through physical and chemical means. These include plasma treatment to increase surface energy and functional group density, laser texturing to create micropatterns that inhibit bacterial adhesion, and chemical etching to increase surface area for antimicrobial agent binding. The interaction between the modified surface and the antimicrobial coating determines durability, with compatibility between substrate material properties and modification techniques being crucial for long-term performance.
- Antimicrobial coating durability and material compatibility: The durability of antimicrobial coatings depends on the interaction between the coating and substrate material, with factors including adhesion strength, mechanical stress resistance, and chemical stability affecting performance. Material compatibility issues arise from differences in surface energy, chemical reactivity, and thermal expansion coefficients between coating and substrate. Advanced formulations incorporate adhesion promoters, cross-linking agents, and surface primers to enhance coating-substrate interactions, while testing protocols evaluate coating performance under various environmental conditions to ensure long-term antimicrobial efficacy.
02 Polymer-based antimicrobial surface technologies
Polymer-based antimicrobial coatings incorporate active ingredients within polymer matrices to create surfaces that resist microbial colonization. These systems can be designed as contact-killing surfaces where the polymer itself has antimicrobial properties, or as controlled-release systems that gradually disperse antimicrobial agents. The interaction between the polymer matrix and the antimicrobial agents affects release kinetics, while the interaction with the substrate material determines adhesion strength and coating longevity. Various polymers including quaternary ammonium-containing polymers, chitosan derivatives, and synthetic antimicrobial polymers are used in these applications.Expand Specific Solutions03 Nanoparticle-enhanced antimicrobial coatings
Nanoparticle-enhanced antimicrobial coatings utilize the unique properties of nanomaterials to improve efficacy and durability. These coatings incorporate nanoparticles such as silver nanoparticles, zinc oxide nanoparticles, or copper nanoparticles that provide enhanced antimicrobial activity due to their high surface area and unique physical properties. The interaction between nanoparticles and the coating matrix affects dispersion, stability, and release characteristics. Additionally, the size, shape, and surface chemistry of nanoparticles influence their antimicrobial efficacy and how they interact with both the coating material and the substrate.Expand Specific Solutions04 Biofilm-resistant surface modifications
Biofilm-resistant surface modifications focus on preventing the initial attachment and subsequent development of microbial biofilms. These approaches include creating surfaces with specific topographical features, hydrophobic or hydrophilic properties, or anti-adhesion characteristics that physically prevent microorganisms from attaching. The interaction between the modified surface and the surrounding environment is critical, as factors such as surface energy, roughness, and charge significantly influence microbial attachment. These coatings often combine physical surface modifications with chemical antimicrobial agents to provide dual-mechanism protection against biofilm formation.Expand Specific Solutions05 Environmentally responsive antimicrobial coatings
Environmentally responsive antimicrobial coatings are designed to activate or enhance their antimicrobial properties in response to specific environmental triggers. These smart coatings may respond to changes in pH, temperature, light, or the presence of specific microbial enzymes. The material interactions in these systems are complex, involving both the coating-substrate interface and the coating-environment interface. These coatings often incorporate stimuli-responsive polymers or encapsulated antimicrobial agents that are released only when needed, improving efficiency and reducing the development of antimicrobial resistance while extending the functional lifetime of the coating.Expand Specific Solutions
Leading Companies and Research Institutions in Antimicrobial Coatings
The antimicrobial surface coatings market is currently in a growth phase, with increasing demand driven by healthcare-associated infection concerns and heightened hygiene awareness post-pandemic. The global market is projected to reach approximately $6-8 billion by 2026, growing at a CAGR of 10-12%. Technology maturity varies across different application segments, with companies demonstrating diverse approaches. Industry leaders like Johnson & Johnson Vision Care and Baxter International focus on medical device applications, while BASF and Bayer AG leverage their chemical expertise for broader antimicrobial solutions. Research institutions such as Sichuan University and CSIRO are advancing fundamental material interactions, while specialized firms like BioInteractions Ltd and SRFC Bio are developing next-generation coatings with enhanced efficacy and reduced environmental impact. The competitive landscape shows a mix of established players and innovative startups collaborating to address antimicrobial resistance challenges.
BioInteractions Ltd
Technical Solution: BioInteractions Ltd has developed advanced antimicrobial surface coating technologies based on their proprietary TridAnt technology platform. Their approach involves creating multi-layer polymeric coatings that incorporate active antimicrobial agents with controlled release mechanisms. The TridAnt technology creates a non-leaching, non-toxic surface modification that provides long-lasting protection against a broad spectrum of pathogens including bacteria, fungi, and viruses. The company's coatings utilize a combination of quaternary ammonium compounds, silver ions, and specialized polymers that work synergistically to disrupt microbial cell membranes and inhibit colonization. Their materials are specifically engineered to maintain antimicrobial efficacy even after repeated cleaning cycles and extended use, with documented activity lasting up to 12 months in clinical settings. BioInteractions has focused on optimizing the interface between the coating matrix and the embedded antimicrobial agents to ensure consistent performance without compromising the physical properties of the treated surfaces.
Strengths: Long-lasting antimicrobial activity without leaching toxic compounds; broad-spectrum efficacy against multiple pathogen types; maintains effectiveness after cleaning cycles. Weaknesses: May have higher production costs compared to conventional coatings; potential for development of microbial resistance with extended use; limited application on certain material substrates.
BASF Corp.
Technical Solution: BASF has developed the HyGentic® antimicrobial coating technology platform that leverages their expertise in polymer chemistry and material science. Their approach focuses on creating multifunctional coating systems with integrated antimicrobial properties through precise material interactions. The technology incorporates specially formulated quaternary ammonium compounds chemically bonded to polymer backbones, creating permanent antimicrobial surfaces that don't leach active ingredients. BASF's innovation includes the development of specialized cross-linking mechanisms that ensure antimicrobial compounds remain properly oriented at the surface interface where they can interact with microbial cell membranes. Their research has optimized the charge density and hydrophobic/hydrophilic balance of the polymer systems to maximize antimicrobial efficacy while maintaining excellent material properties. BASF has also pioneered the incorporation of secondary antimicrobial mechanisms, including metal ions and photocatalytic materials, within their coating systems to create synergistic effects against diverse microbial populations. Their technology includes specialized surface modification techniques that enhance adhesion to challenging substrates while maintaining the functional orientation of antimicrobial components.
Strengths: Non-leaching antimicrobial technology provides long-term protection; compatible with existing coating application methods; maintains effectiveness after repeated cleaning cycles. Weaknesses: May have limited efficacy against certain fungal species; requires specific application parameters for optimal performance; higher cost compared to conventional coating systems.
Key Patents and Research on Material Interactions in Antimicrobial Coatings
Antimicrobial coating for long-term disinfection of surfaces
PatentActiveJP2020063260A
Innovation
- Development of colloidal capsules containing biocides within inorganic-organic shells that provide controlled release and contact killing, combining polymers and metal compounds for antimicrobial coatings on surfaces, which can be applied to both porous and non-porous materials.
Antimicrobial coating containing quaternary ammonium resin and its regeneration
PatentInactiveEP2740355A1
Innovation
- A strongly basic anion-exchange resin film or coating, free of transition metals, is applied to medical device surfaces, which can be replenished with antimicrobial anions such as iodine or chloride, providing sustained antimicrobial activity through exposure to agents like bleach or Lugol's iodine.
Environmental Impact and Sustainability Considerations
The environmental impact of antimicrobial surface coatings has become a critical consideration in their development and deployment. Traditional antimicrobial agents, particularly those containing heavy metals like silver, copper, and zinc, pose significant environmental concerns during their lifecycle. When these coatings degrade, they can release toxic compounds into aquatic ecosystems, potentially disrupting microbial communities essential for environmental balance and causing bioaccumulation in various organisms.
Recent research has focused on developing more sustainable alternatives that maintain antimicrobial efficacy while reducing environmental footprint. Biodegradable polymers as carrier matrices represent a promising approach, as they can decompose into non-toxic components after their functional lifespan. Natural antimicrobial compounds derived from plants, such as essential oils and polyphenols, offer renewable alternatives to synthetic biocides, though challenges remain in ensuring their stability and long-term effectiveness.
Life cycle assessment (LCA) studies of antimicrobial coatings reveal significant variations in environmental impact depending on material selection and manufacturing processes. Coatings utilizing nanotechnology present a complex environmental profile—while they may reduce the quantity of antimicrobial agents needed, concerns persist about the potential release of nanoparticles into the environment and their long-term ecological effects, which remain incompletely understood.
Regulatory frameworks worldwide are increasingly incorporating sustainability criteria for antimicrobial products. The European Union's REACH regulation and the United States EPA's safer choice program have established guidelines that encourage the development of environmentally benign antimicrobial technologies. These regulations are driving innovation toward green chemistry principles in coating formulation.
Energy consumption during manufacturing represents another significant environmental consideration. Advanced plasma deposition and other low-temperature coating technologies have emerged as more energy-efficient alternatives to traditional high-temperature processes, substantially reducing the carbon footprint associated with production.
End-of-life management strategies for antimicrobial surfaces are evolving toward circular economy models. Design approaches that facilitate the recovery and recycling of valuable components from expired antimicrobial coatings are gaining traction. Some innovative systems incorporate trigger mechanisms that allow for controlled degradation when exposed to specific environmental conditions, minimizing persistent waste.
The healthcare sector, a primary user of antimicrobial surfaces, is increasingly adopting green procurement policies that prioritize products with demonstrated environmental benefits alongside antimicrobial performance. This market shift is accelerating the transition toward more sustainable antimicrobial coating technologies that balance efficacy, durability, and environmental responsibility.
Recent research has focused on developing more sustainable alternatives that maintain antimicrobial efficacy while reducing environmental footprint. Biodegradable polymers as carrier matrices represent a promising approach, as they can decompose into non-toxic components after their functional lifespan. Natural antimicrobial compounds derived from plants, such as essential oils and polyphenols, offer renewable alternatives to synthetic biocides, though challenges remain in ensuring their stability and long-term effectiveness.
Life cycle assessment (LCA) studies of antimicrobial coatings reveal significant variations in environmental impact depending on material selection and manufacturing processes. Coatings utilizing nanotechnology present a complex environmental profile—while they may reduce the quantity of antimicrobial agents needed, concerns persist about the potential release of nanoparticles into the environment and their long-term ecological effects, which remain incompletely understood.
Regulatory frameworks worldwide are increasingly incorporating sustainability criteria for antimicrobial products. The European Union's REACH regulation and the United States EPA's safer choice program have established guidelines that encourage the development of environmentally benign antimicrobial technologies. These regulations are driving innovation toward green chemistry principles in coating formulation.
Energy consumption during manufacturing represents another significant environmental consideration. Advanced plasma deposition and other low-temperature coating technologies have emerged as more energy-efficient alternatives to traditional high-temperature processes, substantially reducing the carbon footprint associated with production.
End-of-life management strategies for antimicrobial surfaces are evolving toward circular economy models. Design approaches that facilitate the recovery and recycling of valuable components from expired antimicrobial coatings are gaining traction. Some innovative systems incorporate trigger mechanisms that allow for controlled degradation when exposed to specific environmental conditions, minimizing persistent waste.
The healthcare sector, a primary user of antimicrobial surfaces, is increasingly adopting green procurement policies that prioritize products with demonstrated environmental benefits alongside antimicrobial performance. This market shift is accelerating the transition toward more sustainable antimicrobial coating technologies that balance efficacy, durability, and environmental responsibility.
Regulatory Framework for Antimicrobial Surface Technologies
The regulatory landscape for antimicrobial surface technologies has evolved significantly in response to growing concerns about infection control and antimicrobial resistance. In the United States, the Environmental Protection Agency (EPA) regulates antimicrobial surface coatings under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), requiring manufacturers to register products that make public health claims. The EPA's testing protocols specifically evaluate efficacy against target microorganisms and environmental impact, with particular attention to leaching behavior of active ingredients.
The Food and Drug Administration (FDA) maintains oversight when antimicrobial surfaces are incorporated into medical devices, applying more stringent requirements through the 510(k) clearance or Premarket Approval (PMA) pathways. These regulatory frameworks demand comprehensive biocompatibility testing and clinical evidence of safety and effectiveness, especially for implantable devices with antimicrobial properties.
In the European Union, the Biocidal Products Regulation (BPR) governs antimicrobial surface technologies, requiring authorization of active substances and product-specific approvals. The EU's approach emphasizes the precautionary principle, with strict requirements for risk assessment and documentation of environmental fate. Additionally, the Medical Device Regulation (MDR) imposes specific requirements for antimicrobial surfaces in healthcare applications, including post-market surveillance obligations.
The regulatory frameworks in Asia present a more fragmented landscape. Japan's approach through the Ministry of Health, Labour and Welfare focuses on efficacy validation and safety assessment, while China's National Medical Products Administration has recently strengthened requirements for antimicrobial claims, particularly for imported technologies.
International standards organizations play a crucial harmonizing role, with ISO 22196 and JIS Z 2801 providing standardized methods for evaluating antimicrobial activity on surfaces. These standards facilitate global market access by establishing common testing protocols that satisfy multiple regulatory jurisdictions.
Recent regulatory trends indicate increasing scrutiny of long-term efficacy claims and environmental persistence. Regulators are particularly concerned about the potential for antimicrobial coatings to contribute to resistance development, leading to more rigorous requirements for resistance monitoring in product approval applications. The EPA's recent guidance specifically addresses the need for standardized protocols to evaluate resistance potential in environmental applications.
Emerging regulations are also addressing novel antimicrobial mechanisms, such as physical disruption approaches that do not rely on chemical biocides. These technologies face regulatory uncertainty as they often fall outside traditional antimicrobial product categories, creating challenges for market entry despite their potential advantages in resistance management.
The Food and Drug Administration (FDA) maintains oversight when antimicrobial surfaces are incorporated into medical devices, applying more stringent requirements through the 510(k) clearance or Premarket Approval (PMA) pathways. These regulatory frameworks demand comprehensive biocompatibility testing and clinical evidence of safety and effectiveness, especially for implantable devices with antimicrobial properties.
In the European Union, the Biocidal Products Regulation (BPR) governs antimicrobial surface technologies, requiring authorization of active substances and product-specific approvals. The EU's approach emphasizes the precautionary principle, with strict requirements for risk assessment and documentation of environmental fate. Additionally, the Medical Device Regulation (MDR) imposes specific requirements for antimicrobial surfaces in healthcare applications, including post-market surveillance obligations.
The regulatory frameworks in Asia present a more fragmented landscape. Japan's approach through the Ministry of Health, Labour and Welfare focuses on efficacy validation and safety assessment, while China's National Medical Products Administration has recently strengthened requirements for antimicrobial claims, particularly for imported technologies.
International standards organizations play a crucial harmonizing role, with ISO 22196 and JIS Z 2801 providing standardized methods for evaluating antimicrobial activity on surfaces. These standards facilitate global market access by establishing common testing protocols that satisfy multiple regulatory jurisdictions.
Recent regulatory trends indicate increasing scrutiny of long-term efficacy claims and environmental persistence. Regulators are particularly concerned about the potential for antimicrobial coatings to contribute to resistance development, leading to more rigorous requirements for resistance monitoring in product approval applications. The EPA's recent guidance specifically addresses the need for standardized protocols to evaluate resistance potential in environmental applications.
Emerging regulations are also addressing novel antimicrobial mechanisms, such as physical disruption approaches that do not rely on chemical biocides. These technologies face regulatory uncertainty as they often fall outside traditional antimicrobial product categories, creating challenges for market entry despite their potential advantages in resistance management.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!