Research on the Biocompatibility of Antimicrobial Surface Coatings
SEP 29, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Antimicrobial Coating Biocompatibility Background and Objectives
Antimicrobial surface coatings have emerged as a critical technology in combating pathogenic microorganisms across various sectors including healthcare, food processing, and consumer products. The evolution of these coatings traces back to early silver-based antimicrobial treatments, progressing through copper compounds, quaternary ammonium compounds, and now advancing into nanotechnology-enhanced solutions and biomimetic approaches.
The development trajectory has been significantly accelerated in recent years due to global health challenges, particularly the COVID-19 pandemic, which highlighted the urgent need for effective antimicrobial surfaces in public spaces and healthcare environments. This has catalyzed research interest and funding allocation toward more sophisticated and biocompatible antimicrobial coating technologies.
Current technological trends indicate a shift from conventional biocide-releasing mechanisms toward more sustainable, long-lasting solutions that maintain antimicrobial efficacy while minimizing environmental impact and potential toxicity. The integration of smart materials capable of targeted antimicrobial action represents the cutting edge of this field, with significant implications for reducing healthcare-associated infections and improving public health outcomes.
The primary objective of this technical research is to comprehensively evaluate the biocompatibility aspects of contemporary antimicrobial surface coatings. Specifically, we aim to assess how these coatings interact with human tissues and cells, their potential for cytotoxicity, inflammatory responses, and long-term safety profiles when deployed in various application environments.
Additionally, this research seeks to identify the correlation between antimicrobial efficacy and biocompatibility parameters, exploring the delicate balance that must be maintained to ensure both effective microbial control and human safety. This includes examining the leaching behavior of active ingredients, degradation patterns over time, and potential for developing microbial resistance.
Furthermore, we intend to establish standardized testing protocols for biocompatibility assessment specific to antimicrobial coatings, addressing the current gaps in regulatory frameworks and industry standards. This will contribute to more reliable comparative analyses between different coating technologies and accelerate the path to market for promising innovations.
The expected outcomes of this research include identifying optimal material compositions and surface modification techniques that maximize antimicrobial performance while maintaining excellent biocompatibility profiles. These findings will guide future research directions and inform strategic development decisions for next-generation antimicrobial coating technologies that can be safely implemented across diverse application domains.
The development trajectory has been significantly accelerated in recent years due to global health challenges, particularly the COVID-19 pandemic, which highlighted the urgent need for effective antimicrobial surfaces in public spaces and healthcare environments. This has catalyzed research interest and funding allocation toward more sophisticated and biocompatible antimicrobial coating technologies.
Current technological trends indicate a shift from conventional biocide-releasing mechanisms toward more sustainable, long-lasting solutions that maintain antimicrobial efficacy while minimizing environmental impact and potential toxicity. The integration of smart materials capable of targeted antimicrobial action represents the cutting edge of this field, with significant implications for reducing healthcare-associated infections and improving public health outcomes.
The primary objective of this technical research is to comprehensively evaluate the biocompatibility aspects of contemporary antimicrobial surface coatings. Specifically, we aim to assess how these coatings interact with human tissues and cells, their potential for cytotoxicity, inflammatory responses, and long-term safety profiles when deployed in various application environments.
Additionally, this research seeks to identify the correlation between antimicrobial efficacy and biocompatibility parameters, exploring the delicate balance that must be maintained to ensure both effective microbial control and human safety. This includes examining the leaching behavior of active ingredients, degradation patterns over time, and potential for developing microbial resistance.
Furthermore, we intend to establish standardized testing protocols for biocompatibility assessment specific to antimicrobial coatings, addressing the current gaps in regulatory frameworks and industry standards. This will contribute to more reliable comparative analyses between different coating technologies and accelerate the path to market for promising innovations.
The expected outcomes of this research include identifying optimal material compositions and surface modification techniques that maximize antimicrobial performance while maintaining excellent biocompatibility profiles. These findings will guide future research directions and inform strategic development decisions for next-generation antimicrobial coating technologies that can be safely implemented across diverse application domains.
Market Demand Analysis for Biocompatible Antimicrobial Surfaces
The global market for biocompatible antimicrobial surfaces has experienced significant growth in recent years, driven primarily by increasing healthcare-associated infections (HAIs) and the rising demand for implantable medical devices. The market value reached approximately $2.8 billion in 2022 and is projected to grow at a compound annual growth rate of 7.5% through 2030, reflecting the urgent need for effective infection control solutions.
Healthcare facilities represent the largest market segment, accounting for nearly 45% of the total demand. This is attributed to the critical need to prevent nosocomial infections in hospitals and clinics, where surface contamination remains a persistent challenge. The COVID-19 pandemic has further accelerated this demand, as healthcare providers worldwide have heightened their focus on infection prevention protocols.
The medical device industry constitutes the second-largest market segment. With over 20 million implantable devices used annually worldwide, the need for biocompatible surfaces that can prevent bacterial colonization without triggering adverse immune responses has become paramount. Orthopedic implants, cardiovascular devices, and dental implants are particularly driving this demand due to their long-term placement in the human body.
Consumer preferences are increasingly shifting toward products with proven antimicrobial properties, especially in high-touch environments. Market research indicates that 78% of consumers now consider antimicrobial properties an important factor when purchasing products for healthcare settings, compared to just 32% five years ago.
Regionally, North America dominates the market with a 38% share, followed by Europe at 29% and Asia-Pacific at 24%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 9.2% annually, driven by expanding healthcare infrastructure, increasing medical tourism, and growing awareness about infection control.
Regulatory considerations are significantly shaping market dynamics. The FDA's increased scrutiny of antimicrobial claims and the European Union's Medical Device Regulation (MDR) implementation have created more stringent requirements for market entry. This has led to a notable trend where products with comprehensive biocompatibility testing and clinical validation command premium pricing, often 30-40% higher than alternatives with limited testing.
Industry surveys reveal that end-users prioritize long-term efficacy (cited by 82% of respondents), absence of toxicity concerns (76%), and cost-effectiveness (68%) when selecting antimicrobial surface technologies. This indicates a clear market preference for solutions that balance antimicrobial performance with proven biocompatibility rather than focusing solely on pathogen elimination capabilities.
Healthcare facilities represent the largest market segment, accounting for nearly 45% of the total demand. This is attributed to the critical need to prevent nosocomial infections in hospitals and clinics, where surface contamination remains a persistent challenge. The COVID-19 pandemic has further accelerated this demand, as healthcare providers worldwide have heightened their focus on infection prevention protocols.
The medical device industry constitutes the second-largest market segment. With over 20 million implantable devices used annually worldwide, the need for biocompatible surfaces that can prevent bacterial colonization without triggering adverse immune responses has become paramount. Orthopedic implants, cardiovascular devices, and dental implants are particularly driving this demand due to their long-term placement in the human body.
Consumer preferences are increasingly shifting toward products with proven antimicrobial properties, especially in high-touch environments. Market research indicates that 78% of consumers now consider antimicrobial properties an important factor when purchasing products for healthcare settings, compared to just 32% five years ago.
Regionally, North America dominates the market with a 38% share, followed by Europe at 29% and Asia-Pacific at 24%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 9.2% annually, driven by expanding healthcare infrastructure, increasing medical tourism, and growing awareness about infection control.
Regulatory considerations are significantly shaping market dynamics. The FDA's increased scrutiny of antimicrobial claims and the European Union's Medical Device Regulation (MDR) implementation have created more stringent requirements for market entry. This has led to a notable trend where products with comprehensive biocompatibility testing and clinical validation command premium pricing, often 30-40% higher than alternatives with limited testing.
Industry surveys reveal that end-users prioritize long-term efficacy (cited by 82% of respondents), absence of toxicity concerns (76%), and cost-effectiveness (68%) when selecting antimicrobial surface technologies. This indicates a clear market preference for solutions that balance antimicrobial performance with proven biocompatibility rather than focusing solely on pathogen elimination capabilities.
Current Status and Challenges in Antimicrobial Coating Development
The global landscape of antimicrobial coating development has witnessed significant advancements in recent years, with research institutions and companies across North America, Europe, and Asia-Pacific regions making substantial contributions. Currently, silver-based coatings dominate the commercial market due to their broad-spectrum antimicrobial properties and established efficacy. Quaternary ammonium compounds (QACs) and copper-based technologies also maintain significant market presence, particularly in healthcare settings.
Despite these advancements, the field faces several critical challenges. Biocompatibility remains a primary concern, as many effective antimicrobial agents exhibit cytotoxicity to human cells at concentrations required for microbial inhibition. This creates a narrow therapeutic window that limits clinical applications, particularly for implantable devices and wound care products where tissue integration is essential.
Antimicrobial resistance (AMR) presents another significant challenge. The widespread use of antimicrobial coatings containing conventional biocides may contribute to the development of resistant microbial strains. Recent studies have documented instances of bacterial adaptation to silver nanoparticles and QACs, raising concerns about long-term efficacy and potential public health implications.
Durability and sustained release kinetics continue to pose technical difficulties. Many current coating technologies demonstrate excellent initial antimicrobial activity but suffer from rapid depletion of active agents or degradation under physiological conditions. This results in diminished effectiveness over time, particularly problematic for long-term implantable devices where coating integrity must be maintained for years rather than days or weeks.
Regulatory hurdles further complicate development efforts. Different regions maintain varying standards for biocompatibility testing and antimicrobial efficacy claims. The FDA in the United States has established stringent requirements for medical device coatings, while the European Union's Medical Device Regulation (MDR) imposes additional documentation and testing requirements that extend development timelines and increase costs.
Manufacturing scalability presents technical challenges, particularly for complex coating technologies involving multiple components or sophisticated surface modifications. Ensuring consistent quality, thickness, and antimicrobial performance across large production batches remains difficult, especially for coatings requiring precise nanoscale features or controlled release mechanisms.
Environmental concerns are increasingly influencing development directions, with growing scrutiny of leaching antimicrobials and their potential ecological impacts. Regulatory bodies worldwide are implementing stricter controls on biocide release, pushing researchers toward more environmentally sustainable alternatives that maintain efficacy while minimizing environmental footprint.
Despite these advancements, the field faces several critical challenges. Biocompatibility remains a primary concern, as many effective antimicrobial agents exhibit cytotoxicity to human cells at concentrations required for microbial inhibition. This creates a narrow therapeutic window that limits clinical applications, particularly for implantable devices and wound care products where tissue integration is essential.
Antimicrobial resistance (AMR) presents another significant challenge. The widespread use of antimicrobial coatings containing conventional biocides may contribute to the development of resistant microbial strains. Recent studies have documented instances of bacterial adaptation to silver nanoparticles and QACs, raising concerns about long-term efficacy and potential public health implications.
Durability and sustained release kinetics continue to pose technical difficulties. Many current coating technologies demonstrate excellent initial antimicrobial activity but suffer from rapid depletion of active agents or degradation under physiological conditions. This results in diminished effectiveness over time, particularly problematic for long-term implantable devices where coating integrity must be maintained for years rather than days or weeks.
Regulatory hurdles further complicate development efforts. Different regions maintain varying standards for biocompatibility testing and antimicrobial efficacy claims. The FDA in the United States has established stringent requirements for medical device coatings, while the European Union's Medical Device Regulation (MDR) imposes additional documentation and testing requirements that extend development timelines and increase costs.
Manufacturing scalability presents technical challenges, particularly for complex coating technologies involving multiple components or sophisticated surface modifications. Ensuring consistent quality, thickness, and antimicrobial performance across large production batches remains difficult, especially for coatings requiring precise nanoscale features or controlled release mechanisms.
Environmental concerns are increasingly influencing development directions, with growing scrutiny of leaching antimicrobials and their potential ecological impacts. Regulatory bodies worldwide are implementing stricter controls on biocide release, pushing researchers toward more environmentally sustainable alternatives that maintain efficacy while minimizing environmental footprint.
Current Technical Solutions for Biocompatible Antimicrobial Surfaces
01 Metal-based antimicrobial coatings
Metal-based antimicrobial coatings utilize silver, copper, zinc, and other metal ions or nanoparticles to provide effective antimicrobial properties while maintaining biocompatibility. These metals can be incorporated into various polymer matrices or applied directly to surfaces. The controlled release of metal ions creates a hostile environment for microorganisms while remaining safe for human contact. These coatings are particularly useful for medical devices and implants where both antimicrobial efficacy and tissue compatibility are essential.- Metal-based antimicrobial coatings: Metal-based antimicrobial coatings utilize silver, copper, zinc, or other metal ions to provide broad-spectrum antimicrobial activity while maintaining biocompatibility. These metals can be incorporated as nanoparticles, ions, or complexes within various coating matrices. The controlled release of metal ions provides long-lasting protection against bacteria, fungi, and viruses without significant cytotoxicity to human cells when properly formulated. These coatings are particularly valuable for medical devices and implants where both antimicrobial efficacy and tissue compatibility are essential.
- Polymer-based antimicrobial coatings: Polymer-based antimicrobial coatings incorporate biocompatible polymers such as polyurethanes, silicones, and hydrogels that can be functionalized with antimicrobial agents. These polymers provide a matrix for controlled release of active ingredients while maintaining surface integrity and biocompatibility. Some polymers themselves possess inherent antimicrobial properties through positively charged groups that disrupt bacterial cell membranes. The advantage of polymer-based systems is their versatility in application methods, durability, and ability to be tailored for specific biocompatibility requirements in various medical and industrial applications.
- Natural compound-based antimicrobial coatings: Natural compound-based antimicrobial coatings utilize plant extracts, essential oils, enzymes, and peptides to provide antimicrobial protection with enhanced biocompatibility. These natural compounds often have multiple mechanisms of action against pathogens while exhibiting low toxicity to human cells. Chitosan, derived from crustacean shells, is particularly notable for its antimicrobial properties and excellent biocompatibility. These coatings are advantageous in applications where minimal immune response is critical, such as wound dressings, food packaging, and medical textiles.
- Surface modification techniques for biocompatible antimicrobial coatings: Various surface modification techniques can enhance both the antimicrobial efficacy and biocompatibility of coatings. These include plasma treatment, layer-by-layer deposition, chemical vapor deposition, and surface grafting. These methods allow for precise control over surface properties such as charge, hydrophobicity, and roughness, which influence both antimicrobial activity and cell interactions. By creating micro or nanostructured surfaces, these techniques can provide antimicrobial properties through physical mechanisms without relying solely on chemical agents, thereby improving long-term biocompatibility and reducing the risk of antimicrobial resistance.
- Testing and evaluation methods for antimicrobial coating biocompatibility: Standardized testing and evaluation methods are essential for assessing both the antimicrobial efficacy and biocompatibility of surface coatings. These include in vitro cytotoxicity assays, hemolysis tests, protein adsorption studies, and cell adhesion/proliferation assessments. More advanced evaluations involve inflammatory response testing, genotoxicity studies, and in vivo biocompatibility assessments. Long-term leaching studies help determine the stability of antimicrobial agents within the coating and potential systemic effects. These comprehensive testing protocols ensure that antimicrobial coatings maintain their effectiveness against pathogens while remaining safe for human contact in their intended applications.
02 Quaternary ammonium compound coatings
Quaternary ammonium compounds (QACs) are widely used in antimicrobial surface coatings due to their broad-spectrum activity against bacteria, fungi, and some viruses. These compounds can be covalently bonded to surfaces or incorporated into polymer matrices to create durable antimicrobial coatings. The positively charged nitrogen in QACs disrupts microbial cell membranes, leading to cell death. These coatings demonstrate good biocompatibility with human tissues while maintaining long-lasting antimicrobial properties, making them suitable for healthcare settings and medical devices.Expand Specific Solutions03 Chitosan-based biocompatible antimicrobial coatings
Chitosan, a natural polysaccharide derived from chitin, serves as an excellent base for biocompatible antimicrobial coatings. Its inherent antimicrobial properties, combined with excellent biocompatibility and biodegradability, make it ideal for medical applications. Chitosan can be modified with various functional groups to enhance its antimicrobial efficacy while maintaining its biocompatibility. These coatings work by disrupting bacterial cell membranes and can be applied to various surfaces including implants, wound dressings, and medical devices.Expand Specific Solutions04 Hydrogel-based antimicrobial coatings
Hydrogel-based antimicrobial coatings combine the benefits of hydrogels (high water content, biocompatibility, and flexibility) with antimicrobial agents to create effective surface treatments. These coatings can incorporate various antimicrobial agents such as antibiotics, antimicrobial peptides, or metal ions within their three-dimensional network. The hydrogel matrix allows for controlled release of antimicrobial agents while providing a biocompatible interface with tissues. These coatings are particularly useful for wound dressings, contact lenses, and tissue engineering scaffolds where both moisture retention and antimicrobial properties are desired.Expand Specific Solutions05 Enzyme-based antimicrobial surface treatments
Enzyme-based antimicrobial surface treatments utilize natural enzymes to target and destroy bacterial biofilms and prevent microbial colonization. These enzymes can include lysozymes, proteases, and DNases that break down essential components of bacterial cell walls or biofilm matrices. The highly specific action of enzymes provides excellent biocompatibility as they typically do not harm human cells while effectively targeting microbial structures. These coatings can be designed to release enzymes gradually or be activated under specific conditions, providing smart antimicrobial protection for medical devices and implants.Expand Specific Solutions
Key Industry Players in Biocompatible Antimicrobial Coatings
The biocompatible antimicrobial surface coatings market is currently in a growth phase, driven by increasing healthcare-associated infections and demand for implantable medical devices. The global market is estimated at $1.5-2 billion with projected annual growth of 8-10%. Technologically, the field shows moderate maturity with established players like BASF, Eastman Chemical, and Surmodics offering commercial solutions, while research institutions (MIT, University of Michigan, CNRS) continue advancing fundamental science. Innovation hotspots include BioInteractions Ltd. and Orthobond Corp. focusing on specialized biocompatible coatings, while Medtronic MiniMed and Shandong Weigao Group apply these technologies in medical devices. Emerging players like Jiangsu Biosurf Biotech are developing next-generation coatings with enhanced antimicrobial properties while maintaining excellent biocompatibility.
BioInteractions Ltd
Technical Solution: BioInteractions Ltd has developed proprietary antimicrobial coating technologies specifically designed for medical devices. Their flagship technology, TridAnt, is a non-leaching, non-toxic antimicrobial coating that provides long-lasting protection against a broad spectrum of bacteria, fungi, and viruses. The coating utilizes a multi-mechanism approach combining contact killing, anti-adhesion properties, and biofilm prevention. Their technology incorporates quaternary ammonium compounds and hydrophilic polymers in a unique matrix that maintains activity for extended periods without releasing toxic compounds into the surrounding tissues. Clinical studies have demonstrated efficacy against common pathogens including MRSA and E. coli while maintaining excellent biocompatibility with human tissues. The coating can be applied to various substrates including polymers, metals, and ceramics used in implantable and non-implantable medical devices.
Strengths: Non-leaching technology provides long-term antimicrobial efficacy without depleting active ingredients; excellent biocompatibility profile with minimal inflammatory response; versatile application across multiple substrate materials. Weaknesses: May require specialized application processes; higher cost compared to conventional coatings; performance may vary depending on environmental conditions and mechanical stress.
BASF Corp.
Technical Solution: BASF Corp. has pioneered advanced antimicrobial surface coating technologies through their Functional Surface Solutions division. Their approach centers on polymer-based antimicrobial coatings incorporating silver nanoparticles and organic antimicrobial compounds in controlled-release formulations. These coatings feature a proprietary matrix system that gradually releases active agents at concentrations effective against microbes while remaining below cytotoxic thresholds for mammalian cells. BASF has developed specialized testing protocols to evaluate both antimicrobial efficacy and biocompatibility simultaneously, allowing for optimization of the release kinetics. Their technology includes surface-grafted polymers with quaternary ammonium functionalities that provide contact-killing properties without leaching. Recent innovations include photocatalytic titanium dioxide formulations that activate antimicrobial properties upon exposure to light while maintaining excellent tissue compatibility. These coatings have been successfully applied to medical textiles, wound dressings, and hospital surface materials with demonstrated reduction in healthcare-associated infections.
Strengths: Extensive polymer chemistry expertise allows precise control of release kinetics; comprehensive testing capabilities for both antimicrobial efficacy and biocompatibility; scalable manufacturing processes suitable for commercial applications. Weaknesses: Silver-based technologies may face regulatory challenges in some markets; potential for development of microbial resistance with prolonged use; higher cost compared to conventional non-antimicrobial coatings.
Critical Patents and Research in Antimicrobial Coating Biocompatibility
Antimicrobial coatings comprising quaternary silanes
PatentActiveUS10258046B2
Innovation
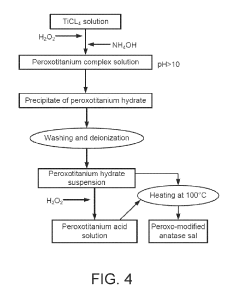
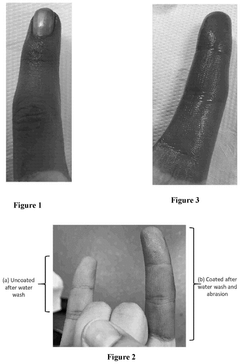
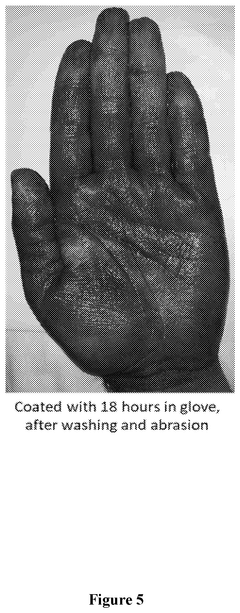
- A method involving the application of a composition comprising organosilanes, such as 3-(trihydroxysilyl)propyl dimethyloctadecyl ammonium chloride, triethanolamine, and a titanyl sol-gel, specifically an aqueous mixture of peroxotitanium acid and peroxo-modified anatase sol, to form a durable and residual antimicrobial coating that maintains efficacy against E. coli and S. epidermidis even after water rinsing or abrasion.
Coatings, formulations, uses and coating methods
PatentPendingUS20240336807A1
Innovation
- The use of alkyl urea polyalkylene imine polymers, optionally combined with anionic and cationic polymers, and guanidine compounds, to create coatings that inhibit biofilm formation and disrupt existing biofilms, offering biocidal and biostatic effects on both non-living surfaces and skin, through application methods such as spraying, dipping, or layering.
Safety and Toxicity Assessment Methodologies
The assessment of safety and toxicity for antimicrobial surface coatings requires rigorous methodological approaches to ensure biocompatibility with human tissues and environmental systems. Current methodologies follow a multi-tiered framework that begins with in vitro screening tests, progresses to ex vivo tissue models, and culminates in comprehensive in vivo evaluations. These methodologies are designed to identify potential cytotoxicity, genotoxicity, immunogenicity, and long-term exposure effects.
In vitro cytotoxicity assays represent the first line of evaluation, typically employing cell culture models such as fibroblasts, keratinocytes, and macrophages to assess direct cellular responses to coating materials. Standard protocols include MTT/XTT assays for metabolic activity, LDH release for membrane integrity, and live/dead staining techniques. These methods provide rapid screening capabilities but must be interpreted with caution due to their limited representation of complex biological interactions.
Advanced ex vivo models have emerged as critical intermediate assessment tools, bridging the gap between simplified cell cultures and complex living systems. Three-dimensional tissue constructs, organ-on-chip technologies, and explant cultures offer more physiologically relevant platforms for evaluating tissue-specific responses to antimicrobial coatings. These models can detect subtle toxicity mechanisms that might be overlooked in traditional cell culture systems.
In vivo assessment methodologies remain the gold standard for comprehensive safety evaluation, despite ethical considerations and resource intensiveness. These typically include implantation studies in small animal models with histopathological analysis of surrounding tissues, systemic toxicity evaluations through blood chemistry and organ function tests, and immunological response assessments. Importantly, long-term studies examining chronic exposure effects have become increasingly emphasized in regulatory frameworks.
Leaching and degradation studies constitute another critical component of safety assessment, focusing on the potential release of coating components into surrounding tissues or environments. Analytical techniques such as high-performance liquid chromatography (HPLC), mass spectrometry, and atomic absorption spectroscopy are employed to quantify leachable compounds and degradation products under physiologically relevant conditions.
Standardization efforts by organizations including ISO, ASTM, and regulatory bodies have established harmonized testing protocols that enhance reproducibility and regulatory acceptance. The ISO 10993 series specifically addresses biological evaluation of medical devices, providing structured approaches to toxicity assessment of surface coatings. These standards continue to evolve as new understanding of biointerface interactions emerges.
Emerging methodologies incorporating high-throughput screening approaches, toxicogenomics, and computational toxicology models are revolutionizing safety assessment practices. These advanced techniques enable more comprehensive hazard identification while potentially reducing animal testing requirements through predictive modeling and in silico approaches.
In vitro cytotoxicity assays represent the first line of evaluation, typically employing cell culture models such as fibroblasts, keratinocytes, and macrophages to assess direct cellular responses to coating materials. Standard protocols include MTT/XTT assays for metabolic activity, LDH release for membrane integrity, and live/dead staining techniques. These methods provide rapid screening capabilities but must be interpreted with caution due to their limited representation of complex biological interactions.
Advanced ex vivo models have emerged as critical intermediate assessment tools, bridging the gap between simplified cell cultures and complex living systems. Three-dimensional tissue constructs, organ-on-chip technologies, and explant cultures offer more physiologically relevant platforms for evaluating tissue-specific responses to antimicrobial coatings. These models can detect subtle toxicity mechanisms that might be overlooked in traditional cell culture systems.
In vivo assessment methodologies remain the gold standard for comprehensive safety evaluation, despite ethical considerations and resource intensiveness. These typically include implantation studies in small animal models with histopathological analysis of surrounding tissues, systemic toxicity evaluations through blood chemistry and organ function tests, and immunological response assessments. Importantly, long-term studies examining chronic exposure effects have become increasingly emphasized in regulatory frameworks.
Leaching and degradation studies constitute another critical component of safety assessment, focusing on the potential release of coating components into surrounding tissues or environments. Analytical techniques such as high-performance liquid chromatography (HPLC), mass spectrometry, and atomic absorption spectroscopy are employed to quantify leachable compounds and degradation products under physiologically relevant conditions.
Standardization efforts by organizations including ISO, ASTM, and regulatory bodies have established harmonized testing protocols that enhance reproducibility and regulatory acceptance. The ISO 10993 series specifically addresses biological evaluation of medical devices, providing structured approaches to toxicity assessment of surface coatings. These standards continue to evolve as new understanding of biointerface interactions emerges.
Emerging methodologies incorporating high-throughput screening approaches, toxicogenomics, and computational toxicology models are revolutionizing safety assessment practices. These advanced techniques enable more comprehensive hazard identification while potentially reducing animal testing requirements through predictive modeling and in silico approaches.
Regulatory Framework for Medical Surface Coatings
The regulatory landscape for antimicrobial surface coatings in medical applications is complex and multifaceted, requiring manufacturers to navigate various approval pathways depending on the intended use and jurisdiction. In the United States, the Food and Drug Administration (FDA) classifies antimicrobial coatings based on their mechanism of action and claims. Coatings that make specific antimicrobial claims are typically regulated as medical devices under the 510(k) premarket notification process, requiring demonstration of substantial equivalence to legally marketed devices.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which impose stringent requirements for safety and performance of medical devices, including those with antimicrobial coatings. These regulations emphasize the importance of biocompatibility testing according to ISO 10993 standards, particularly focusing on cytotoxicity, sensitization, and irritation potential.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for antimicrobial medical devices, requiring comprehensive biocompatibility data and evidence of antimicrobial efficacy without promoting antimicrobial resistance. Similarly, China's National Medical Products Administration (NMPA) has developed regulatory frameworks that address both the safety and effectiveness of antimicrobial coatings.
International standards play a crucial role in harmonizing regulatory approaches across different jurisdictions. ISO 10993 series, particularly ISO 10993-1 for biological evaluation of medical devices, provides a framework for biocompatibility assessment. Additionally, ASTM E2180 and JIS Z 2801 standards offer standardized methods for evaluating antimicrobial activity on surfaces.
Recent regulatory trends indicate a growing emphasis on the environmental impact of antimicrobial agents, with restrictions on certain biocides under regulations such as the EU Biocidal Products Regulation (BPR). Regulatory bodies are increasingly requiring manufacturers to demonstrate that antimicrobial coatings do not contribute to the development of antimicrobial resistance, a significant global health concern.
The regulatory pathway for novel antimicrobial coating technologies often involves early engagement with regulatory authorities through pre-submission consultations. This approach helps manufacturers understand specific testing requirements and potential regulatory challenges. For combination products that incorporate both device and drug components, additional regulatory considerations apply, potentially involving multiple regulatory pathways.
Compliance with these regulatory frameworks necessitates robust quality management systems and post-market surveillance to monitor the long-term safety and performance of antimicrobial coatings in clinical settings. Manufacturers must maintain vigilance regarding evolving regulatory requirements and scientific understanding of biocompatibility and antimicrobial resistance mechanisms.
The European Union has implemented the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which impose stringent requirements for safety and performance of medical devices, including those with antimicrobial coatings. These regulations emphasize the importance of biocompatibility testing according to ISO 10993 standards, particularly focusing on cytotoxicity, sensitization, and irritation potential.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for antimicrobial medical devices, requiring comprehensive biocompatibility data and evidence of antimicrobial efficacy without promoting antimicrobial resistance. Similarly, China's National Medical Products Administration (NMPA) has developed regulatory frameworks that address both the safety and effectiveness of antimicrobial coatings.
International standards play a crucial role in harmonizing regulatory approaches across different jurisdictions. ISO 10993 series, particularly ISO 10993-1 for biological evaluation of medical devices, provides a framework for biocompatibility assessment. Additionally, ASTM E2180 and JIS Z 2801 standards offer standardized methods for evaluating antimicrobial activity on surfaces.
Recent regulatory trends indicate a growing emphasis on the environmental impact of antimicrobial agents, with restrictions on certain biocides under regulations such as the EU Biocidal Products Regulation (BPR). Regulatory bodies are increasingly requiring manufacturers to demonstrate that antimicrobial coatings do not contribute to the development of antimicrobial resistance, a significant global health concern.
The regulatory pathway for novel antimicrobial coating technologies often involves early engagement with regulatory authorities through pre-submission consultations. This approach helps manufacturers understand specific testing requirements and potential regulatory challenges. For combination products that incorporate both device and drug components, additional regulatory considerations apply, potentially involving multiple regulatory pathways.
Compliance with these regulatory frameworks necessitates robust quality management systems and post-market surveillance to monitor the long-term safety and performance of antimicrobial coatings in clinical settings. Manufacturers must maintain vigilance regarding evolving regulatory requirements and scientific understanding of biocompatibility and antimicrobial resistance mechanisms.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!