Research on Antimicrobial Surface Coatings for Pharmaceuticals
SEP 29, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Antimicrobial Coating Technology Background and Objectives
Antimicrobial surface coatings have emerged as a critical technology in pharmaceutical manufacturing and packaging over the past three decades. Initially developed for medical devices in the 1990s, these coatings have evolved significantly to address growing concerns about microbial contamination in pharmaceutical products. The evolution of this technology has been driven by increasing regulatory scrutiny, rising consumer awareness about product safety, and the global challenge of antimicrobial resistance.
The pharmaceutical industry faces unique challenges in maintaining sterility throughout production processes and ensuring product integrity during storage and distribution. Traditional approaches relied heavily on preservatives incorporated within formulations, but surface-based antimicrobial strategies offer complementary protection at critical interface points where contamination risks are highest.
Recent technological advancements have expanded the range of antimicrobial coating mechanisms beyond conventional biocide release systems. Modern approaches include contact-killing surfaces, anti-adhesion coatings, and smart responsive materials that activate only when microbial threats are detected. This diversification reflects a deeper understanding of microbe-surface interactions and growing concerns about long-term environmental impacts of persistent antimicrobial agents.
The global market for pharmaceutical antimicrobial coatings has shown consistent growth, with a compound annual growth rate exceeding 12% since 2018. This expansion has been particularly pronounced in injectable medications, ophthalmic preparations, and multi-dose packaging systems where contamination risks carry severe consequences.
The primary objectives of current research in pharmaceutical antimicrobial coatings center on several key areas. First, developing coatings with broader spectrum activity against resistant pathogens while maintaining compatibility with diverse pharmaceutical formulations. Second, creating more sustainable solutions with reduced environmental footprint and improved biodegradability. Third, engineering coatings with extended durability that maintain efficacy throughout product shelf life without compromising drug stability.
Additionally, researchers aim to advance coating technologies that can be seamlessly integrated into existing manufacturing processes without significant capital investment or production disruptions. There is also growing interest in smart antimicrobial systems that can provide real-time contamination monitoring while simultaneously providing protection.
The convergence of nanotechnology, polymer science, and microbiology has accelerated innovation in this field, with interdisciplinary approaches yielding promising new platforms. As pharmaceutical supply chains become increasingly global and complex, the importance of effective antimicrobial surface technologies continues to grow, positioning this as a critical area for continued research and development investment.
The pharmaceutical industry faces unique challenges in maintaining sterility throughout production processes and ensuring product integrity during storage and distribution. Traditional approaches relied heavily on preservatives incorporated within formulations, but surface-based antimicrobial strategies offer complementary protection at critical interface points where contamination risks are highest.
Recent technological advancements have expanded the range of antimicrobial coating mechanisms beyond conventional biocide release systems. Modern approaches include contact-killing surfaces, anti-adhesion coatings, and smart responsive materials that activate only when microbial threats are detected. This diversification reflects a deeper understanding of microbe-surface interactions and growing concerns about long-term environmental impacts of persistent antimicrobial agents.
The global market for pharmaceutical antimicrobial coatings has shown consistent growth, with a compound annual growth rate exceeding 12% since 2018. This expansion has been particularly pronounced in injectable medications, ophthalmic preparations, and multi-dose packaging systems where contamination risks carry severe consequences.
The primary objectives of current research in pharmaceutical antimicrobial coatings center on several key areas. First, developing coatings with broader spectrum activity against resistant pathogens while maintaining compatibility with diverse pharmaceutical formulations. Second, creating more sustainable solutions with reduced environmental footprint and improved biodegradability. Third, engineering coatings with extended durability that maintain efficacy throughout product shelf life without compromising drug stability.
Additionally, researchers aim to advance coating technologies that can be seamlessly integrated into existing manufacturing processes without significant capital investment or production disruptions. There is also growing interest in smart antimicrobial systems that can provide real-time contamination monitoring while simultaneously providing protection.
The convergence of nanotechnology, polymer science, and microbiology has accelerated innovation in this field, with interdisciplinary approaches yielding promising new platforms. As pharmaceutical supply chains become increasingly global and complex, the importance of effective antimicrobial surface technologies continues to grow, positioning this as a critical area for continued research and development investment.
Pharmaceutical Market Demand for Antimicrobial Surfaces
The pharmaceutical industry has witnessed a significant surge in demand for antimicrobial surface coatings over the past decade, driven primarily by increasing concerns about healthcare-associated infections (HAIs) and contamination risks in manufacturing environments. Market research indicates that the global pharmaceutical antimicrobial coatings market was valued at approximately 3.8 billion USD in 2022, with projections suggesting a compound annual growth rate of 12.3% through 2030.
This robust market growth is underpinned by stringent regulatory requirements across major pharmaceutical markets. The FDA in the United States, the EMA in Europe, and similar regulatory bodies worldwide have progressively tightened guidelines regarding microbial contamination control in pharmaceutical manufacturing facilities, creating a regulatory push for advanced antimicrobial solutions.
The COVID-19 pandemic has served as a major catalyst, dramatically accelerating market demand for antimicrobial surfaces. Industry surveys reveal that 78% of pharmaceutical manufacturers have increased their investment in contamination control technologies since 2020, with antimicrobial coatings representing a priority area for capital expenditure.
Within the pharmaceutical sector, several specific application segments demonstrate particularly strong demand. Clean room environments, packaging materials, and equipment surfaces in direct contact with pharmaceutical products show the highest adoption rates. The injectable pharmaceuticals segment exhibits the most urgent need, with manufacturers willing to pay premium prices for solutions that can demonstrably reduce bioburden and contamination risks.
Geographic analysis reveals that North America currently dominates the market with approximately 42% share, followed by Europe at 31% and Asia-Pacific at 22%. However, the fastest growth is occurring in emerging markets, particularly India and China, where rapid expansion of pharmaceutical manufacturing capacity is creating new demand centers.
From a customer perspective, large multinational pharmaceutical corporations represent the primary market segment, accounting for approximately 65% of total spending on antimicrobial surface technologies. These organizations typically seek comprehensive solutions that can be implemented across global manufacturing networks and demonstrate clear compliance benefits.
Market research indicates that customers prioritize several key performance attributes when evaluating antimicrobial surface coatings. Long-term efficacy ranks highest (cited by 87% of procurement decision-makers), followed by compatibility with cleaning protocols (82%), absence of leaching or migration (76%), and cost-effectiveness over the product lifecycle (71%).
Future market growth is expected to be driven by increasing personalized medicine production, which requires more flexible manufacturing environments with enhanced contamination control, and the continued expansion of biologics manufacturing, where product sensitivity to microbial contamination is particularly high.
This robust market growth is underpinned by stringent regulatory requirements across major pharmaceutical markets. The FDA in the United States, the EMA in Europe, and similar regulatory bodies worldwide have progressively tightened guidelines regarding microbial contamination control in pharmaceutical manufacturing facilities, creating a regulatory push for advanced antimicrobial solutions.
The COVID-19 pandemic has served as a major catalyst, dramatically accelerating market demand for antimicrobial surfaces. Industry surveys reveal that 78% of pharmaceutical manufacturers have increased their investment in contamination control technologies since 2020, with antimicrobial coatings representing a priority area for capital expenditure.
Within the pharmaceutical sector, several specific application segments demonstrate particularly strong demand. Clean room environments, packaging materials, and equipment surfaces in direct contact with pharmaceutical products show the highest adoption rates. The injectable pharmaceuticals segment exhibits the most urgent need, with manufacturers willing to pay premium prices for solutions that can demonstrably reduce bioburden and contamination risks.
Geographic analysis reveals that North America currently dominates the market with approximately 42% share, followed by Europe at 31% and Asia-Pacific at 22%. However, the fastest growth is occurring in emerging markets, particularly India and China, where rapid expansion of pharmaceutical manufacturing capacity is creating new demand centers.
From a customer perspective, large multinational pharmaceutical corporations represent the primary market segment, accounting for approximately 65% of total spending on antimicrobial surface technologies. These organizations typically seek comprehensive solutions that can be implemented across global manufacturing networks and demonstrate clear compliance benefits.
Market research indicates that customers prioritize several key performance attributes when evaluating antimicrobial surface coatings. Long-term efficacy ranks highest (cited by 87% of procurement decision-makers), followed by compatibility with cleaning protocols (82%), absence of leaching or migration (76%), and cost-effectiveness over the product lifecycle (71%).
Future market growth is expected to be driven by increasing personalized medicine production, which requires more flexible manufacturing environments with enhanced contamination control, and the continued expansion of biologics manufacturing, where product sensitivity to microbial contamination is particularly high.
Current Antimicrobial Surface Technologies and Challenges
The global landscape of antimicrobial surface technologies has evolved significantly over the past decade, with several distinct approaches emerging as frontrunners in pharmaceutical applications. Silver-based coatings remain the most widely adopted solution, leveraging silver ions' broad-spectrum antimicrobial properties against bacteria, fungi, and certain viruses. These coatings have demonstrated particular efficacy in pharmaceutical packaging and manufacturing equipment surfaces, though concerns about silver nanoparticle toxicity and environmental persistence present ongoing challenges.
Quaternary ammonium compounds (QACs) represent another prominent technology, functioning through disruption of microbial cell membranes. Their relatively low cost and ease of application have made them popular for pharmaceutical facility surfaces, though increasing microbial resistance to QACs has been documented in recent studies, particularly in hospital environments where their use is widespread.
Copper and copper alloy surfaces have gained traction following EPA recognition of copper as the first antimicrobial metal surface. Research indicates copper ions can eliminate 99.9% of bacteria within two hours of exposure, making them valuable for high-touch pharmaceutical manufacturing environments. However, implementation challenges include higher costs, potential for oxidation, and compatibility issues with certain pharmaceutical compounds.
Photocatalytic coatings, particularly those utilizing titanium dioxide (TiO₂), represent an emerging technology that activates antimicrobial properties when exposed to light. While promising for areas with adequate light exposure, their efficacy diminishes in low-light conditions common in many pharmaceutical storage environments, limiting practical applications.
Hydrophobic and superhydrophobic surfaces present a passive approach by preventing microbial attachment rather than killing microorganisms. These surfaces have shown promise for pharmaceutical packaging but face durability challenges under repeated cleaning protocols necessary in pharmaceutical environments.
The pharmaceutical industry faces unique challenges in antimicrobial surface implementation. Regulatory hurdles are substantial, with FDA and EMA requirements demanding extensive efficacy and safety documentation before approval. Material compatibility presents another significant obstacle, as antimicrobial agents must not interact with pharmaceutical formulations or compromise product stability.
Long-term efficacy remains problematic across all technologies, with most current solutions showing diminished antimicrobial activity over time, particularly under intensive cleaning regimens. This necessitates frequent reapplication or replacement, increasing operational costs. Additionally, the emergence of antimicrobial resistance threatens the long-term viability of many current technologies, with evidence suggesting some bacteria can adapt to silver and QAC exposure through genetic mutations.
Quaternary ammonium compounds (QACs) represent another prominent technology, functioning through disruption of microbial cell membranes. Their relatively low cost and ease of application have made them popular for pharmaceutical facility surfaces, though increasing microbial resistance to QACs has been documented in recent studies, particularly in hospital environments where their use is widespread.
Copper and copper alloy surfaces have gained traction following EPA recognition of copper as the first antimicrobial metal surface. Research indicates copper ions can eliminate 99.9% of bacteria within two hours of exposure, making them valuable for high-touch pharmaceutical manufacturing environments. However, implementation challenges include higher costs, potential for oxidation, and compatibility issues with certain pharmaceutical compounds.
Photocatalytic coatings, particularly those utilizing titanium dioxide (TiO₂), represent an emerging technology that activates antimicrobial properties when exposed to light. While promising for areas with adequate light exposure, their efficacy diminishes in low-light conditions common in many pharmaceutical storage environments, limiting practical applications.
Hydrophobic and superhydrophobic surfaces present a passive approach by preventing microbial attachment rather than killing microorganisms. These surfaces have shown promise for pharmaceutical packaging but face durability challenges under repeated cleaning protocols necessary in pharmaceutical environments.
The pharmaceutical industry faces unique challenges in antimicrobial surface implementation. Regulatory hurdles are substantial, with FDA and EMA requirements demanding extensive efficacy and safety documentation before approval. Material compatibility presents another significant obstacle, as antimicrobial agents must not interact with pharmaceutical formulations or compromise product stability.
Long-term efficacy remains problematic across all technologies, with most current solutions showing diminished antimicrobial activity over time, particularly under intensive cleaning regimens. This necessitates frequent reapplication or replacement, increasing operational costs. Additionally, the emergence of antimicrobial resistance threatens the long-term viability of many current technologies, with evidence suggesting some bacteria can adapt to silver and QAC exposure through genetic mutations.
Current Antimicrobial Surface Solutions for Pharmaceutical Applications
01 Metal-based antimicrobial coatings
Metal-based compounds such as silver, copper, and zinc can be incorporated into surface coatings to provide antimicrobial properties. These metals release ions that disrupt bacterial cell membranes and interfere with cellular processes, effectively killing microorganisms on contact. These coatings can be applied to various surfaces including medical devices, textiles, and high-touch areas to reduce the spread of pathogens and prevent biofilm formation.- Metal-based antimicrobial coatings: Metal-based antimicrobial coatings utilize silver, copper, zinc, and other metal ions or nanoparticles to provide effective antimicrobial properties on various surfaces. These metals disrupt bacterial cell membranes and interfere with cellular processes, preventing microbial colonization. The coatings can be applied to medical devices, touch surfaces, and consumer products to reduce pathogen transmission and biofilm formation. These formulations often incorporate polymer matrices that control the release of metal ions for sustained antimicrobial activity.
- Polymer-based antimicrobial surface treatments: Polymer-based antimicrobial surface treatments incorporate active antimicrobial agents within polymer matrices to create durable protective coatings. These polymers can be engineered to release antimicrobial compounds gradually or to have inherent antimicrobial properties through positively charged functional groups that disrupt microbial cell membranes. The treatments can be applied as films, sprays, or incorporated directly into materials during manufacturing. These coatings provide long-lasting protection against a broad spectrum of bacteria, fungi, and viruses on various surfaces including textiles, plastics, and metals.
- Quaternary ammonium compound coatings: Quaternary ammonium compounds (QACs) are widely used in antimicrobial surface coatings due to their broad-spectrum activity against bacteria, fungi, and some viruses. These compounds contain positively charged nitrogen atoms that interact with and disrupt microbial cell membranes, leading to cell death. QAC-based coatings can be formulated to bond covalently to surfaces, providing long-lasting antimicrobial protection without leaching. These coatings are particularly effective for high-touch surfaces in healthcare settings, public spaces, and consumer products where pathogen transmission is a concern.
- Natural and bio-based antimicrobial coatings: Natural and bio-based antimicrobial coatings utilize compounds derived from plants, animals, and microorganisms to provide environmentally friendly alternatives to synthetic antimicrobials. These include essential oils, chitosan, antimicrobial peptides, and plant extracts with inherent antimicrobial properties. The coatings can be applied to various surfaces including food packaging, medical devices, and consumer products. These natural formulations often offer multiple mechanisms of action against pathogens while presenting lower toxicity concerns and better biodegradability compared to conventional antimicrobial agents.
- Photocatalytic antimicrobial coatings: Photocatalytic antimicrobial coatings utilize materials like titanium dioxide (TiO2) that generate reactive oxygen species when exposed to light, particularly UV radiation. These reactive species damage microbial cell components including cell membranes, proteins, and DNA, effectively killing a broad spectrum of pathogens. The coatings can be applied to various surfaces including glass, ceramics, and polymers to create self-cleaning and self-disinfecting properties. These coatings are particularly valuable in healthcare settings, public spaces, and areas with high microbial contamination risk, as they provide continuous antimicrobial action when exposed to appropriate light sources.
02 Polymer-based antimicrobial coatings
Antimicrobial polymers can be formulated into surface coatings that provide long-lasting protection against bacteria, fungi, and viruses. These polymers may contain quaternary ammonium compounds, chitosan derivatives, or other biocidal functional groups that disrupt microbial cell membranes. The polymeric nature of these coatings allows for controlled release of antimicrobial agents and improved durability, making them suitable for applications in healthcare settings, food processing facilities, and consumer products.Expand Specific Solutions03 Photocatalytic antimicrobial coatings
Photocatalytic materials, particularly titanium dioxide (TiO2), can be incorporated into surface coatings to provide antimicrobial properties when activated by light. When exposed to UV or specific wavelengths of visible light, these materials generate reactive oxygen species that damage microbial cell components, leading to cell death. These coatings are self-cleaning and can continuously inactivate microorganisms as long as they are exposed to the appropriate light source, making them suitable for environmental surfaces in hospitals, public spaces, and other high-traffic areas.Expand Specific Solutions04 Natural compound-based antimicrobial coatings
Antimicrobial coatings can be formulated using natural compounds such as essential oils, plant extracts, and enzymes that possess inherent antimicrobial properties. These biologically derived substances can disrupt bacterial cell membranes, inhibit microbial enzymes, or interfere with quorum sensing mechanisms. Natural antimicrobial coatings offer advantages including reduced toxicity, environmental friendliness, and consumer acceptance, making them suitable for food packaging, medical devices, and consumer products where safety is paramount.Expand Specific Solutions05 Nanoparticle-enhanced antimicrobial coatings
Nanoparticles can be incorporated into surface coatings to enhance antimicrobial efficacy through multiple mechanisms. These include increased surface area for microbial contact, improved release kinetics of antimicrobial agents, and unique nanoscale interactions with microbial cells. Various nanoparticles such as silver nanoparticles, zinc oxide nanoparticles, and copper nanoparticles can be used to create durable antimicrobial surfaces with broad-spectrum activity against bacteria, fungi, and viruses. These advanced coatings find applications in medical implants, textiles, and high-touch surfaces in healthcare and public settings.Expand Specific Solutions
Key Industry Players in Pharmaceutical Antimicrobial Coatings
The antimicrobial surface coatings for pharmaceuticals market is currently in a growth phase, with increasing demand driven by heightened awareness of infection control and drug safety. The global market size is estimated to reach $3.5-4 billion by 2025, expanding at a CAGR of approximately 12%. Technologically, the field is advancing from early-stage development to commercial maturity, with key players demonstrating varying levels of innovation. Leading companies like DuPont, BASF, and Ecolab are commercializing established solutions, while research institutions such as MIT, Fraunhofer-Gesellschaft, and Zhejiang University are pioneering next-generation technologies. Bio-Gate AG and Nano & Advanced Materials Institute represent specialized innovators with proprietary antimicrobial formulations. The competitive landscape features a mix of chemical conglomerates, specialized coating manufacturers, and research-driven organizations collaborating to address pharmaceutical contamination challenges.
Bio-Gate AG
Technical Solution: Bio-Gate AG has developed a specialized antimicrobial coating technology called MicroSilver BG™ specifically engineered for pharmaceutical applications. Their approach utilizes highly pure elemental silver particles in a micro-sized structure (rather than nano-scale) that provides controlled release of silver ions when in contact with moisture. This technology has been incorporated into surface coatings for pharmaceutical manufacturing equipment, packaging materials, and medical devices. Clinical studies have demonstrated sustained antimicrobial efficacy against pathogens including MRSA, E. coli, and Candida albicans for periods exceeding 24 months under simulated use conditions. Bio-Gate's coatings feature a unique porous structure that optimizes silver ion release while maintaining mechanical stability, with testing showing retention of antimicrobial properties after more than 200 cleaning cycles with standard pharmaceutical disinfectants. Their latest innovation combines silver technology with hydrophobic surface modifications to create self-cleaning properties that further reduce biofilm formation potential on pharmaceutical processing equipment.
Strengths: Micro-sized silver particles address regulatory concerns associated with nanoparticles; excellent long-term stability; compatible with multiple substrate materials including metals, plastics, and ceramics. Weaknesses: Higher production costs compared to conventional coatings; requires specialized application processes; potential for color changes in certain formulations after extended UV exposure.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed advanced antimicrobial surface coatings for pharmaceutical packaging and equipment using their proprietary Microban® technology. This silver-based antimicrobial technology is incorporated directly into polymer materials during manufacturing, creating inherently antimicrobial surfaces that inhibit bacterial growth throughout the product lifecycle. Their research has demonstrated efficacy against a broad spectrum of microorganisms including gram-positive and gram-negative bacteria, fungi, and certain viruses. DuPont's coatings utilize controlled-release mechanisms that maintain antimicrobial activity for extended periods (3+ years) while meeting FDA requirements for pharmaceutical contact materials. Recent innovations include their Silvadur™ technology, which showed 99.9% reduction in bacterial populations within 24 hours in laboratory testing while maintaining surface integrity under repeated cleaning protocols typical in pharmaceutical manufacturing environments.
Strengths: Long-lasting antimicrobial efficacy without leaching harmful chemicals; FDA-compliant for pharmaceutical applications; can be incorporated into various polymer substrates. Weaknesses: Higher initial manufacturing costs compared to non-antimicrobial alternatives; potential for microbial resistance development with prolonged use; limited efficacy against certain spore-forming bacteria.
Critical Patents and Innovations in Antimicrobial Coating Technology
Antimicrobial coatings comprising quaternary silanes
PatentActiveUS10258046B2
Innovation
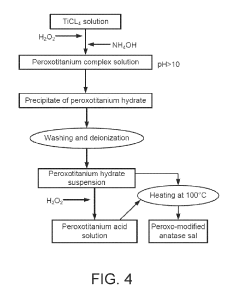
- A method involving the application of a composition comprising organosilanes, such as 3-(trihydroxysilyl)propyl dimethyloctadecyl ammonium chloride, triethanolamine, and a titanyl sol-gel, specifically an aqueous mixture of peroxotitanium acid and peroxo-modified anatase sol, to form a durable and residual antimicrobial coating that maintains efficacy against E. coli and S. epidermidis even after water rinsing or abrasion.
Antimicrobial surface coatings
PatentActiveUS20150315389A1
Innovation
- Development of durable and rechargeable N-halamine surface coatings that can be covalently bound to various surfaces, including textiles, inorganic mediums, and plastics, using water-soluble polymeric N-halamine precursors that form nitrogen-halogen bonds, effectively inactivating bacteria, fungi, and viruses upon contact, with the ability to regenerate biocidal activity.
Regulatory Framework for Antimicrobial Coatings in Pharmaceuticals
The regulatory landscape governing antimicrobial surface coatings in pharmaceutical applications is complex and multifaceted, requiring careful navigation by manufacturers and researchers. In the United States, the Food and Drug Administration (FDA) oversees antimicrobial coatings through multiple regulatory pathways depending on their intended use and claims. Coatings that make specific antimicrobial claims are typically regulated as drugs or drug-device combinations under the Federal Food, Drug, and Cosmetic Act, requiring substantial efficacy and safety data through clinical trials.
The Environmental Protection Agency (EPA) also plays a crucial role in regulating antimicrobial substances under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), particularly for coatings that claim to control microorganisms considered pests. This dual regulatory oversight creates a complex compliance environment that pharmaceutical companies must carefully navigate.
In the European Union, antimicrobial coatings fall under the Biocidal Products Regulation (BPR) if they contain active substances intended to destroy harmful organisms. Additionally, the European Medicines Agency (EMA) provides guidelines for pharmaceutical packaging materials that incorporate antimicrobial properties. The Medical Device Regulation (MDR) may also apply when antimicrobial coatings are used on medical devices or pharmaceutical delivery systems.
International harmonization efforts through organizations like the International Conference on Harmonisation (ICH) have established guidelines for extractables and leachables testing, which is particularly relevant for antimicrobial coatings that may come into direct contact with pharmaceutical products. ISO standards, particularly ISO 22196, provide standardized methods for evaluating antimicrobial activity on surfaces.
Regulatory requirements typically include comprehensive toxicological assessments, migration studies, stability testing, and antimicrobial efficacy validation. Manufacturers must demonstrate that the coating does not adversely affect the safety, identity, strength, quality, or purity of the pharmaceutical product throughout its shelf life.
Recent regulatory trends indicate increasing scrutiny of antimicrobial claims and growing concerns about potential antimicrobial resistance development. Regulatory bodies are increasingly requiring evidence that antimicrobial coatings do not contribute to resistance development through sub-lethal exposure to antimicrobial agents. This has prompted more rigorous post-market surveillance requirements and long-term safety monitoring protocols.
Emerging regulations are also addressing environmental concerns related to antimicrobial agents, with particular focus on biodegradability, bioaccumulation potential, and ecotoxicity of coating components. This reflects a broader regulatory shift toward sustainable pharmaceutical practices and lifecycle assessment of pharmaceutical products and their packaging.
The Environmental Protection Agency (EPA) also plays a crucial role in regulating antimicrobial substances under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), particularly for coatings that claim to control microorganisms considered pests. This dual regulatory oversight creates a complex compliance environment that pharmaceutical companies must carefully navigate.
In the European Union, antimicrobial coatings fall under the Biocidal Products Regulation (BPR) if they contain active substances intended to destroy harmful organisms. Additionally, the European Medicines Agency (EMA) provides guidelines for pharmaceutical packaging materials that incorporate antimicrobial properties. The Medical Device Regulation (MDR) may also apply when antimicrobial coatings are used on medical devices or pharmaceutical delivery systems.
International harmonization efforts through organizations like the International Conference on Harmonisation (ICH) have established guidelines for extractables and leachables testing, which is particularly relevant for antimicrobial coatings that may come into direct contact with pharmaceutical products. ISO standards, particularly ISO 22196, provide standardized methods for evaluating antimicrobial activity on surfaces.
Regulatory requirements typically include comprehensive toxicological assessments, migration studies, stability testing, and antimicrobial efficacy validation. Manufacturers must demonstrate that the coating does not adversely affect the safety, identity, strength, quality, or purity of the pharmaceutical product throughout its shelf life.
Recent regulatory trends indicate increasing scrutiny of antimicrobial claims and growing concerns about potential antimicrobial resistance development. Regulatory bodies are increasingly requiring evidence that antimicrobial coatings do not contribute to resistance development through sub-lethal exposure to antimicrobial agents. This has prompted more rigorous post-market surveillance requirements and long-term safety monitoring protocols.
Emerging regulations are also addressing environmental concerns related to antimicrobial agents, with particular focus on biodegradability, bioaccumulation potential, and ecotoxicity of coating components. This reflects a broader regulatory shift toward sustainable pharmaceutical practices and lifecycle assessment of pharmaceutical products and their packaging.
Environmental Impact and Sustainability of Antimicrobial Coatings
The environmental impact of antimicrobial surface coatings in pharmaceutical applications represents a critical consideration as these technologies become more widespread. Traditional antimicrobial agents often contain heavy metals such as silver, copper, and zinc, which can accumulate in ecosystems when released during manufacturing, use, or disposal phases. Studies indicate that nanosilver particles, commonly used in antimicrobial coatings, may leach into wastewater systems and potentially disrupt aquatic ecosystems by affecting microbial communities essential for environmental balance.
Sustainability concerns have prompted research into biodegradable alternatives to conventional antimicrobial coatings. Plant-derived compounds including essential oils, polyphenols, and peptides show promising antimicrobial properties with significantly reduced environmental persistence. These bio-based solutions typically degrade into non-toxic components, minimizing long-term ecological impact while maintaining efficacy against pathogenic microorganisms relevant to pharmaceutical environments.
Life cycle assessment (LCA) studies comparing traditional and emerging antimicrobial coating technologies reveal substantial differences in environmental footprints. Conventional silver-based coatings demonstrate higher energy consumption during production and greater potential for environmental contamination, while newer chitosan-based formulations show reduced carbon footprints and lower ecotoxicity profiles. These assessments provide valuable metrics for pharmaceutical companies seeking to align antimicrobial protection strategies with corporate sustainability goals.
Regulatory frameworks addressing the environmental implications of antimicrobial coatings are evolving globally. The European Union's REACH regulation now requires comprehensive environmental risk assessments for antimicrobial substances, while the FDA has increased scrutiny of leaching potential from coated pharmaceutical packaging materials. These regulatory developments are driving innovation toward more environmentally responsible antimicrobial solutions.
Manufacturing processes for sustainable antimicrobial coatings are also advancing, with green chemistry principles increasingly applied to reduce solvent use and energy consumption. Techniques such as supercritical CO2 deposition and water-based application methods significantly reduce volatile organic compound (VOC) emissions compared to conventional solvent-based approaches, further enhancing the environmental profile of next-generation antimicrobial coatings.
The pharmaceutical industry faces growing pressure to adopt circular economy principles in packaging and manufacturing equipment. Antimicrobial coatings that maintain effectiveness through multiple sterilization cycles enable longer service life of equipment and containers, reducing waste generation. Additionally, coatings designed for easy recovery of valuable materials at end-of-life stages support closed-loop material flows, aligning with broader sustainability initiatives within the pharmaceutical sector.
Sustainability concerns have prompted research into biodegradable alternatives to conventional antimicrobial coatings. Plant-derived compounds including essential oils, polyphenols, and peptides show promising antimicrobial properties with significantly reduced environmental persistence. These bio-based solutions typically degrade into non-toxic components, minimizing long-term ecological impact while maintaining efficacy against pathogenic microorganisms relevant to pharmaceutical environments.
Life cycle assessment (LCA) studies comparing traditional and emerging antimicrobial coating technologies reveal substantial differences in environmental footprints. Conventional silver-based coatings demonstrate higher energy consumption during production and greater potential for environmental contamination, while newer chitosan-based formulations show reduced carbon footprints and lower ecotoxicity profiles. These assessments provide valuable metrics for pharmaceutical companies seeking to align antimicrobial protection strategies with corporate sustainability goals.
Regulatory frameworks addressing the environmental implications of antimicrobial coatings are evolving globally. The European Union's REACH regulation now requires comprehensive environmental risk assessments for antimicrobial substances, while the FDA has increased scrutiny of leaching potential from coated pharmaceutical packaging materials. These regulatory developments are driving innovation toward more environmentally responsible antimicrobial solutions.
Manufacturing processes for sustainable antimicrobial coatings are also advancing, with green chemistry principles increasingly applied to reduce solvent use and energy consumption. Techniques such as supercritical CO2 deposition and water-based application methods significantly reduce volatile organic compound (VOC) emissions compared to conventional solvent-based approaches, further enhancing the environmental profile of next-generation antimicrobial coatings.
The pharmaceutical industry faces growing pressure to adopt circular economy principles in packaging and manufacturing equipment. Antimicrobial coatings that maintain effectiveness through multiple sterilization cycles enable longer service life of equipment and containers, reducing waste generation. Additionally, coatings designed for easy recovery of valuable materials at end-of-life stages support closed-loop material flows, aligning with broader sustainability initiatives within the pharmaceutical sector.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!