Regulatory Parameters for Safe Use of Antimicrobial Surface Coatings
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Antimicrobial Coating Technology Background and Objectives
Antimicrobial surface coatings have emerged as a critical technology in the global fight against pathogenic microorganisms, particularly in healthcare settings, food processing facilities, and public spaces. The development of these coatings traces back to the early 1990s, with significant advancements occurring in the past two decades due to increased concerns about hospital-acquired infections and foodborne illnesses. The evolution of this technology has been characterized by a shift from simple biocidal agents to more sophisticated, controlled-release systems and contact-killing surfaces.
The technological trajectory has moved from conventional antimicrobial agents such as silver and copper to more advanced nanomaterials, photocatalytic compounds, and enzyme-based systems. Recent innovations have focused on developing coatings that maintain efficacy over extended periods while minimizing environmental impact and potential for antimicrobial resistance development.
Current research emphasizes the development of regulatory frameworks that can effectively balance antimicrobial efficacy with human and environmental safety. This includes establishing standardized testing protocols, exposure limits, and classification systems for different types of antimicrobial coatings based on their mechanisms of action and potential risks.
The primary technical objectives in this field include developing parameters for assessing long-term efficacy under real-world conditions, establishing safety thresholds for various antimicrobial agents in different application contexts, and creating harmonized international standards for evaluation and approval processes. Additionally, there is a growing focus on understanding the potential for antimicrobial resistance development resulting from widespread coating use.
Another critical goal is to establish clear guidelines for lifecycle assessment of these coatings, including production, application, use phase, and disposal or recycling. This holistic approach aims to prevent unintended consequences such as environmental contamination or occupational exposure risks.
The field is also witnessing increased attention to the development of "smart" antimicrobial coatings that can respond to environmental triggers or microbial presence, thereby optimizing antimicrobial action while minimizing unnecessary release of active compounds. This represents a promising direction for addressing regulatory concerns about continuous exposure to antimicrobial agents.
As global health challenges continue to evolve, including the emergence of new pathogens and antimicrobial-resistant strains, the regulatory framework for antimicrobial coatings must balance innovation with precaution. The ultimate objective is to establish science-based parameters that enable the safe and effective deployment of this technology across diverse applications while protecting public health and environmental integrity.
The technological trajectory has moved from conventional antimicrobial agents such as silver and copper to more advanced nanomaterials, photocatalytic compounds, and enzyme-based systems. Recent innovations have focused on developing coatings that maintain efficacy over extended periods while minimizing environmental impact and potential for antimicrobial resistance development.
Current research emphasizes the development of regulatory frameworks that can effectively balance antimicrobial efficacy with human and environmental safety. This includes establishing standardized testing protocols, exposure limits, and classification systems for different types of antimicrobial coatings based on their mechanisms of action and potential risks.
The primary technical objectives in this field include developing parameters for assessing long-term efficacy under real-world conditions, establishing safety thresholds for various antimicrobial agents in different application contexts, and creating harmonized international standards for evaluation and approval processes. Additionally, there is a growing focus on understanding the potential for antimicrobial resistance development resulting from widespread coating use.
Another critical goal is to establish clear guidelines for lifecycle assessment of these coatings, including production, application, use phase, and disposal or recycling. This holistic approach aims to prevent unintended consequences such as environmental contamination or occupational exposure risks.
The field is also witnessing increased attention to the development of "smart" antimicrobial coatings that can respond to environmental triggers or microbial presence, thereby optimizing antimicrobial action while minimizing unnecessary release of active compounds. This represents a promising direction for addressing regulatory concerns about continuous exposure to antimicrobial agents.
As global health challenges continue to evolve, including the emergence of new pathogens and antimicrobial-resistant strains, the regulatory framework for antimicrobial coatings must balance innovation with precaution. The ultimate objective is to establish science-based parameters that enable the safe and effective deployment of this technology across diverse applications while protecting public health and environmental integrity.
Market Analysis for Antimicrobial Surface Solutions
The global market for antimicrobial surface solutions has experienced significant growth in recent years, driven primarily by increasing awareness of infection control and hygiene standards across multiple sectors. The market value reached approximately $3.6 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 12.1% through 2028, potentially reaching $7.2 billion by the end of the forecast period.
Healthcare facilities represent the largest market segment, accounting for roughly 38% of the total market share. This dominance stems from stringent infection control protocols and the critical need to prevent healthcare-associated infections (HAIs). The food processing industry follows as the second-largest consumer of antimicrobial surface solutions, representing about 24% of the market, where contamination prevention directly impacts product safety and shelf life.
Regional analysis indicates North America currently leads the market with approximately 35% share, followed closely by Europe at 30%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 14.3% annually, primarily driven by rapid healthcare infrastructure development, increasing healthcare expenditure, and growing awareness about infection control in countries like China, India, and Japan.
Consumer preferences are increasingly shifting toward environmentally sustainable antimicrobial solutions. Products featuring non-toxic, biodegradable components have seen a 27% increase in demand over the past two years. This trend aligns with stricter regulatory frameworks being implemented globally regarding chemical safety and environmental impact.
The COVID-19 pandemic served as a significant market accelerator, with a 34% spike in demand observed during 2020-2021. While growth has normalized somewhat post-pandemic, baseline demand remains substantially higher than pre-pandemic levels, indicating a permanent shift in hygiene consciousness among institutional buyers and consumers alike.
Key market restraints include regulatory hurdles for new antimicrobial compounds, with approval timelines averaging 18-24 months in developed markets. Additionally, price sensitivity remains high in emerging economies, where cost often outweighs performance considerations in purchasing decisions.
Market fragmentation is notable, with the top five manufacturers controlling approximately 43% of the global market. However, numerous specialized regional players collectively hold significant market share, particularly in applications requiring customized solutions for specific industries or environments.
Healthcare facilities represent the largest market segment, accounting for roughly 38% of the total market share. This dominance stems from stringent infection control protocols and the critical need to prevent healthcare-associated infections (HAIs). The food processing industry follows as the second-largest consumer of antimicrobial surface solutions, representing about 24% of the market, where contamination prevention directly impacts product safety and shelf life.
Regional analysis indicates North America currently leads the market with approximately 35% share, followed closely by Europe at 30%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 14.3% annually, primarily driven by rapid healthcare infrastructure development, increasing healthcare expenditure, and growing awareness about infection control in countries like China, India, and Japan.
Consumer preferences are increasingly shifting toward environmentally sustainable antimicrobial solutions. Products featuring non-toxic, biodegradable components have seen a 27% increase in demand over the past two years. This trend aligns with stricter regulatory frameworks being implemented globally regarding chemical safety and environmental impact.
The COVID-19 pandemic served as a significant market accelerator, with a 34% spike in demand observed during 2020-2021. While growth has normalized somewhat post-pandemic, baseline demand remains substantially higher than pre-pandemic levels, indicating a permanent shift in hygiene consciousness among institutional buyers and consumers alike.
Key market restraints include regulatory hurdles for new antimicrobial compounds, with approval timelines averaging 18-24 months in developed markets. Additionally, price sensitivity remains high in emerging economies, where cost often outweighs performance considerations in purchasing decisions.
Market fragmentation is notable, with the top five manufacturers controlling approximately 43% of the global market. However, numerous specialized regional players collectively hold significant market share, particularly in applications requiring customized solutions for specific industries or environments.
Current Regulatory Landscape and Technical Challenges
The regulatory landscape for antimicrobial surface coatings is complex and varies significantly across different regions and jurisdictions. In the United States, these coatings fall under the purview of multiple regulatory bodies, primarily the Environmental Protection Agency (EPA) under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), which regulates antimicrobial products making public health claims. Additionally, the Food and Drug Administration (FDA) oversees coatings used in food contact surfaces and medical devices, requiring extensive safety and efficacy data.
In the European Union, the Biocidal Products Regulation (BPR) governs antimicrobial coatings, implementing a two-tier approval system: active substance approval at the EU level and product authorization at the member state level. This creates a more stringent framework compared to other regions, particularly regarding the use of silver, copper, and quaternary ammonium compounds in coatings.
Asia-Pacific regions demonstrate varying regulatory maturity, with Japan and South Korea having established frameworks similar to Western standards, while emerging markets often have less defined parameters. China has recently strengthened its regulatory approach through the New Chemical Substance Notification (NCSN) system, though enforcement remains inconsistent.
Technical challenges in meeting these regulatory requirements are substantial. Leaching of active ingredients presents a primary concern, as antimicrobial agents must remain effective while not migrating excessively into the environment or contacting surfaces. Current testing methodologies lack standardization across jurisdictions, creating compliance difficulties for global manufacturers. The ISO 22196 and JIS Z 2801 standards are widely used but criticized for poor correlation with real-world conditions.
Long-term efficacy verification poses another significant challenge. Regulatory bodies increasingly demand evidence of sustained antimicrobial activity throughout the product lifecycle, yet accelerated aging tests often fail to accurately predict performance in diverse environmental conditions. This creates uncertainty in product development and regulatory submission processes.
The toxicological assessment requirements have become more rigorous, particularly regarding potential endocrine disruption and environmental persistence. Many traditional antimicrobial compounds face scrutiny under newer regulatory frameworks, forcing manufacturers to reformulate established products. The EU's REACH regulation and similar initiatives globally have significantly impacted material selection options.
Cross-border regulatory harmonization remains elusive despite industry efforts. The lack of mutual recognition agreements between major markets necessitates multiple testing protocols and submission packages, increasing development costs and time-to-market. This regulatory fragmentation particularly affects innovative small and medium enterprises attempting to enter the antimicrobial coatings market.
In the European Union, the Biocidal Products Regulation (BPR) governs antimicrobial coatings, implementing a two-tier approval system: active substance approval at the EU level and product authorization at the member state level. This creates a more stringent framework compared to other regions, particularly regarding the use of silver, copper, and quaternary ammonium compounds in coatings.
Asia-Pacific regions demonstrate varying regulatory maturity, with Japan and South Korea having established frameworks similar to Western standards, while emerging markets often have less defined parameters. China has recently strengthened its regulatory approach through the New Chemical Substance Notification (NCSN) system, though enforcement remains inconsistent.
Technical challenges in meeting these regulatory requirements are substantial. Leaching of active ingredients presents a primary concern, as antimicrobial agents must remain effective while not migrating excessively into the environment or contacting surfaces. Current testing methodologies lack standardization across jurisdictions, creating compliance difficulties for global manufacturers. The ISO 22196 and JIS Z 2801 standards are widely used but criticized for poor correlation with real-world conditions.
Long-term efficacy verification poses another significant challenge. Regulatory bodies increasingly demand evidence of sustained antimicrobial activity throughout the product lifecycle, yet accelerated aging tests often fail to accurately predict performance in diverse environmental conditions. This creates uncertainty in product development and regulatory submission processes.
The toxicological assessment requirements have become more rigorous, particularly regarding potential endocrine disruption and environmental persistence. Many traditional antimicrobial compounds face scrutiny under newer regulatory frameworks, forcing manufacturers to reformulate established products. The EU's REACH regulation and similar initiatives globally have significantly impacted material selection options.
Cross-border regulatory harmonization remains elusive despite industry efforts. The lack of mutual recognition agreements between major markets necessitates multiple testing protocols and submission packages, increasing development costs and time-to-market. This regulatory fragmentation particularly affects innovative small and medium enterprises attempting to enter the antimicrobial coatings market.
Current Antimicrobial Coating Compliance Frameworks
01 Regulatory compliance for antimicrobial coatings
Antimicrobial surface coatings must comply with various regulatory frameworks depending on their intended use and jurisdiction. These regulations often include safety assessments, efficacy testing protocols, and environmental impact evaluations. Manufacturers must adhere to specific guidelines from regulatory bodies such as the EPA, FDA, or equivalent international authorities when formulating and marketing antimicrobial coatings, particularly for medical devices, food contact surfaces, or public spaces.- FDA and EPA regulatory compliance for antimicrobial coatings: Antimicrobial surface coatings must comply with regulatory frameworks established by agencies such as the FDA and EPA. These regulations govern the safety assessment, efficacy testing, and marketing claims for products intended to prevent microbial growth on surfaces. Compliance requirements vary depending on the intended use, with medical devices facing stricter scrutiny than general consumer products. Manufacturers must demonstrate that antimicrobial agents remain stable and effective throughout the product's lifecycle while meeting leaching and toxicity standards.
- Testing protocols and efficacy standards for antimicrobial claims: Standardized testing protocols are required to validate antimicrobial efficacy claims for surface coatings. These include JIS Z 2801, ISO 22196, and ASTM E2180 for measuring antimicrobial activity on treated surfaces. Testing must demonstrate significant reduction in microbial populations under specified conditions and timeframes. Regulatory bodies require documentation of kill rates against specific pathogens, durability of antimicrobial properties after cleaning cycles, and environmental exposure. Long-term efficacy testing and accelerated aging studies are essential for regulatory approval.
- Environmental impact and toxicity requirements: Regulatory parameters for antimicrobial surface coatings include strict environmental impact and toxicity assessments. Manufacturers must provide data on biodegradability, bioaccumulation potential, and ecotoxicity of active ingredients. Leaching studies are required to determine the release rate of antimicrobial agents into the environment. Products must comply with regulations such as REACH in Europe and the Toxic Substances Control Act in the US. Risk assessments must demonstrate that the coatings pose minimal harm to aquatic organisms, soil microbiota, and non-target species.
- Material safety and biocompatibility requirements: Antimicrobial coatings for medical devices and food-contact surfaces must meet stringent biocompatibility and safety standards. ISO 10993 series provides guidelines for biological evaluation of medical devices with antimicrobial properties. Testing includes cytotoxicity, sensitization, irritation, and systemic toxicity assessments. For food-contact applications, materials must comply with FDA 21 CFR regulations or EU Food Contact Materials Regulation. Manufacturers must demonstrate that antimicrobial agents do not migrate to food in quantities that exceed acceptable limits or alter food composition, taste, or odor.
- Labeling and marketing claim restrictions: Regulatory frameworks impose strict requirements on labeling and marketing claims for antimicrobial surface coatings. Products cannot make claims about preventing disease unless specifically approved as medical devices or public health products. General antimicrobial claims must be supported by scientific evidence and clearly state the specific organisms affected. The EPA requires registration of products making public health claims under FIFRA. Labels must include active ingredient information, safety precautions, application instructions, and limitations of protection. Manufacturers must avoid misleading statements about the duration or scope of antimicrobial protection.
02 Testing standards for antimicrobial efficacy
Standardized testing protocols are essential for validating the efficacy of antimicrobial surface coatings. These include methods for measuring microbial reduction rates, durability of antimicrobial properties under various conditions, and long-term performance evaluation. Testing must follow established standards such as ISO, ASTM, or JIS methodologies to ensure consistent and comparable results across different antimicrobial coating technologies.Expand Specific Solutions03 Environmental and toxicological considerations
Regulatory parameters for antimicrobial coatings include environmental impact assessments and toxicological evaluations. These parameters govern the acceptable levels of leaching, bioaccumulation potential, and ecological effects of active ingredients. Manufacturers must demonstrate that their antimicrobial coatings do not pose unacceptable risks to human health or the environment through comprehensive safety data and risk assessments throughout the product lifecycle.Expand Specific Solutions04 Material compatibility and application methods
Regulatory frameworks address the compatibility of antimicrobial agents with various substrate materials and application methods. Parameters include adhesion properties, stability during application processes, and potential interactions with underlying materials. Regulations may specify acceptable application techniques, curing conditions, and quality control measures to ensure consistent antimicrobial performance while maintaining the integrity of the coated surface.Expand Specific Solutions05 Labeling and marketing claim requirements
Regulatory parameters govern the claims that can be made about antimicrobial surface coatings in marketing materials and product labels. These include specific language requirements, substantiation of efficacy claims, and limitations on public health assertions. Manufacturers must provide sufficient scientific evidence to support their claims and follow jurisdiction-specific guidelines regarding how antimicrobial properties can be communicated to consumers or professional users.Expand Specific Solutions
Key Patents and Scientific Literature on Safety Parameters
Antimicrobial surface coatings
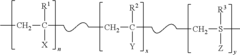
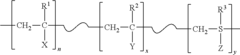
PatentActiveUS20150315389A1
Innovation
- Development of durable and rechargeable N-halamine surface coatings that can be covalently bound to various surfaces, including textiles, inorganic mediums, and plastics, using water-soluble polymeric N-halamine precursors that form nitrogen-halogen bonds, effectively inactivating bacteria, fungi, and viruses upon contact, with the ability to regenerate biocidal activity.
Antimicrobial coating for long-term disinfection of surfaces
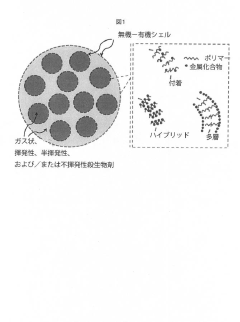
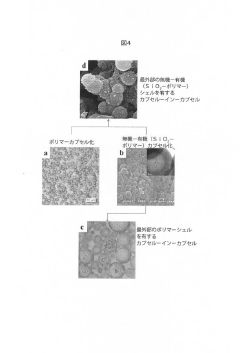
PatentActiveJP2020063260A
Innovation
- Development of colloidal capsules containing biocides within inorganic-organic shells that provide controlled release and contact killing, combining polymers and metal compounds for antimicrobial coatings on surfaces, which can be applied to both porous and non-porous materials.
Environmental Impact Assessment of Antimicrobial Coatings
The environmental impact of antimicrobial surface coatings represents a critical consideration in regulatory frameworks governing their safe use. These coatings, while offering significant benefits in infection control and hygiene maintenance, introduce complex environmental concerns that must be thoroughly assessed before widespread implementation.
Antimicrobial coatings typically contain active ingredients such as silver nanoparticles, copper compounds, quaternary ammonium compounds, or triclosan that can leach into the environment through various pathways. Studies indicate that aquatic ecosystems are particularly vulnerable, with evidence showing bioaccumulation of silver and copper ions in fish and other aquatic organisms, potentially disrupting ecological balance and biodiversity.
Soil contamination presents another significant concern, as antimicrobial agents may persist in soil matrices, affecting microbial communities essential for nutrient cycling and plant health. Research has demonstrated that certain antimicrobial compounds can remain active in soil for extended periods, ranging from months to years depending on environmental conditions and compound stability.
The manufacturing processes for these coatings also contribute to their environmental footprint through energy consumption, chemical waste generation, and greenhouse gas emissions. Life cycle assessments reveal that the environmental impact varies significantly based on production methods, with sol-gel techniques generally showing lower environmental burdens compared to vapor deposition methods.
Waste management challenges emerge at the end of product lifecycles, as antimicrobial-treated surfaces may require specialized disposal protocols to prevent environmental contamination. The potential for these compounds to interfere with wastewater treatment processes further complicates their environmental profile, as they may reduce the efficiency of biological treatment systems.
Regulatory frameworks increasingly incorporate environmental impact assessments as mandatory components for approval of antimicrobial coatings. The European Union's Biocidal Products Regulation (BPR) and the U.S. Environmental Protection Agency's pesticide registration requirements both mandate comprehensive environmental fate and ecotoxicity data. These regulations typically evaluate persistence, bioaccumulation potential, and toxicity to non-target organisms.
Emerging research focuses on developing environmentally friendly alternatives, including biodegradable antimicrobial compounds, biomimetic approaches, and controlled-release technologies that minimize environmental exposure while maintaining efficacy. These innovations aim to address the regulatory challenges by reducing environmental risks without compromising antimicrobial performance.
The establishment of standardized testing protocols for environmental impact assessment represents a crucial development in regulatory science. These protocols enable consistent evaluation across different antimicrobial technologies and provide reliable data for regulatory decision-making, ultimately supporting the development of environmentally sustainable antimicrobial surface solutions.
Antimicrobial coatings typically contain active ingredients such as silver nanoparticles, copper compounds, quaternary ammonium compounds, or triclosan that can leach into the environment through various pathways. Studies indicate that aquatic ecosystems are particularly vulnerable, with evidence showing bioaccumulation of silver and copper ions in fish and other aquatic organisms, potentially disrupting ecological balance and biodiversity.
Soil contamination presents another significant concern, as antimicrobial agents may persist in soil matrices, affecting microbial communities essential for nutrient cycling and plant health. Research has demonstrated that certain antimicrobial compounds can remain active in soil for extended periods, ranging from months to years depending on environmental conditions and compound stability.
The manufacturing processes for these coatings also contribute to their environmental footprint through energy consumption, chemical waste generation, and greenhouse gas emissions. Life cycle assessments reveal that the environmental impact varies significantly based on production methods, with sol-gel techniques generally showing lower environmental burdens compared to vapor deposition methods.
Waste management challenges emerge at the end of product lifecycles, as antimicrobial-treated surfaces may require specialized disposal protocols to prevent environmental contamination. The potential for these compounds to interfere with wastewater treatment processes further complicates their environmental profile, as they may reduce the efficiency of biological treatment systems.
Regulatory frameworks increasingly incorporate environmental impact assessments as mandatory components for approval of antimicrobial coatings. The European Union's Biocidal Products Regulation (BPR) and the U.S. Environmental Protection Agency's pesticide registration requirements both mandate comprehensive environmental fate and ecotoxicity data. These regulations typically evaluate persistence, bioaccumulation potential, and toxicity to non-target organisms.
Emerging research focuses on developing environmentally friendly alternatives, including biodegradable antimicrobial compounds, biomimetic approaches, and controlled-release technologies that minimize environmental exposure while maintaining efficacy. These innovations aim to address the regulatory challenges by reducing environmental risks without compromising antimicrobial performance.
The establishment of standardized testing protocols for environmental impact assessment represents a crucial development in regulatory science. These protocols enable consistent evaluation across different antimicrobial technologies and provide reliable data for regulatory decision-making, ultimately supporting the development of environmentally sustainable antimicrobial surface solutions.
Public Health Implications and Risk Management Strategies
The widespread implementation of antimicrobial surface coatings presents significant public health implications that require comprehensive risk management strategies. These coatings, while offering potential benefits in reducing pathogen transmission, also introduce complex considerations for population health outcomes and regulatory oversight.
Antimicrobial surface technologies can substantially reduce healthcare-associated infections (HAIs), which affect approximately 1.7 million patients annually in the United States alone. Studies indicate that properly implemented antimicrobial surfaces can achieve 15-35% reduction in surface contamination, potentially translating to meaningful decreases in infection rates across healthcare settings, public transportation, and commercial facilities.
However, these benefits must be balanced against potential risks. Long-term exposure to certain antimicrobial compounds may contribute to antimicrobial resistance development—a critical global health concern. Research suggests that sublethal concentrations of biocides can induce cross-resistance to clinically important antibiotics through shared resistance mechanisms, necessitating careful monitoring and mitigation strategies.
Environmental health considerations also warrant attention, as leaching of antimicrobial agents into water systems could disrupt ecological balances and potentially affect non-target organisms. Lifecycle assessments indicate that some silver-based coatings may release nanoparticles during weathering processes, with downstream environmental implications that remain incompletely characterized.
Risk management frameworks for antimicrobial coatings should incorporate multi-tiered approaches. Surveillance systems must be established to monitor both efficacy against target pathogens and potential emergence of resistant strains. These systems should include regular environmental sampling and susceptibility testing to detect early warning signs of resistance development.
Exposure assessment protocols represent another critical component, particularly for vulnerable populations such as immunocompromised patients, children, and pregnant women. Standardized methodologies for evaluating cumulative exposure across multiple coated surfaces in various settings will enable more accurate risk characterization and appropriate mitigation measures.
Public communication strategies constitute an essential element of risk management. Clear messaging regarding appropriate expectations, limitations, and complementary infection control practices helps prevent over-reliance on antimicrobial surfaces and maintains adherence to fundamental hygiene practices. Transparency about the scientific evidence supporting specific applications builds trust and facilitates appropriate implementation decisions.
Antimicrobial surface technologies can substantially reduce healthcare-associated infections (HAIs), which affect approximately 1.7 million patients annually in the United States alone. Studies indicate that properly implemented antimicrobial surfaces can achieve 15-35% reduction in surface contamination, potentially translating to meaningful decreases in infection rates across healthcare settings, public transportation, and commercial facilities.
However, these benefits must be balanced against potential risks. Long-term exposure to certain antimicrobial compounds may contribute to antimicrobial resistance development—a critical global health concern. Research suggests that sublethal concentrations of biocides can induce cross-resistance to clinically important antibiotics through shared resistance mechanisms, necessitating careful monitoring and mitigation strategies.
Environmental health considerations also warrant attention, as leaching of antimicrobial agents into water systems could disrupt ecological balances and potentially affect non-target organisms. Lifecycle assessments indicate that some silver-based coatings may release nanoparticles during weathering processes, with downstream environmental implications that remain incompletely characterized.
Risk management frameworks for antimicrobial coatings should incorporate multi-tiered approaches. Surveillance systems must be established to monitor both efficacy against target pathogens and potential emergence of resistant strains. These systems should include regular environmental sampling and susceptibility testing to detect early warning signs of resistance development.
Exposure assessment protocols represent another critical component, particularly for vulnerable populations such as immunocompromised patients, children, and pregnant women. Standardized methodologies for evaluating cumulative exposure across multiple coated surfaces in various settings will enable more accurate risk characterization and appropriate mitigation measures.
Public communication strategies constitute an essential element of risk management. Clear messaging regarding appropriate expectations, limitations, and complementary infection control practices helps prevent over-reliance on antimicrobial surfaces and maintains adherence to fundamental hygiene practices. Transparency about the scientific evidence supporting specific applications builds trust and facilitates appropriate implementation decisions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!