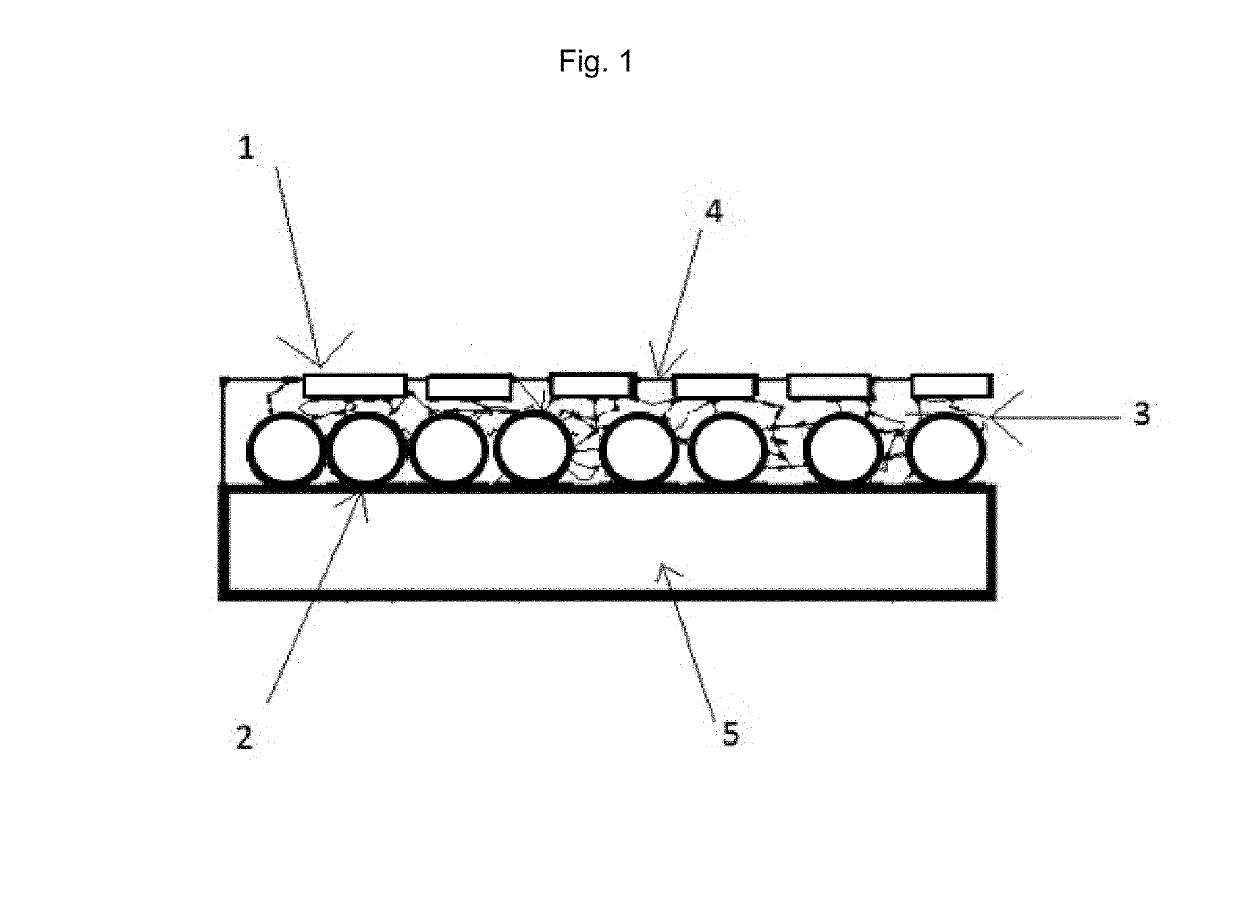
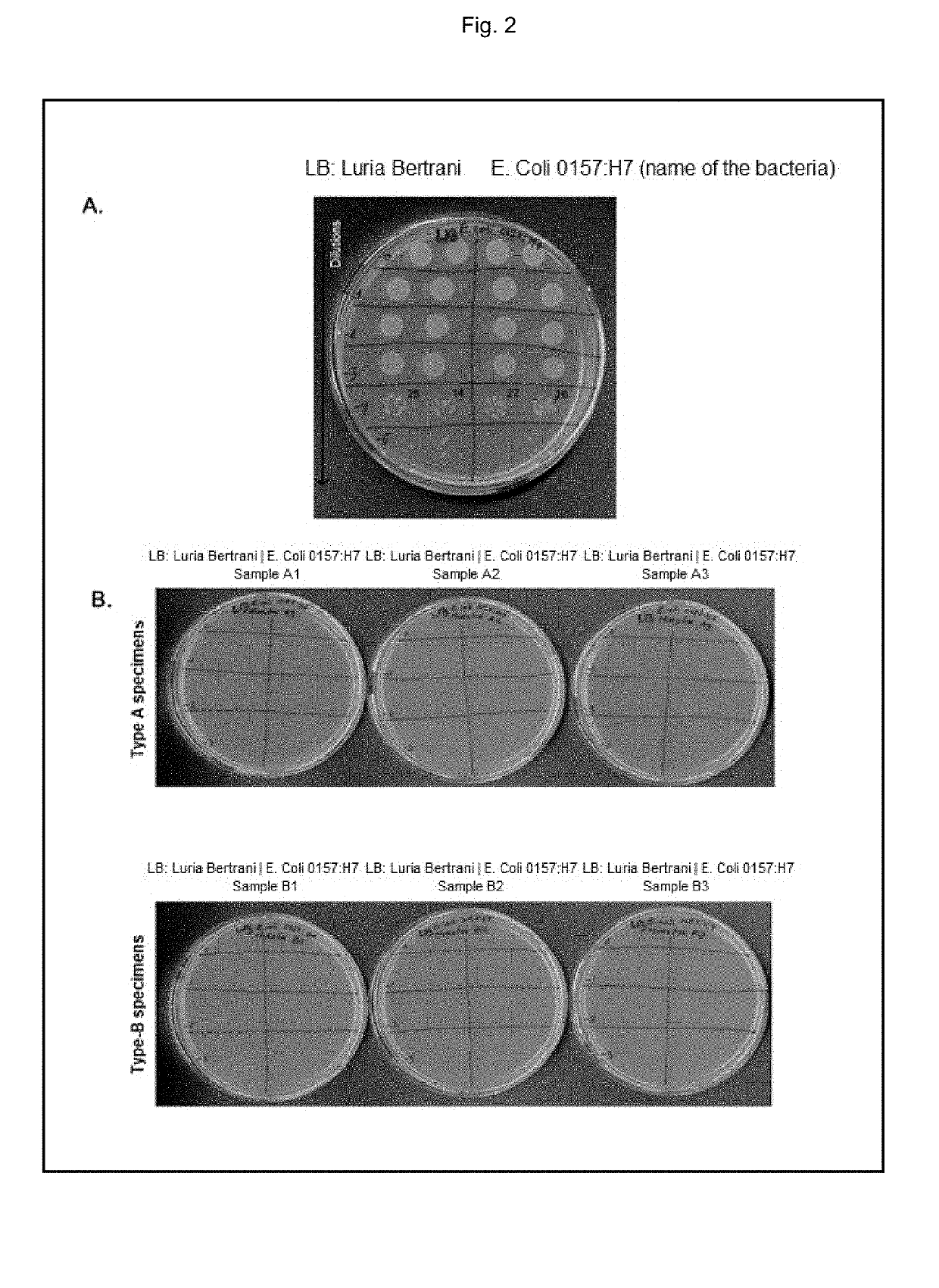
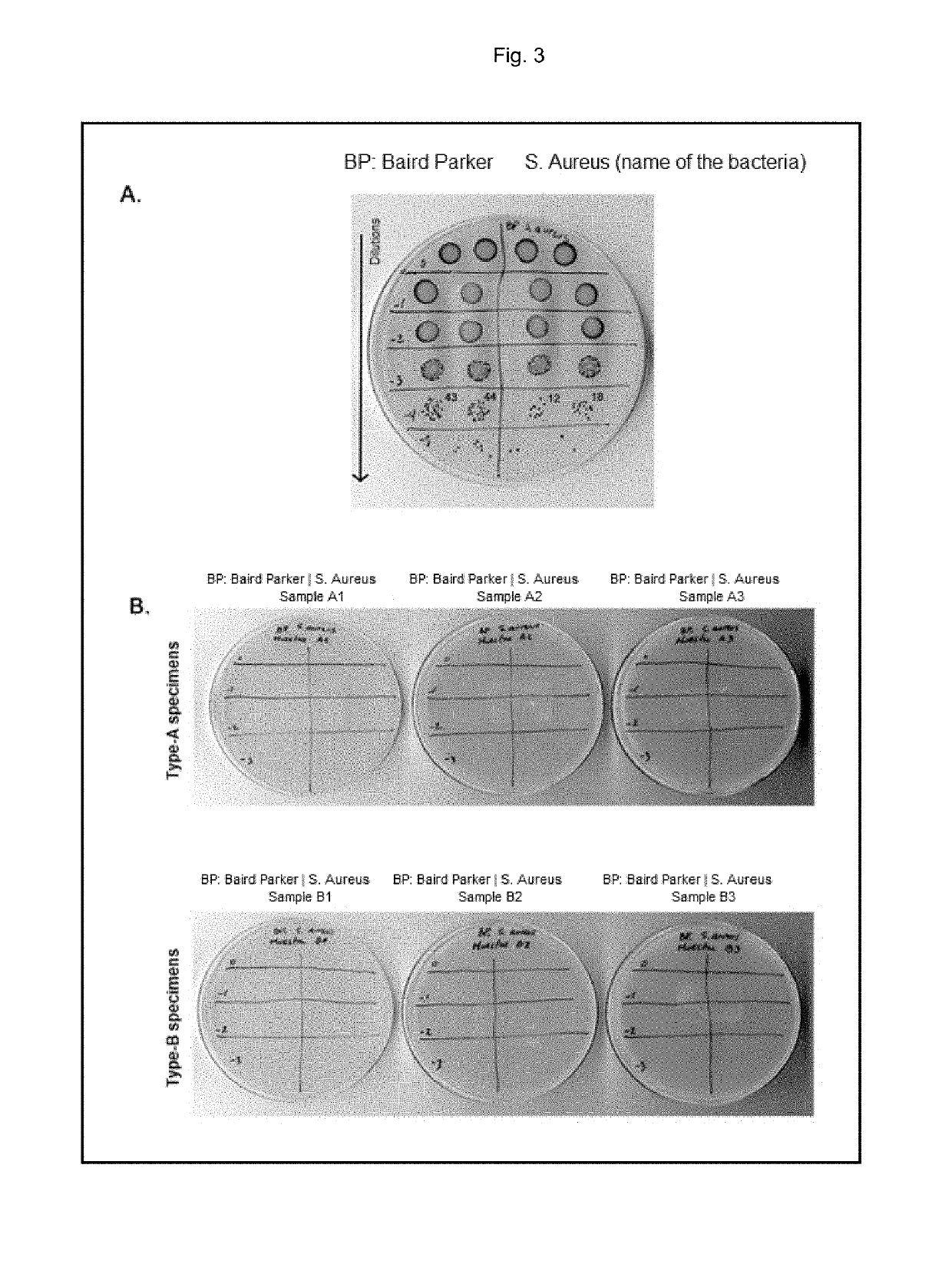
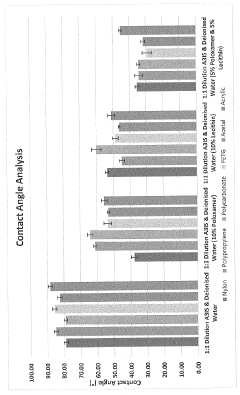
What Makes Antimicrobial Surface Coatings Effective in Hospitals
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Antimicrobial Coating Technology Background and Objectives
Antimicrobial surface coatings have emerged as a critical technology in healthcare settings, evolving significantly over the past several decades. The concept originated in the 1970s with simple antimicrobial additives, but has since transformed into sophisticated nanotechnology-based solutions. This evolution has been driven by the persistent challenge of healthcare-associated infections (HAIs), which affect approximately 1 in 31 hospital patients globally and result in significant mortality rates and economic burden exceeding $45 billion annually in the United States alone.
The technological trajectory has been marked by several pivotal developments, including the transition from silver-based compounds to copper alloys in the 1990s, followed by the integration of quaternary ammonium compounds in the early 2000s. The past decade has witnessed revolutionary advancements in photocatalytic coatings, particularly those utilizing titanium dioxide, which activate antimicrobial properties when exposed to light.
Current research is increasingly focused on "smart" responsive coatings that can release antimicrobial agents on demand when detecting microbial presence, as well as multi-functional surfaces that combine antimicrobial properties with anti-fouling capabilities. These developments reflect the industry's recognition that effective hospital antimicrobial coatings must address both immediate pathogen elimination and long-term prevention of biofilm formation.
The primary objectives of antimicrobial coating technology development include achieving broad-spectrum efficacy against bacteria, viruses, and fungi, particularly targeting resistant strains such as MRSA and C. difficile that pose significant threats in hospital environments. Additionally, there is a growing emphasis on developing coatings with sustained antimicrobial activity that can maintain effectiveness for extended periods, ideally matching the lifecycle of the coated surfaces.
Environmental and health safety considerations have become increasingly prominent, with research objectives shifting toward non-toxic, environmentally sustainable solutions that minimize the potential for antimicrobial resistance development. This represents a significant departure from earlier generations of coatings that often relied on heavy metals or potentially harmful chemical agents.
Cost-effectiveness and scalability also feature prominently in current technological objectives, as widespread implementation across healthcare facilities requires solutions that are economically viable while maintaining performance standards. The industry is working toward standardized testing protocols and regulatory frameworks to ensure consistent evaluation of antimicrobial efficacy claims, addressing a historical challenge in this field.
The technological trajectory has been marked by several pivotal developments, including the transition from silver-based compounds to copper alloys in the 1990s, followed by the integration of quaternary ammonium compounds in the early 2000s. The past decade has witnessed revolutionary advancements in photocatalytic coatings, particularly those utilizing titanium dioxide, which activate antimicrobial properties when exposed to light.
Current research is increasingly focused on "smart" responsive coatings that can release antimicrobial agents on demand when detecting microbial presence, as well as multi-functional surfaces that combine antimicrobial properties with anti-fouling capabilities. These developments reflect the industry's recognition that effective hospital antimicrobial coatings must address both immediate pathogen elimination and long-term prevention of biofilm formation.
The primary objectives of antimicrobial coating technology development include achieving broad-spectrum efficacy against bacteria, viruses, and fungi, particularly targeting resistant strains such as MRSA and C. difficile that pose significant threats in hospital environments. Additionally, there is a growing emphasis on developing coatings with sustained antimicrobial activity that can maintain effectiveness for extended periods, ideally matching the lifecycle of the coated surfaces.
Environmental and health safety considerations have become increasingly prominent, with research objectives shifting toward non-toxic, environmentally sustainable solutions that minimize the potential for antimicrobial resistance development. This represents a significant departure from earlier generations of coatings that often relied on heavy metals or potentially harmful chemical agents.
Cost-effectiveness and scalability also feature prominently in current technological objectives, as widespread implementation across healthcare facilities requires solutions that are economically viable while maintaining performance standards. The industry is working toward standardized testing protocols and regulatory frameworks to ensure consistent evaluation of antimicrobial efficacy claims, addressing a historical challenge in this field.
Hospital Infection Control Market Analysis
The hospital infection control market has experienced significant growth in recent years, driven primarily by increasing awareness of healthcare-associated infections (HAIs) and their substantial economic burden on healthcare systems worldwide. The global market for hospital infection control solutions was valued at approximately 32.6 billion USD in 2022 and is projected to reach 44.7 billion USD by 2028, representing a compound annual growth rate (CAGR) of 5.4% during the forecast period.
Antimicrobial surface coatings represent one of the fastest-growing segments within this market, with an estimated market size of 3.8 billion USD in 2022. This segment is expected to grow at a CAGR of 7.2% through 2028, outpacing the overall infection control market growth. The heightened demand for these coatings stems from their proven efficacy in reducing pathogen transmission via high-touch surfaces in healthcare environments.
Regional analysis reveals North America currently dominates the hospital infection control market, accounting for approximately 38% of global market share. This dominance is attributed to stringent regulatory frameworks, advanced healthcare infrastructure, and higher healthcare expenditure. Europe follows closely at 29% market share, with particular growth observed in Germany, France, and the UK. The Asia-Pacific region, while currently representing about 22% of the market, is projected to witness the highest growth rate of 8.3% annually through 2028, driven by improving healthcare infrastructure and increasing awareness in countries like China, India, and Japan.
The competitive landscape features both established players and innovative startups. Major companies including 3M, Ecolab, BD, Biomerieux, and Johnson & Johnson collectively hold approximately 45% market share. However, the antimicrobial coatings segment specifically has seen the emergence of specialized firms focusing exclusively on novel coating technologies, creating a more fragmented competitive environment in this subsector.
Customer segmentation analysis indicates that large teaching hospitals and academic medical centers represent the early adopters of advanced antimicrobial coating solutions, accounting for approximately 42% of current market demand. Community hospitals constitute about 35% of the market, while long-term care facilities represent a growing segment at 18%, with particularly strong growth potential due to their vulnerable patient populations.
Market drivers include increasing regulatory pressure to reduce HAI rates, growing patient awareness and expectations regarding infection prevention, and the rising economic burden of treating HAIs, estimated at 28.4 billion USD annually in the US alone. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of comprehensive infection control strategies in healthcare settings.
Antimicrobial surface coatings represent one of the fastest-growing segments within this market, with an estimated market size of 3.8 billion USD in 2022. This segment is expected to grow at a CAGR of 7.2% through 2028, outpacing the overall infection control market growth. The heightened demand for these coatings stems from their proven efficacy in reducing pathogen transmission via high-touch surfaces in healthcare environments.
Regional analysis reveals North America currently dominates the hospital infection control market, accounting for approximately 38% of global market share. This dominance is attributed to stringent regulatory frameworks, advanced healthcare infrastructure, and higher healthcare expenditure. Europe follows closely at 29% market share, with particular growth observed in Germany, France, and the UK. The Asia-Pacific region, while currently representing about 22% of the market, is projected to witness the highest growth rate of 8.3% annually through 2028, driven by improving healthcare infrastructure and increasing awareness in countries like China, India, and Japan.
The competitive landscape features both established players and innovative startups. Major companies including 3M, Ecolab, BD, Biomerieux, and Johnson & Johnson collectively hold approximately 45% market share. However, the antimicrobial coatings segment specifically has seen the emergence of specialized firms focusing exclusively on novel coating technologies, creating a more fragmented competitive environment in this subsector.
Customer segmentation analysis indicates that large teaching hospitals and academic medical centers represent the early adopters of advanced antimicrobial coating solutions, accounting for approximately 42% of current market demand. Community hospitals constitute about 35% of the market, while long-term care facilities represent a growing segment at 18%, with particularly strong growth potential due to their vulnerable patient populations.
Market drivers include increasing regulatory pressure to reduce HAI rates, growing patient awareness and expectations regarding infection prevention, and the rising economic burden of treating HAIs, estimated at 28.4 billion USD annually in the US alone. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of comprehensive infection control strategies in healthcare settings.
Current Antimicrobial Surface Technologies and Challenges
Antimicrobial surface technologies in healthcare settings have evolved significantly over the past decade, with several distinct approaches currently dominating the market. Contact-killing surfaces incorporating copper and silver ions represent one of the most established technologies, with copper alloys receiving EPA registration for antimicrobial claims. These surfaces work through ion release mechanisms that disrupt bacterial cell membranes and interfere with essential cellular processes. However, their effectiveness diminishes over time due to oxidation and surface wear.
Polymer-based antimicrobial coatings represent another major category, including quaternary ammonium compounds (QACs) and polycationic surfaces that physically rupture microbial cell membranes through electrostatic interactions. While these coatings offer durability advantages, concerns about potential antimicrobial resistance development have emerged in recent studies, particularly with QACs that are also common in disinfectants.
Light-activated antimicrobial surfaces, particularly those utilizing titanium dioxide photocatalysts and photodynamic materials, have gained traction for their ability to generate reactive oxygen species that damage multiple cellular targets simultaneously. These technologies offer the advantage of broad-spectrum activity without contributing to antimicrobial resistance, though their dependence on light activation limits application in certain hospital environments.
Despite technological advances, significant challenges persist in antimicrobial surface implementation. Durability remains a primary concern, with many coatings demonstrating reduced efficacy after repeated cleaning cycles or mechanical abrasion. This is particularly problematic in high-touch areas where antimicrobial protection is most needed but also most likely to degrade rapidly.
Biofilm formation presents another substantial challenge, as mature biofilms can resist antimicrobial effects that would kill planktonic bacteria. Current technologies often fail to address the complex extracellular polymeric substances that protect microorganisms within biofilms, limiting long-term effectiveness in real-world hospital environments.
Regulatory hurdles further complicate technology adoption, with varying standards across regions creating inconsistent approval pathways. The EPA and FDA in the United States maintain different requirements for antimicrobial claims, while the European Union's Biocidal Products Regulation imposes additional constraints on active substance approval.
Cost-effectiveness represents a significant barrier to widespread implementation, with many advanced antimicrobial technologies requiring substantial initial investment without clearly established return on investment metrics. Healthcare facilities struggle to justify these costs without standardized methods for evaluating real-world clinical impact on healthcare-associated infection rates.
The environmental impact of antimicrobial technologies has also emerged as a growing concern, particularly regarding the potential leaching of active compounds into wastewater systems and subsequent ecological effects. Sustainable alternatives that maintain efficacy while minimizing environmental footprint remain underdeveloped in the current technological landscape.
Polymer-based antimicrobial coatings represent another major category, including quaternary ammonium compounds (QACs) and polycationic surfaces that physically rupture microbial cell membranes through electrostatic interactions. While these coatings offer durability advantages, concerns about potential antimicrobial resistance development have emerged in recent studies, particularly with QACs that are also common in disinfectants.
Light-activated antimicrobial surfaces, particularly those utilizing titanium dioxide photocatalysts and photodynamic materials, have gained traction for their ability to generate reactive oxygen species that damage multiple cellular targets simultaneously. These technologies offer the advantage of broad-spectrum activity without contributing to antimicrobial resistance, though their dependence on light activation limits application in certain hospital environments.
Despite technological advances, significant challenges persist in antimicrobial surface implementation. Durability remains a primary concern, with many coatings demonstrating reduced efficacy after repeated cleaning cycles or mechanical abrasion. This is particularly problematic in high-touch areas where antimicrobial protection is most needed but also most likely to degrade rapidly.
Biofilm formation presents another substantial challenge, as mature biofilms can resist antimicrobial effects that would kill planktonic bacteria. Current technologies often fail to address the complex extracellular polymeric substances that protect microorganisms within biofilms, limiting long-term effectiveness in real-world hospital environments.
Regulatory hurdles further complicate technology adoption, with varying standards across regions creating inconsistent approval pathways. The EPA and FDA in the United States maintain different requirements for antimicrobial claims, while the European Union's Biocidal Products Regulation imposes additional constraints on active substance approval.
Cost-effectiveness represents a significant barrier to widespread implementation, with many advanced antimicrobial technologies requiring substantial initial investment without clearly established return on investment metrics. Healthcare facilities struggle to justify these costs without standardized methods for evaluating real-world clinical impact on healthcare-associated infection rates.
The environmental impact of antimicrobial technologies has also emerged as a growing concern, particularly regarding the potential leaching of active compounds into wastewater systems and subsequent ecological effects. Sustainable alternatives that maintain efficacy while minimizing environmental footprint remain underdeveloped in the current technological landscape.
Current Antimicrobial Coating Implementation Strategies
01 Metal-based antimicrobial coatings
Metal-based antimicrobial coatings utilize silver, copper, zinc, and other metal ions or nanoparticles to provide effective antimicrobial properties on various surfaces. These metals disrupt bacterial cell membranes and interfere with cellular processes, preventing microbial growth and colonization. The coatings can be applied to medical devices, touch surfaces, and industrial equipment to reduce infection risks and provide long-lasting protection against a broad spectrum of pathogens.- Metal-based antimicrobial coatings: Metal-based antimicrobial coatings utilize silver, copper, zinc, and other metal ions or nanoparticles to provide effective surface protection against a wide range of microorganisms. These metals disrupt bacterial cell membranes, interfere with enzyme functions, and prevent biofilm formation. The controlled release of metal ions provides long-lasting antimicrobial activity, making these coatings suitable for healthcare settings, food processing equipment, and high-touch surfaces in public spaces.
- Quaternary ammonium compound coatings: Quaternary ammonium compounds (QACs) are effective antimicrobial agents that can be incorporated into surface coatings. These positively charged molecules disrupt microbial cell membranes, leading to cell death. QAC-based coatings provide broad-spectrum activity against bacteria, fungi, and some viruses. These coatings can be formulated to bond permanently to surfaces, creating a durable antimicrobial barrier that remains effective even after multiple cleaning cycles, making them ideal for high-traffic areas and frequently touched surfaces.
- Polymer-based antimicrobial coatings: Polymer-based antimicrobial coatings incorporate active ingredients within a polymer matrix that provides controlled release of antimicrobial agents. These coatings can be designed with specific release profiles to maintain effectiveness over extended periods. Some polymers themselves possess inherent antimicrobial properties. The polymer matrix also enhances durability and adhesion to various substrates, protecting surfaces from microbial colonization while withstanding environmental factors and cleaning procedures.
- Natural and plant-derived antimicrobial coatings: Natural and plant-derived compounds offer environmentally friendly alternatives for antimicrobial surface coatings. Essential oils, plant extracts, and naturally occurring compounds like chitosan demonstrate significant antimicrobial activity against various pathogens. These bio-based coatings address growing concerns about chemical exposure and environmental impact while providing effective protection. The complex mixture of active compounds in natural extracts can also help prevent the development of microbial resistance, offering sustainable solutions for antimicrobial surface protection.
- Photocatalytic antimicrobial coatings: Photocatalytic antimicrobial coatings utilize materials like titanium dioxide that generate reactive oxygen species when exposed to light. These reactive species effectively destroy microorganisms on contact by oxidizing their cellular components. The self-cleaning nature of these coatings provides continuous antimicrobial action as long as light exposure continues. These coatings are particularly effective in well-lit environments and can be enhanced with dopants to improve activity under visible light, making them suitable for both indoor and outdoor applications.
02 Polymer-based antimicrobial surface treatments
Polymer-based antimicrobial surface treatments incorporate active antimicrobial agents within polymer matrices to create durable protective coatings. These formulations can include quaternary ammonium compounds, chitosan derivatives, or other biocides embedded in polymers like polyurethane, silicone, or acrylic. The polymer matrix provides controlled release of the antimicrobial agents while maintaining surface integrity. These coatings are particularly effective for high-touch surfaces in healthcare settings, food processing facilities, and public spaces.Expand Specific Solutions03 Natural and plant-derived antimicrobial coatings
Natural and plant-derived antimicrobial coatings utilize extracts from plants, essential oils, and other naturally occurring compounds to inhibit microbial growth on surfaces. These environmentally friendly alternatives offer antimicrobial efficacy while reducing reliance on synthetic chemicals. Components like thymol, carvacrol, eugenol, and plant polyphenols disrupt bacterial cell membranes and metabolic processes. These coatings are particularly valuable for food contact surfaces, packaging materials, and applications where environmental sustainability is prioritized.Expand Specific Solutions04 Photocatalytic antimicrobial surface technologies
Photocatalytic antimicrobial surface technologies utilize materials like titanium dioxide that, when exposed to light, generate reactive oxygen species that destroy microorganisms. These self-cleaning surfaces remain continuously active as long as they receive appropriate light exposure. The photocatalytic reaction breaks down organic contaminants and inactivates bacteria, viruses, and fungi without depleting the active ingredient. These coatings are particularly effective for environmental surfaces in hospitals, public facilities, and areas with good light exposure.Expand Specific Solutions05 Testing and evaluation methods for antimicrobial coatings
Standardized testing and evaluation methods are essential for determining the effectiveness of antimicrobial surface coatings. These include protocols for measuring zone of inhibition, bacterial adhesion reduction, biofilm prevention, and long-term antimicrobial activity under various environmental conditions. Testing methods also evaluate durability factors such as wear resistance, chemical stability, and efficacy after repeated cleaning cycles. These standardized approaches enable comparison between different antimicrobial technologies and ensure that coatings maintain their effectiveness throughout their intended service life.Expand Specific Solutions
Leading Manufacturers and Research Institutions Analysis
The antimicrobial surface coatings market for hospitals is currently in a growth phase, with increasing adoption driven by heightened infection control concerns. The global market is expanding rapidly, estimated to reach several billion dollars by 2025, with a CAGR of 12-15%. Technologically, the field shows varying maturity levels across different approaches. Leading pharmaceutical companies like Novartis AG and Baxter International have established antimicrobial coating divisions, while specialized firms such as Bio-Gate AG, Quick-Med Technologies, and Polyamyna Nanotech are driving innovation with proprietary technologies. Academic institutions including MIT, Zhejiang University, and Empa are advancing fundamental research in novel coating materials. The industry is seeing convergence between traditional chemical approaches and emerging nanotechnology solutions, with silver-based technologies (from companies like Silver Bullet Therapeutics) currently dominating commercial applications.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have developed a groundbreaking "SLIPS" (Slippery Liquid-Infused Porous Surfaces) technology that represents a paradigm shift in antimicrobial surface design for healthcare settings. Unlike traditional antimicrobial coatings that kill microbes through chemical action, SLIPS creates ultra-slippery surfaces that prevent bacterial attachment and biofilm formation through physical means. The technology consists of a micro/nanoporous substrate infused with a lubricating liquid that creates a smooth, defect-free surface where bacteria cannot adhere. MIT's research demonstrates that SLIPS surfaces reduce bacterial adhesion by over 99.9% compared to untreated surfaces, with effectiveness against both gram-positive and gram-negative bacteria, including antibiotic-resistant strains. A key innovation is the self-healing property of these surfaces - when the surface is damaged, the lubricating liquid spontaneously refills the damaged area, maintaining antimicrobial functionality. Clinical testing in hospital environments has shown that SLIPS-treated surfaces maintain their anti-fouling properties for extended periods even with regular cleaning protocols and high-touch usage.
Strengths: Novel physical approach eliminates risk of antimicrobial resistance development; self-healing properties provide extended functional lifetime; effective against biofilm formation which is resistant to traditional antimicrobials. Weaknesses: More complex manufacturing process compared to conventional coatings; potential compatibility issues with certain hospital cleaning chemicals; higher initial implementation costs compared to traditional antimicrobial surfaces.
Quick-Med Technologies, Inc.
Technical Solution: Quick-Med Technologies has developed NIMBUS® (Novel Intrinsically Microbicidal Polymers), a revolutionary non-leaching antimicrobial technology specifically engineered for hospital environments. Unlike conventional antimicrobial coatings that release active agents, NIMBUS® technology permanently bonds antimicrobial compounds to substrate materials, creating surfaces that kill microorganisms on contact without releasing any chemicals. The technology utilizes positively charged polymers that attract negatively charged microbial cell membranes, causing cell disruption and death upon contact. This mechanism provides broad-spectrum activity against bacteria, fungi, and certain enveloped viruses. Clinical studies in hospital settings have demonstrated that NIMBUS®-treated surfaces maintain greater than 99.99% reduction in bacterial counts even after 100+ cleaning cycles with hospital-grade disinfectants. A significant advantage of this technology is its non-leaching nature, which prevents the development of antimicrobial resistance and eliminates concerns about chemical exposure to patients and healthcare workers. The technology can be applied to various hospital surfaces including textiles, plastics, and metals, providing versatile implementation options.
Strengths: Non-leaching mechanism eliminates risk of antimicrobial agents entering the environment; exceptional durability through multiple cleaning cycles; reduced potential for development of antimicrobial resistance. Weaknesses: Limited effectiveness against certain non-enveloped viruses; higher initial implementation costs; requires specific application processes for optimal performance on different substrate materials.
Key Antimicrobial Mechanisms and Efficacy Research
Antimicrobial composition for coating surfaces
PatentInactiveUS20190246644A1
Innovation
- A composition combining micronized, high-purity metallic copper particles of various shapes and sizes with a solvent-free polymer binder, applied cold and hardened by polymerization, ensuring comprehensive coverage and long-lasting antimicrobial action.
Antimicrobial coating composition
PatentWO2023227599A1
Innovation
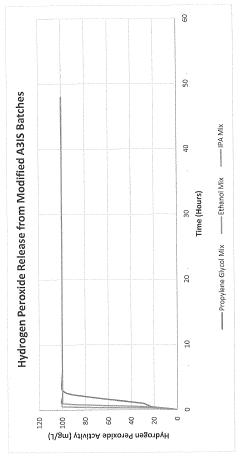
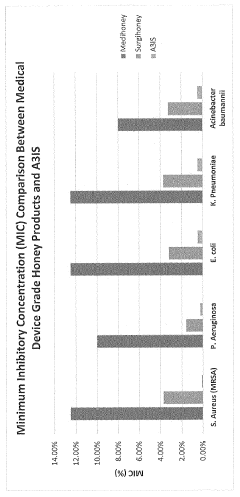
- An antimicrobial coating composition comprising A3IS (an oxidoreductase enzyme system with hydrogen peroxide and silicone, which provides a sustained release of hydrogen peroxide, preventing bacterial attachment and biofilm formation, and is less toxic and more cost-effective than traditional coatings.
Regulatory Framework for Healthcare Surface Materials
The regulatory landscape governing antimicrobial surface materials in healthcare settings is complex and multifaceted, involving various national and international bodies. In the United States, the Environmental Protection Agency (EPA) regulates antimicrobial coatings under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), requiring manufacturers to register products and substantiate efficacy claims. The EPA's testing protocols specifically evaluate the performance of these coatings against healthcare-associated pathogens, establishing minimum standards for hospital implementation.
Concurrently, the Food and Drug Administration (FDA) oversees antimicrobial coatings that make medical claims or are integrated into medical devices. These products must undergo rigorous premarket approval processes, including clinical trials demonstrating both safety and efficacy in healthcare environments. The FDA's guidance documents specifically address leaching concerns and potential development of antimicrobial resistance.
In Europe, the Biocidal Products Regulation (BPR) governs antimicrobial surface technologies, requiring extensive safety data and standardized efficacy testing. The European Committee for Standardization (CEN) has developed specific testing methodologies (EN 13697, EN 16615) for evaluating surface disinfectants and antimicrobial coatings in medical settings, providing benchmarks for performance assessment.
International standards organizations, including ISO and ASTM International, have established testing protocols specifically for antimicrobial surfaces in healthcare environments. ISO 22196 and ASTM E2180 are particularly relevant for evaluating antimicrobial activity on surfaces, though critics note these laboratory tests may not fully replicate real-world hospital conditions with variable humidity, temperature, and organic soil loads.
Healthcare accreditation bodies increasingly incorporate antimicrobial surface requirements into their facility standards. The Joint Commission and Healthcare Facilities Accreditation Program have developed guidelines addressing the implementation and maintenance of antimicrobial surfaces as part of infection prevention strategies.
Recent regulatory trends indicate a shift toward lifecycle assessment requirements, evaluating the environmental impact of antimicrobial coatings from production through disposal. Several jurisdictions now mandate disclosure of active ingredients and potential environmental effects, particularly for nano-silver and triclosan-based products which have raised ecotoxicological concerns.
Compliance with these regulatory frameworks presents significant challenges for manufacturers, requiring substantial investment in testing and documentation. However, these standards are essential for ensuring that antimicrobial surface coatings deployed in hospitals deliver their promised benefits without introducing new risks to patients, healthcare workers, or the environment.
Concurrently, the Food and Drug Administration (FDA) oversees antimicrobial coatings that make medical claims or are integrated into medical devices. These products must undergo rigorous premarket approval processes, including clinical trials demonstrating both safety and efficacy in healthcare environments. The FDA's guidance documents specifically address leaching concerns and potential development of antimicrobial resistance.
In Europe, the Biocidal Products Regulation (BPR) governs antimicrobial surface technologies, requiring extensive safety data and standardized efficacy testing. The European Committee for Standardization (CEN) has developed specific testing methodologies (EN 13697, EN 16615) for evaluating surface disinfectants and antimicrobial coatings in medical settings, providing benchmarks for performance assessment.
International standards organizations, including ISO and ASTM International, have established testing protocols specifically for antimicrobial surfaces in healthcare environments. ISO 22196 and ASTM E2180 are particularly relevant for evaluating antimicrobial activity on surfaces, though critics note these laboratory tests may not fully replicate real-world hospital conditions with variable humidity, temperature, and organic soil loads.
Healthcare accreditation bodies increasingly incorporate antimicrobial surface requirements into their facility standards. The Joint Commission and Healthcare Facilities Accreditation Program have developed guidelines addressing the implementation and maintenance of antimicrobial surfaces as part of infection prevention strategies.
Recent regulatory trends indicate a shift toward lifecycle assessment requirements, evaluating the environmental impact of antimicrobial coatings from production through disposal. Several jurisdictions now mandate disclosure of active ingredients and potential environmental effects, particularly for nano-silver and triclosan-based products which have raised ecotoxicological concerns.
Compliance with these regulatory frameworks presents significant challenges for manufacturers, requiring substantial investment in testing and documentation. However, these standards are essential for ensuring that antimicrobial surface coatings deployed in hospitals deliver their promised benefits without introducing new risks to patients, healthcare workers, or the environment.
Cost-Benefit Analysis of Antimicrobial Surface Implementations
Implementing antimicrobial surfaces in healthcare facilities requires careful financial analysis to justify the initial investment. The cost structure includes immediate expenses such as material procurement, installation labor, and potential facility downtime during implementation. Premium antimicrobial coatings typically command a 15-30% price premium over standard surfaces, with installation costs varying based on application method and surface area coverage.
Maintenance expenses represent another significant cost factor, as some antimicrobial technologies require periodic reapplication or special cleaning protocols to maintain efficacy. These ongoing costs must be factored into long-term financial planning, with maintenance intervals ranging from 6 months to 5 years depending on the specific technology employed.
The benefit side of the equation presents compelling financial advantages. Studies from major healthcare institutions demonstrate reduced healthcare-associated infection (HAI) rates ranging from 15-40% following comprehensive antimicrobial surface implementation. Each prevented infection represents substantial cost savings, with average HAI treatment costs estimated between $10,000-$45,000 per case depending on infection type and severity.
Staff productivity gains constitute another quantifiable benefit, as reduced infection rates correlate with decreased staff time dedicated to infection management protocols. Facilities implementing comprehensive antimicrobial surface strategies report nursing time savings of 2-5 hours per patient with prevented infections.
Return on investment (ROI) calculations typically show breakeven periods of 8-24 months for high-touch surface implementations in critical care environments. Less intensive applications may require longer payback periods of 2-4 years. Several healthcare systems report positive ROI within the first year of implementation when targeting high-risk areas with elevated infection rates.
Risk mitigation value must also be considered, as HAIs increasingly impact hospital reimbursement rates and public quality metrics. The financial impact of reputation damage from high infection rates can exceed direct treatment costs, particularly in competitive healthcare markets where quality metrics influence patient choice and insurance contracts.
Implementation strategies that prioritize high-touch surfaces in high-risk departments typically yield the most favorable cost-benefit ratios. Phased implementation approaches allow facilities to distribute costs while gathering facility-specific efficacy data to justify expanded applications.
Maintenance expenses represent another significant cost factor, as some antimicrobial technologies require periodic reapplication or special cleaning protocols to maintain efficacy. These ongoing costs must be factored into long-term financial planning, with maintenance intervals ranging from 6 months to 5 years depending on the specific technology employed.
The benefit side of the equation presents compelling financial advantages. Studies from major healthcare institutions demonstrate reduced healthcare-associated infection (HAI) rates ranging from 15-40% following comprehensive antimicrobial surface implementation. Each prevented infection represents substantial cost savings, with average HAI treatment costs estimated between $10,000-$45,000 per case depending on infection type and severity.
Staff productivity gains constitute another quantifiable benefit, as reduced infection rates correlate with decreased staff time dedicated to infection management protocols. Facilities implementing comprehensive antimicrobial surface strategies report nursing time savings of 2-5 hours per patient with prevented infections.
Return on investment (ROI) calculations typically show breakeven periods of 8-24 months for high-touch surface implementations in critical care environments. Less intensive applications may require longer payback periods of 2-4 years. Several healthcare systems report positive ROI within the first year of implementation when targeting high-risk areas with elevated infection rates.
Risk mitigation value must also be considered, as HAIs increasingly impact hospital reimbursement rates and public quality metrics. The financial impact of reputation damage from high infection rates can exceed direct treatment costs, particularly in competitive healthcare markets where quality metrics influence patient choice and insurance contracts.
Implementation strategies that prioritize high-touch surfaces in high-risk departments typically yield the most favorable cost-benefit ratios. Phased implementation approaches allow facilities to distribute costs while gathering facility-specific efficacy data to justify expanded applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!