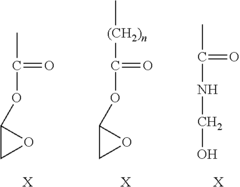
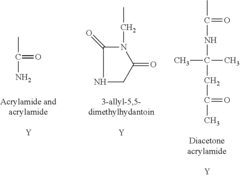
Antimicrobial Surface Coatings in Compliance with FDA Regulations
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Antimicrobial Coatings Background and Objectives
Antimicrobial surface coatings have emerged as a critical technology in healthcare settings, food processing facilities, and public spaces to combat the spread of harmful microorganisms. The evolution of these coatings traces back to the early 2000s when initial silver-based antimicrobial technologies gained traction. Since then, the field has expanded significantly, incorporating diverse active ingredients such as quaternary ammonium compounds, copper, zinc oxide, and advanced nanomaterials.
The technological trajectory has shifted from simple contact-killing mechanisms to more sophisticated approaches including controlled-release systems, photocatalytic surfaces, and biomimetic designs. This evolution reflects growing concerns about healthcare-associated infections (HAIs), antibiotic resistance, and the need for passive infection control measures that do not require active human intervention.
FDA regulatory oversight has become increasingly stringent, particularly following the COVID-19 pandemic, which highlighted the critical importance of surface contamination in disease transmission. Current FDA frameworks categorize antimicrobial coatings based on their intended use, with medical devices facing the most rigorous scrutiny under the premarket approval (PMA) or 510(k) pathways.
The primary technical objectives of this research are to develop antimicrobial surface coatings that demonstrate broad-spectrum efficacy against bacteria, viruses, and fungi while maintaining long-term durability and stability. These coatings must achieve a minimum 3-log reduction in microbial load within 2 hours of contact and maintain efficacy for at least 6 months under normal use conditions.
Additionally, the coatings must comply with FDA safety requirements, particularly regarding leaching of active ingredients, biocompatibility, and potential for inducing antimicrobial resistance. For medical applications, the coatings should withstand standard sterilization procedures without degradation of antimicrobial properties.
A significant technical challenge involves balancing antimicrobial efficacy with human safety considerations. The ideal coating should demonstrate selective toxicity—highly effective against microorganisms while remaining non-toxic to human cells. This selective action must be achieved without contributing to the development of resistant microbial strains.
The research aims to establish clear correlations between surface chemistry, topography, and antimicrobial performance to enable predictive modeling of new formulations. Furthermore, standardized testing protocols aligned with FDA requirements will be developed to accelerate regulatory approval processes for novel antimicrobial coating technologies.
Ultimately, this research seeks to create a new generation of antimicrobial coatings that can be seamlessly integrated into existing manufacturing processes while meeting increasingly stringent regulatory requirements, thereby addressing the growing market demand for safer surfaces in healthcare and public environments.
The technological trajectory has shifted from simple contact-killing mechanisms to more sophisticated approaches including controlled-release systems, photocatalytic surfaces, and biomimetic designs. This evolution reflects growing concerns about healthcare-associated infections (HAIs), antibiotic resistance, and the need for passive infection control measures that do not require active human intervention.
FDA regulatory oversight has become increasingly stringent, particularly following the COVID-19 pandemic, which highlighted the critical importance of surface contamination in disease transmission. Current FDA frameworks categorize antimicrobial coatings based on their intended use, with medical devices facing the most rigorous scrutiny under the premarket approval (PMA) or 510(k) pathways.
The primary technical objectives of this research are to develop antimicrobial surface coatings that demonstrate broad-spectrum efficacy against bacteria, viruses, and fungi while maintaining long-term durability and stability. These coatings must achieve a minimum 3-log reduction in microbial load within 2 hours of contact and maintain efficacy for at least 6 months under normal use conditions.
Additionally, the coatings must comply with FDA safety requirements, particularly regarding leaching of active ingredients, biocompatibility, and potential for inducing antimicrobial resistance. For medical applications, the coatings should withstand standard sterilization procedures without degradation of antimicrobial properties.
A significant technical challenge involves balancing antimicrobial efficacy with human safety considerations. The ideal coating should demonstrate selective toxicity—highly effective against microorganisms while remaining non-toxic to human cells. This selective action must be achieved without contributing to the development of resistant microbial strains.
The research aims to establish clear correlations between surface chemistry, topography, and antimicrobial performance to enable predictive modeling of new formulations. Furthermore, standardized testing protocols aligned with FDA requirements will be developed to accelerate regulatory approval processes for novel antimicrobial coating technologies.
Ultimately, this research seeks to create a new generation of antimicrobial coatings that can be seamlessly integrated into existing manufacturing processes while meeting increasingly stringent regulatory requirements, thereby addressing the growing market demand for safer surfaces in healthcare and public environments.
Market Analysis for FDA-Compliant Antimicrobial Surfaces
The global market for antimicrobial surface coatings has experienced significant growth in recent years, driven primarily by increasing healthcare-associated infections (HAIs) and growing awareness of surface transmission of pathogens. The FDA-compliant antimicrobial surfaces market was valued at approximately $3.6 billion in 2022 and is projected to reach $6.3 billion by 2028, representing a compound annual growth rate (CAGR) of 9.8%.
Healthcare facilities constitute the largest market segment, accounting for roughly 45% of the total market share. This dominance stems from stringent infection control protocols and the high cost of HAIs to healthcare systems. Hospitals alone spend an estimated $28-45 billion annually managing preventable infections in the United States.
The food processing industry represents the second-largest market segment at 22%, where FDA compliance is particularly critical due to direct contact with consumable products. The remaining market share is distributed across pharmaceutical manufacturing (15%), consumer goods (10%), and other industries (8%).
Regionally, North America leads the market with approximately 38% share, followed by Europe (29%), Asia-Pacific (24%), and rest of the world (9%). The United States specifically accounts for about 32% of the global market, largely due to its robust healthcare infrastructure and stringent regulatory environment.
Market demand is increasingly shifting toward long-lasting antimicrobial solutions that maintain efficacy over extended periods while meeting FDA safety standards. Customers are willing to pay premium prices for coatings that demonstrate both durability and compliance, with surveys indicating that 78% of healthcare procurement officers prioritize regulatory compliance over cost considerations.
The COVID-19 pandemic has accelerated market growth, with a 34% increase in antimicrobial coating applications between 2019 and 2021. This trend is expected to continue as public health awareness remains heightened and infection control protocols become standardized across industries.
Key market drivers include increasing regulatory pressure, rising healthcare costs associated with infections, consumer demand for safer products, and technological advancements enabling more effective and compliant solutions. Barriers to market entry include lengthy FDA approval processes, which typically take 12-24 months, and the high cost of clinical validation studies, averaging $2-5 million per new antimicrobial technology.
The market is also witnessing a shift toward environmentally sustainable antimicrobial technologies that avoid traditional biocides while maintaining FDA compliance, with this eco-friendly segment growing at 14.3% annually, outpacing the overall market growth rate.
Healthcare facilities constitute the largest market segment, accounting for roughly 45% of the total market share. This dominance stems from stringent infection control protocols and the high cost of HAIs to healthcare systems. Hospitals alone spend an estimated $28-45 billion annually managing preventable infections in the United States.
The food processing industry represents the second-largest market segment at 22%, where FDA compliance is particularly critical due to direct contact with consumable products. The remaining market share is distributed across pharmaceutical manufacturing (15%), consumer goods (10%), and other industries (8%).
Regionally, North America leads the market with approximately 38% share, followed by Europe (29%), Asia-Pacific (24%), and rest of the world (9%). The United States specifically accounts for about 32% of the global market, largely due to its robust healthcare infrastructure and stringent regulatory environment.
Market demand is increasingly shifting toward long-lasting antimicrobial solutions that maintain efficacy over extended periods while meeting FDA safety standards. Customers are willing to pay premium prices for coatings that demonstrate both durability and compliance, with surveys indicating that 78% of healthcare procurement officers prioritize regulatory compliance over cost considerations.
The COVID-19 pandemic has accelerated market growth, with a 34% increase in antimicrobial coating applications between 2019 and 2021. This trend is expected to continue as public health awareness remains heightened and infection control protocols become standardized across industries.
Key market drivers include increasing regulatory pressure, rising healthcare costs associated with infections, consumer demand for safer products, and technological advancements enabling more effective and compliant solutions. Barriers to market entry include lengthy FDA approval processes, which typically take 12-24 months, and the high cost of clinical validation studies, averaging $2-5 million per new antimicrobial technology.
The market is also witnessing a shift toward environmentally sustainable antimicrobial technologies that avoid traditional biocides while maintaining FDA compliance, with this eco-friendly segment growing at 14.3% annually, outpacing the overall market growth rate.
Current Antimicrobial Coating Technologies and Barriers
The antimicrobial coating market has witnessed significant growth in recent years, driven by increasing concerns about healthcare-associated infections and the need for safer surfaces in medical environments. Currently, several technologies dominate the antimicrobial coating landscape, each with distinct mechanisms of action and regulatory considerations.
Silver-based coatings represent one of the most established technologies, utilizing silver ions' ability to disrupt bacterial cell membranes and metabolic processes. These coatings have demonstrated broad-spectrum efficacy against bacteria, fungi, and certain viruses. However, concerns regarding silver nanoparticle toxicity and environmental persistence have prompted increased regulatory scrutiny, particularly for direct food-contact applications.
Quaternary ammonium compounds (QACs) offer another widely implemented solution, functioning through disruption of microbial cell membranes. While effective against many pathogens, QACs face challenges related to potential antimicrobial resistance development and cytotoxicity at higher concentrations, raising concerns for FDA approval in certain medical device applications.
Copper and copper alloy surfaces have gained traction following EPA registration as antimicrobial materials. These surfaces continuously kill bacteria through multiple mechanisms, including membrane damage and oxidative stress. Despite proven efficacy, the relatively high cost of implementation and potential for surface discoloration have limited widespread adoption in healthcare settings.
Photocatalytic coatings, typically based on titanium dioxide, represent an emerging technology that generates reactive oxygen species upon light exposure. While promising for environmental applications, their dependence on light activation limits utility in many medical contexts, and questions regarding long-term stability remain unresolved.
Significant regulatory barriers exist across these technologies. The FDA's regulatory framework for antimicrobial coatings varies based on intended use, with medical devices facing particularly stringent requirements under the 510(k) or PMA pathways. Manufacturers must navigate complex efficacy testing protocols while demonstrating that antimicrobial agents do not leach at harmful levels or interfere with the primary function of the coated device.
Technical challenges further complicate development efforts. Ensuring coating durability under repeated cleaning and disinfection protocols remains problematic, with many current solutions showing diminished efficacy over time. Adhesion to diverse substrate materials presents another obstacle, particularly for complex medical devices with multiple material components.
The balance between antimicrobial efficacy and biocompatibility represents perhaps the most significant barrier. Coatings potent enough to rapidly eliminate pathogens often demonstrate cytotoxicity toward human cells, creating a narrow therapeutic window that must be carefully navigated to achieve FDA compliance while maintaining clinical utility.
Silver-based coatings represent one of the most established technologies, utilizing silver ions' ability to disrupt bacterial cell membranes and metabolic processes. These coatings have demonstrated broad-spectrum efficacy against bacteria, fungi, and certain viruses. However, concerns regarding silver nanoparticle toxicity and environmental persistence have prompted increased regulatory scrutiny, particularly for direct food-contact applications.
Quaternary ammonium compounds (QACs) offer another widely implemented solution, functioning through disruption of microbial cell membranes. While effective against many pathogens, QACs face challenges related to potential antimicrobial resistance development and cytotoxicity at higher concentrations, raising concerns for FDA approval in certain medical device applications.
Copper and copper alloy surfaces have gained traction following EPA registration as antimicrobial materials. These surfaces continuously kill bacteria through multiple mechanisms, including membrane damage and oxidative stress. Despite proven efficacy, the relatively high cost of implementation and potential for surface discoloration have limited widespread adoption in healthcare settings.
Photocatalytic coatings, typically based on titanium dioxide, represent an emerging technology that generates reactive oxygen species upon light exposure. While promising for environmental applications, their dependence on light activation limits utility in many medical contexts, and questions regarding long-term stability remain unresolved.
Significant regulatory barriers exist across these technologies. The FDA's regulatory framework for antimicrobial coatings varies based on intended use, with medical devices facing particularly stringent requirements under the 510(k) or PMA pathways. Manufacturers must navigate complex efficacy testing protocols while demonstrating that antimicrobial agents do not leach at harmful levels or interfere with the primary function of the coated device.
Technical challenges further complicate development efforts. Ensuring coating durability under repeated cleaning and disinfection protocols remains problematic, with many current solutions showing diminished efficacy over time. Adhesion to diverse substrate materials presents another obstacle, particularly for complex medical devices with multiple material components.
The balance between antimicrobial efficacy and biocompatibility represents perhaps the most significant barrier. Coatings potent enough to rapidly eliminate pathogens often demonstrate cytotoxicity toward human cells, creating a narrow therapeutic window that must be carefully navigated to achieve FDA compliance while maintaining clinical utility.
Existing FDA-Approved Antimicrobial Surface Solutions
01 Metal-based antimicrobial coatings
Metal-based antimicrobial coatings utilize silver, copper, zinc, and other metal ions or nanoparticles to provide long-lasting protection against a wide range of microorganisms. These metals disrupt bacterial cell membranes, interfere with enzyme functions, and prevent biofilm formation. The coatings can be applied to various surfaces including medical devices, touch surfaces, and industrial equipment, offering both contact-killing properties and controlled ion release mechanisms for sustained antimicrobial activity.- Metal-based antimicrobial coatings: Metal-based antimicrobial coatings utilize silver, copper, zinc, and other metal ions or nanoparticles to provide broad-spectrum antimicrobial activity. These metals disrupt bacterial cell membranes, interfere with enzyme functions, and generate reactive oxygen species that kill microorganisms. Such coatings can be applied to various surfaces including medical devices, textiles, and high-touch areas to provide long-lasting protection against bacteria, fungi, and certain viruses.
- Polymer-based antimicrobial coatings: Polymer-based antimicrobial coatings incorporate active ingredients within polymer matrices that can release antimicrobial agents in a controlled manner or contain inherently antimicrobial polymers. These coatings may include quaternary ammonium compounds, chitosan derivatives, or other biocidal polymers that disrupt microbial cell membranes. The polymer matrix provides durability while allowing for sustained antimicrobial activity, making these coatings suitable for various applications including healthcare surfaces, consumer products, and industrial equipment.
- Natural compound-based antimicrobial coatings: Natural compound-based antimicrobial coatings utilize plant extracts, essential oils, enzymes, and other naturally derived substances to inhibit microbial growth. These environmentally friendly alternatives often contain components like thymol, carvacrol, eugenol, or plant-derived polyphenols that disrupt bacterial membranes or inhibit vital cellular processes. Such coatings appeal to consumers seeking sustainable and non-toxic solutions for food packaging, household surfaces, and personal care items.
- Photocatalytic antimicrobial coatings: Photocatalytic antimicrobial coatings contain materials like titanium dioxide that, when exposed to light (particularly UV radiation), generate reactive oxygen species that can destroy microorganisms. These coatings provide continuous self-cleaning and disinfecting properties as long as they receive adequate light exposure. The technology is particularly useful for outdoor surfaces, transparent materials, and areas with good lighting conditions, offering a passive approach to maintaining surface hygiene without requiring additional chemical treatments.
- Multi-functional antimicrobial surface coatings: Multi-functional antimicrobial surface coatings combine antimicrobial properties with additional beneficial characteristics such as anti-fouling, self-cleaning, durability enhancement, or corrosion resistance. These advanced formulations may incorporate multiple active ingredients or utilize synergistic effects between components to address various surface protection needs simultaneously. Such coatings are particularly valuable in demanding environments like healthcare facilities, marine applications, or food processing equipment where multiple surface challenges exist.
02 Polymer-based antimicrobial surface treatments
Polymer-based antimicrobial surface treatments incorporate active ingredients within polymer matrices to create durable protective coatings. These formulations can include quaternary ammonium compounds, antimicrobial peptides, or other biocides chemically bonded to or embedded within polymers. The polymer matrix provides controlled release of antimicrobial agents and improves adhesion to various substrates. These coatings offer advantages such as reduced leaching, extended efficacy periods, and compatibility with existing manufacturing processes.Expand Specific Solutions03 Natural compound-based antimicrobial coatings
Natural compound-based antimicrobial coatings utilize plant extracts, essential oils, enzymes, and other biological substances to create environmentally friendly surface protection. These formulations leverage compounds like chitosan, tea tree oil, thymol, and plant-derived polyphenols that have inherent antimicrobial properties. The coatings provide effective protection against bacteria, fungi, and certain viruses while offering advantages such as biodegradability, reduced toxicity, and consumer appeal for applications in food packaging, healthcare settings, and consumer products.Expand Specific Solutions04 Photocatalytic antimicrobial coatings
Photocatalytic antimicrobial coatings utilize materials like titanium dioxide (TiO2) that generate reactive oxygen species when exposed to light, particularly UV radiation. These reactive species effectively destroy microorganisms by oxidizing their cell components. The coatings provide continuous self-cleaning and disinfecting properties as long as they receive adequate light exposure. Advanced formulations incorporate dopants or sensitizers to enhance activity under visible light, making them suitable for indoor applications in healthcare facilities, public spaces, and food processing environments.Expand Specific Solutions05 Multi-functional antimicrobial surface technologies
Multi-functional antimicrobial surface technologies combine antimicrobial properties with additional beneficial characteristics such as anti-fouling, self-cleaning, or enhanced durability. These advanced coatings often utilize layer-by-layer deposition techniques, nanocomposite structures, or stimuli-responsive materials to create surfaces that can adapt to environmental conditions. Applications include medical implants that resist both bacterial colonization and protein adsorption, marine coatings that prevent biofouling while killing microorganisms, and textiles with both antimicrobial and moisture-wicking properties.Expand Specific Solutions
Leading Companies in FDA-Compliant Antimicrobial Coatings
The antimicrobial surface coatings market is currently in a growth phase, with increasing demand driven by healthcare-associated infection concerns and FDA regulatory compliance requirements. The global market is estimated at $3-4 billion annually with projected CAGR of 12-15% through 2027. Leading players represent diverse technological approaches: Bio-Gate AG specializes in silver-based antimicrobial solutions, Kastus Technologies offers patented antiviral coatings for glass and ceramics, while DuPont and BASF provide industrial-scale chemical solutions. Research institutions like Fraunhofer-Gesellschaft, MIT, and CNRS are advancing next-generation technologies. Medical device manufacturers including Medtronic MiniMed and Mentor Worldwide are integrating antimicrobial coatings into FDA-regulated products, while specialized firms like Orthobond focus on proprietary surface modification technologies for implantable devices.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed Silvadur™, an advanced antimicrobial technology platform for surface coatings that utilizes intelligent silver ion delivery systems. Their approach encapsulates silver ions within a polymer delivery system that responds to environmental triggers such as moisture or bacterial presence, releasing antimicrobial agents only when needed. This smart-release technology significantly extends the effective lifespan of the coating while minimizing unnecessary silver exposure. DuPont's manufacturing process incorporates their proprietary stabilization chemistry that prevents silver ion agglomeration, ensuring uniform distribution throughout the coating matrix. The technology has been engineered to maintain efficacy through multiple cleaning cycles and environmental stressors, with testing showing antimicrobial activity retention after 50+ industrial washing cycles. For FDA-regulated applications, DuPont has developed specialized formulations that meet biocompatibility requirements while providing broad-spectrum protection against bacteria, fungi, and certain viruses. Their coatings have been successfully applied to medical textiles, food contact surfaces, and healthcare environment materials with documented >99.9% reduction in microbial populations.
Strengths: Smart-release technology optimizes antimicrobial efficacy while minimizing environmental impact; exceptional durability through cleaning and wear cycles; versatile application across multiple substrate types. Weaknesses: Higher initial implementation costs compared to conventional antimicrobials; complex formulation may require specialized application equipment; regulatory approval pathway varies significantly by specific application.
Medtronic MiniMed, Inc.
Technical Solution: Medtronic MiniMed has pioneered antimicrobial coating technology specifically designed for diabetes management devices and implantable medical technologies. Their approach utilizes a multi-layer coating system incorporating silver nanoparticles embedded in a biocompatible polymer matrix that slowly releases silver ions to create an antimicrobial zone around the device. The coating architecture includes a base adhesion layer, an active antimicrobial middle layer, and a top control layer that regulates ion release rates to maintain effectiveness while meeting FDA safety thresholds. This controlled-release technology has been engineered to provide antimicrobial protection for up to 30 days in implantable devices while preventing biofilm formation. Medtronic's coatings undergo rigorous cytotoxicity, sensitization, and irritation testing to ensure compliance with FDA biocompatibility standards (ISO 10993). Their technology has been successfully implemented in insulin pump infusion sets and continuous glucose monitoring systems, demonstrating significant reduction in infection rates compared to uncoated devices.
Strengths: Specialized for medical device applications with proven clinical efficacy; controlled-release technology balances antimicrobial activity with safety; extensive FDA approval experience and established regulatory pathway. Weaknesses: Silver ion technology has limited duration of effectiveness; potential for silver accumulation in tissues with long-term use; higher production costs compared to non-antimicrobial alternatives.
Key Patents and Research in Antimicrobial Surface Technology
Antimicrobial surface coatings
PatentActiveUS10131731B2
Innovation
- Development of water-soluble polymeric N-halamine precursors that form covalent bonds with surfaces, providing durable and rechargeable antimicrobial functions through a halogenation process, which can be regenerated, and incorporating additional functional groups for enhanced properties like hydrophobicity and anti-static properties.
Antimicrobial coating for inhibition of bacterial adhesion and biofilm formation
PatentInactiveUS20150010715A1
Innovation
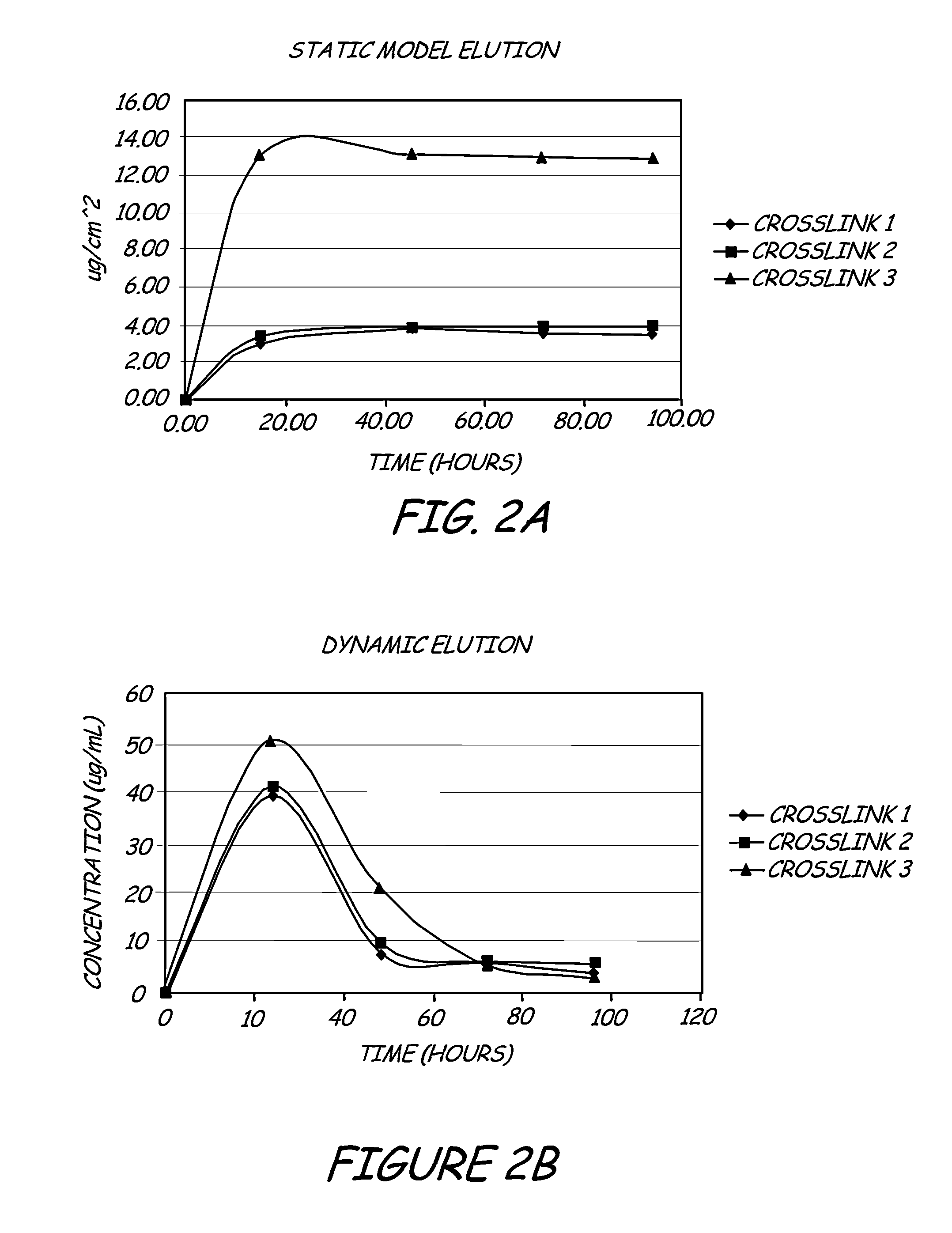
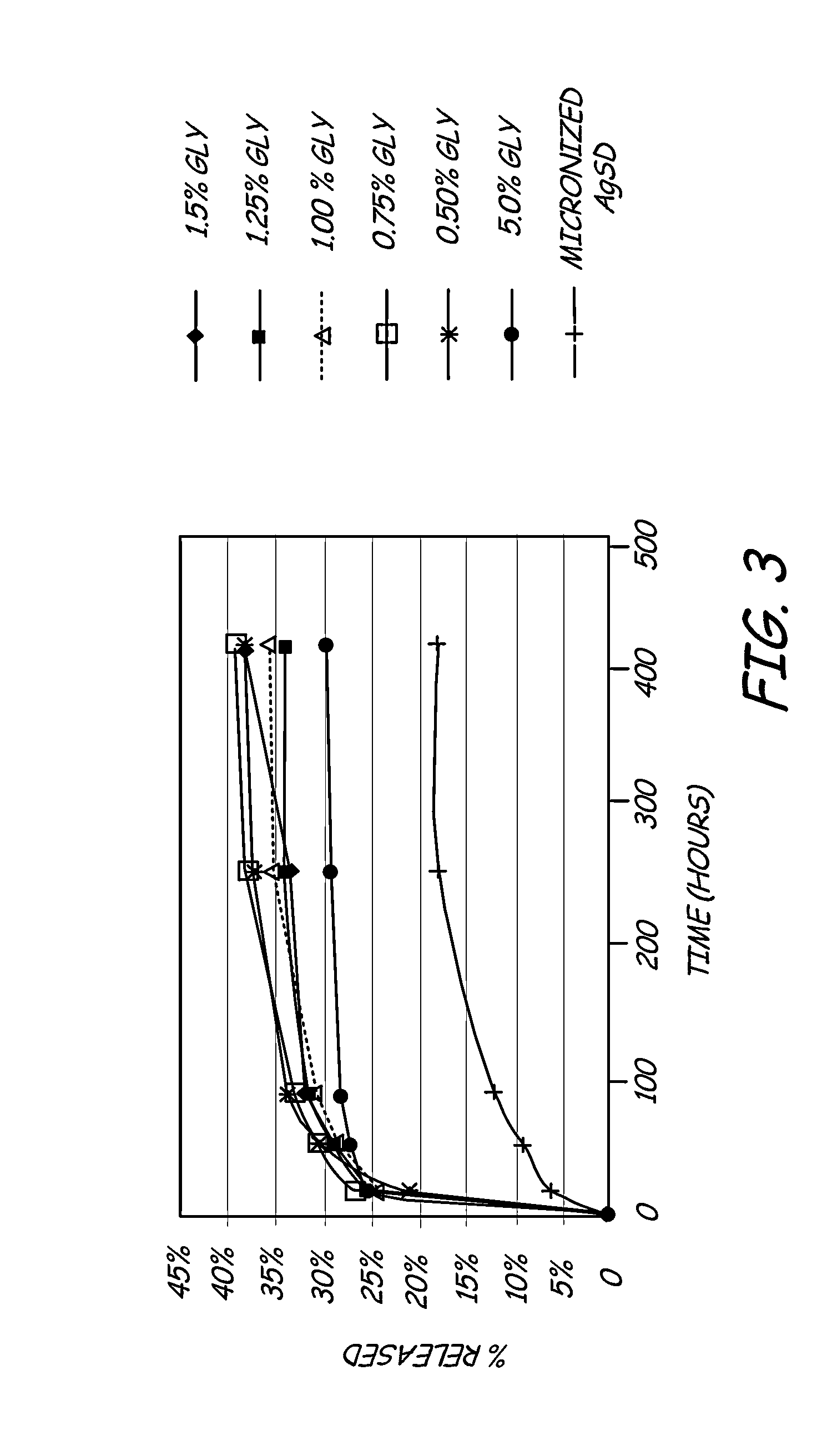
- A hydrophilic polymeric coating with a cross-linked 3D network that maintains a water-insoluble antimicrobial metallic compound, such as silver sulfadiazine, in a solubilized form, allowing high concentrations to be incorporated in thin coatings, inhibiting bacterial adhesion and biofilm formation by releasing bactericidal silver ions.
FDA Regulatory Framework for Antimicrobial Materials
The FDA regulatory framework for antimicrobial materials is built upon a complex system of laws, regulations, and guidance documents designed to ensure the safety and efficacy of products that make antimicrobial claims. The primary legislation governing these materials is the Federal Food, Drug, and Cosmetic Act (FFDCA), which provides the FDA with authority to regulate products based on their intended use and claims made.
For antimicrobial surface coatings, the regulatory pathway depends significantly on the specific claims made about the product. Materials claiming to kill or inhibit the growth of microorganisms on inanimate objects are typically regulated as pesticides under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), administered by the Environmental Protection Agency (EPA), with FDA involvement when these materials contact food or medical devices.
The FDA's Center for Devices and Radiological Health (CDRH) oversees antimicrobial coatings used on medical devices. These coatings must undergo premarket approval processes, with requirements varying based on the device classification and intended use. Class III devices with antimicrobial properties typically require Premarket Approval (PMA), while Class II devices may follow the 510(k) pathway if substantial equivalence to a predicate device can be demonstrated.
For food contact surfaces, the FDA's Center for Food Safety and Applied Nutrition (CFSAN) regulates antimicrobial materials through Food Contact Substance Notifications (FCNs) or Food Additive Petitions. These submissions must include comprehensive safety data, migration studies, and efficacy testing to demonstrate that the antimicrobial coating does not adulterate food or present safety concerns.
The FDA has established specific testing protocols for evaluating antimicrobial efficacy, including the AOAC International methods and ASTM International standards. These protocols assess factors such as log reduction of microbial populations, zone of inhibition, and durability of antimicrobial activity over time and under various environmental conditions.
Recent regulatory developments include the FDA's increased scrutiny of antimicrobial claims, particularly those related to public health significance. The agency has issued guidance documents clarifying the distinction between general antimicrobial properties and specific claims regarding protection against pathogens, with the latter requiring more substantial supporting evidence and potentially different regulatory pathways.
Manufacturers must also comply with labeling requirements that accurately reflect the proven capabilities of their antimicrobial coatings without overstating efficacy or making unsubstantiated claims. The FDA's enforcement actions have targeted products making exaggerated or misleading antimicrobial claims, emphasizing the importance of regulatory compliance in this rapidly evolving field.
For antimicrobial surface coatings, the regulatory pathway depends significantly on the specific claims made about the product. Materials claiming to kill or inhibit the growth of microorganisms on inanimate objects are typically regulated as pesticides under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), administered by the Environmental Protection Agency (EPA), with FDA involvement when these materials contact food or medical devices.
The FDA's Center for Devices and Radiological Health (CDRH) oversees antimicrobial coatings used on medical devices. These coatings must undergo premarket approval processes, with requirements varying based on the device classification and intended use. Class III devices with antimicrobial properties typically require Premarket Approval (PMA), while Class II devices may follow the 510(k) pathway if substantial equivalence to a predicate device can be demonstrated.
For food contact surfaces, the FDA's Center for Food Safety and Applied Nutrition (CFSAN) regulates antimicrobial materials through Food Contact Substance Notifications (FCNs) or Food Additive Petitions. These submissions must include comprehensive safety data, migration studies, and efficacy testing to demonstrate that the antimicrobial coating does not adulterate food or present safety concerns.
The FDA has established specific testing protocols for evaluating antimicrobial efficacy, including the AOAC International methods and ASTM International standards. These protocols assess factors such as log reduction of microbial populations, zone of inhibition, and durability of antimicrobial activity over time and under various environmental conditions.
Recent regulatory developments include the FDA's increased scrutiny of antimicrobial claims, particularly those related to public health significance. The agency has issued guidance documents clarifying the distinction between general antimicrobial properties and specific claims regarding protection against pathogens, with the latter requiring more substantial supporting evidence and potentially different regulatory pathways.
Manufacturers must also comply with labeling requirements that accurately reflect the proven capabilities of their antimicrobial coatings without overstating efficacy or making unsubstantiated claims. The FDA's enforcement actions have targeted products making exaggerated or misleading antimicrobial claims, emphasizing the importance of regulatory compliance in this rapidly evolving field.
Environmental Impact and Sustainability Considerations
The environmental impact of antimicrobial surface coatings has become increasingly significant as healthcare facilities and consumer product manufacturers adopt these technologies. Traditional antimicrobial coatings often contain heavy metals such as silver, copper, and zinc, which can leach into the environment during manufacturing, use, and disposal phases. Studies indicate that silver nanoparticles, commonly used in antimicrobial coatings, may accumulate in aquatic ecosystems and potentially disrupt microbial communities essential for environmental processes.
FDA-compliant antimicrobial coatings must balance efficacy against pathogens with minimal environmental footprint. Recent innovations have focused on developing biodegradable alternatives that maintain antimicrobial properties while reducing persistence in the environment. For instance, naturally derived antimicrobial compounds from plant extracts and essential oils show promising results with significantly lower environmental toxicity compared to metal-based solutions.
Life cycle assessment (LCA) studies of antimicrobial coatings reveal varying environmental impacts depending on production methods, application techniques, and disposal protocols. Coatings utilizing quaternary ammonium compounds typically demonstrate lower carbon footprints during production but may present challenges during end-of-life management. Conversely, some metal oxide-based coatings require energy-intensive manufacturing processes but offer longer service lives, potentially offsetting initial environmental costs.
Water consumption represents another critical environmental consideration, particularly for solution-based coating applications. Advanced application technologies such as powder coating and UV-curing systems have emerged as more sustainable alternatives, reducing volatile organic compound (VOC) emissions and water usage by up to 80% compared to conventional wet application methods.
Regulatory frameworks increasingly emphasize sustainability metrics alongside safety and efficacy requirements. The FDA's Environmental Assessment (EA) guidelines now encourage manufacturers to document potential environmental impacts throughout the product lifecycle. Companies developing next-generation antimicrobial coatings are responding by incorporating green chemistry principles, including solvent-free formulations and renewable raw materials.
Circular economy approaches are gaining traction in the antimicrobial coating sector, with innovations focused on recyclability and material recovery. Some promising developments include detachable coating systems that allow substrate reuse and antimicrobial treatments designed to biodegrade into non-toxic components after their functional lifespan. These advancements align with broader sustainability goals while maintaining compliance with stringent FDA regulations for surface materials in medical and food-contact applications.
FDA-compliant antimicrobial coatings must balance efficacy against pathogens with minimal environmental footprint. Recent innovations have focused on developing biodegradable alternatives that maintain antimicrobial properties while reducing persistence in the environment. For instance, naturally derived antimicrobial compounds from plant extracts and essential oils show promising results with significantly lower environmental toxicity compared to metal-based solutions.
Life cycle assessment (LCA) studies of antimicrobial coatings reveal varying environmental impacts depending on production methods, application techniques, and disposal protocols. Coatings utilizing quaternary ammonium compounds typically demonstrate lower carbon footprints during production but may present challenges during end-of-life management. Conversely, some metal oxide-based coatings require energy-intensive manufacturing processes but offer longer service lives, potentially offsetting initial environmental costs.
Water consumption represents another critical environmental consideration, particularly for solution-based coating applications. Advanced application technologies such as powder coating and UV-curing systems have emerged as more sustainable alternatives, reducing volatile organic compound (VOC) emissions and water usage by up to 80% compared to conventional wet application methods.
Regulatory frameworks increasingly emphasize sustainability metrics alongside safety and efficacy requirements. The FDA's Environmental Assessment (EA) guidelines now encourage manufacturers to document potential environmental impacts throughout the product lifecycle. Companies developing next-generation antimicrobial coatings are responding by incorporating green chemistry principles, including solvent-free formulations and renewable raw materials.
Circular economy approaches are gaining traction in the antimicrobial coating sector, with innovations focused on recyclability and material recovery. Some promising developments include detachable coating systems that allow substrate reuse and antimicrobial treatments designed to biodegrade into non-toxic components after their functional lifespan. These advancements align with broader sustainability goals while maintaining compliance with stringent FDA regulations for surface materials in medical and food-contact applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!