Market Analysis of Antimicrobial Surface Coatings in Healthcare
SEP 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Antimicrobial Coating Technology Background and Objectives
Antimicrobial surface coatings represent a significant technological advancement in healthcare infection control, with roots dating back to the early 20th century. The evolution of these technologies has accelerated dramatically over the past two decades, driven by increasing concerns about healthcare-associated infections (HAIs) and the rise of antimicrobial resistance. Initially focused on silver-based compounds, the field has expanded to encompass a diverse array of chemical, physical, and biological approaches to surface protection.
The technological trajectory has moved from simple antimicrobial additives to sophisticated engineered surfaces with controlled release mechanisms, contact-killing properties, and anti-adhesion characteristics. Recent innovations have introduced smart coatings capable of responding to environmental triggers and nanotechnology-enhanced formulations that dramatically improve efficacy while reducing material requirements.
Current research is increasingly focused on developing sustainable antimicrobial solutions that address growing concerns about environmental impact and potential contribution to antimicrobial resistance. This includes exploration of biomimetic approaches inspired by naturally occurring antimicrobial surfaces found in plants and animals, as well as combination technologies that integrate multiple mechanisms of action to enhance performance and reduce resistance development.
The primary objective of antimicrobial coating technology in healthcare settings is to create persistently safe surfaces that reduce pathogen transmission without requiring constant manual disinfection. Secondary goals include developing solutions that are cost-effective for widespread implementation, durable enough to withstand hospital cleaning protocols, and safe for human contact over extended periods.
Technical challenges driving innovation include achieving long-term antimicrobial efficacy, preventing biofilm formation, maintaining activity in the presence of organic matter, and ensuring compatibility with diverse substrate materials common in healthcare environments. The field is also addressing the need for coatings that can be applied to complex geometries and existing surfaces without specialized equipment.
Global research efforts are increasingly collaborative, with significant contributions from materials science, microbiology, nanotechnology, and surface engineering. Major research hubs have emerged in North America, Europe, and East Asia, with specialized centers focusing on healthcare applications of antimicrobial technologies at universities and research institutions worldwide.
The COVID-19 pandemic has accelerated interest in antimicrobial surfaces, creating new momentum for research and implementation while highlighting the critical importance of environmental control in infection prevention strategies. This has expanded the technology's potential applications beyond traditional healthcare settings to include public spaces and transportation systems.
The technological trajectory has moved from simple antimicrobial additives to sophisticated engineered surfaces with controlled release mechanisms, contact-killing properties, and anti-adhesion characteristics. Recent innovations have introduced smart coatings capable of responding to environmental triggers and nanotechnology-enhanced formulations that dramatically improve efficacy while reducing material requirements.
Current research is increasingly focused on developing sustainable antimicrobial solutions that address growing concerns about environmental impact and potential contribution to antimicrobial resistance. This includes exploration of biomimetic approaches inspired by naturally occurring antimicrobial surfaces found in plants and animals, as well as combination technologies that integrate multiple mechanisms of action to enhance performance and reduce resistance development.
The primary objective of antimicrobial coating technology in healthcare settings is to create persistently safe surfaces that reduce pathogen transmission without requiring constant manual disinfection. Secondary goals include developing solutions that are cost-effective for widespread implementation, durable enough to withstand hospital cleaning protocols, and safe for human contact over extended periods.
Technical challenges driving innovation include achieving long-term antimicrobial efficacy, preventing biofilm formation, maintaining activity in the presence of organic matter, and ensuring compatibility with diverse substrate materials common in healthcare environments. The field is also addressing the need for coatings that can be applied to complex geometries and existing surfaces without specialized equipment.
Global research efforts are increasingly collaborative, with significant contributions from materials science, microbiology, nanotechnology, and surface engineering. Major research hubs have emerged in North America, Europe, and East Asia, with specialized centers focusing on healthcare applications of antimicrobial technologies at universities and research institutions worldwide.
The COVID-19 pandemic has accelerated interest in antimicrobial surfaces, creating new momentum for research and implementation while highlighting the critical importance of environmental control in infection prevention strategies. This has expanded the technology's potential applications beyond traditional healthcare settings to include public spaces and transportation systems.
Healthcare Market Demand Analysis for Antimicrobial Surfaces
The global market for antimicrobial surface coatings in healthcare settings has experienced significant growth in recent years, driven primarily by increasing awareness of healthcare-associated infections (HAIs) and the critical need for improved infection control protocols. Current market valuations indicate that the healthcare antimicrobial coatings sector reached approximately 3.6 billion USD in 2022, with projections suggesting a compound annual growth rate of 12.3% through 2030.
Hospital-acquired infections represent a substantial burden on healthcare systems worldwide, with an estimated 1.7 million patients in the United States alone contracting HAIs annually. The economic impact is equally concerning, with direct costs for treating these infections exceeding 28 billion USD per year. These statistics have created urgent demand for effective preventive solutions, positioning antimicrobial surfaces as a critical intervention strategy.
The COVID-19 pandemic has dramatically accelerated market growth, fundamentally altering perceptions about surface transmission of pathogens. Healthcare facilities have significantly increased investments in infection prevention technologies, with antimicrobial surfaces moving from optional enhancements to essential infrastructure components. Market surveys indicate that 78% of healthcare administrators now prioritize antimicrobial properties when making facility renovation decisions.
Regional analysis reveals varying adoption rates, with North America currently dominating market share at approximately 42%, followed by Europe at 31% and Asia-Pacific showing the fastest growth trajectory at 15.8% annually. This regional disparity reflects differences in healthcare infrastructure development, regulatory frameworks, and public health priorities.
By application segment, high-touch surfaces in patient care areas represent the largest market share (38%), followed by surgical environments (27%), medical device coatings (21%), and general facility surfaces (14%). The remaining market share is distributed across specialized applications such as laboratory environments and pharmaceutical manufacturing facilities.
End-user segmentation shows hospitals consuming 56% of antimicrobial surface products, outpatient clinics accounting for 18%, long-term care facilities at 14%, and other healthcare settings comprising the remaining 12%. Within hospitals, intensive care units and operating theaters demonstrate the highest implementation rates, reflecting their critical infection control requirements.
Market drivers extend beyond infection prevention to include regulatory pressures, with several countries implementing stricter guidelines for healthcare facility surfaces. Additionally, the rising prevalence of antibiotic-resistant organisms has intensified the search for alternative prevention strategies, with antimicrobial surfaces offering a promising complementary approach to traditional infection control measures.
Hospital-acquired infections represent a substantial burden on healthcare systems worldwide, with an estimated 1.7 million patients in the United States alone contracting HAIs annually. The economic impact is equally concerning, with direct costs for treating these infections exceeding 28 billion USD per year. These statistics have created urgent demand for effective preventive solutions, positioning antimicrobial surfaces as a critical intervention strategy.
The COVID-19 pandemic has dramatically accelerated market growth, fundamentally altering perceptions about surface transmission of pathogens. Healthcare facilities have significantly increased investments in infection prevention technologies, with antimicrobial surfaces moving from optional enhancements to essential infrastructure components. Market surveys indicate that 78% of healthcare administrators now prioritize antimicrobial properties when making facility renovation decisions.
Regional analysis reveals varying adoption rates, with North America currently dominating market share at approximately 42%, followed by Europe at 31% and Asia-Pacific showing the fastest growth trajectory at 15.8% annually. This regional disparity reflects differences in healthcare infrastructure development, regulatory frameworks, and public health priorities.
By application segment, high-touch surfaces in patient care areas represent the largest market share (38%), followed by surgical environments (27%), medical device coatings (21%), and general facility surfaces (14%). The remaining market share is distributed across specialized applications such as laboratory environments and pharmaceutical manufacturing facilities.
End-user segmentation shows hospitals consuming 56% of antimicrobial surface products, outpatient clinics accounting for 18%, long-term care facilities at 14%, and other healthcare settings comprising the remaining 12%. Within hospitals, intensive care units and operating theaters demonstrate the highest implementation rates, reflecting their critical infection control requirements.
Market drivers extend beyond infection prevention to include regulatory pressures, with several countries implementing stricter guidelines for healthcare facility surfaces. Additionally, the rising prevalence of antibiotic-resistant organisms has intensified the search for alternative prevention strategies, with antimicrobial surfaces offering a promising complementary approach to traditional infection control measures.
Current Antimicrobial Coating Technologies and Challenges
Antimicrobial surface coatings in healthcare settings have evolved significantly over the past decade, with several technologies currently dominating the market. Silver-based coatings remain the most widely adopted solution, utilizing silver ions or nanoparticles that disrupt bacterial cell membranes and inhibit DNA replication. These coatings demonstrate broad-spectrum efficacy against bacteria, fungi, and certain viruses, though their effectiveness typically diminishes over time as silver ions are depleted.
Copper and copper alloy surfaces represent another established technology, leveraging copper's inherent antimicrobial properties through contact killing mechanisms. Studies have shown that copper surfaces can reduce bacterial loads by up to 83% in hospital settings compared to standard surfaces. However, implementation costs and aesthetic considerations have limited widespread adoption despite their proven efficacy.
Quaternary ammonium compound (QAC) coatings function by disrupting microbial cell membranes through their positively charged molecules. These coatings offer the advantage of relatively low production costs and ease of application to various surfaces, but concerns regarding potential antimicrobial resistance development have emerged with their prolonged use.
Titanium dioxide (TiO₂) photocatalytic coatings represent an advancing technology that generates reactive oxygen species when exposed to light, effectively destroying microorganisms. While highly effective in well-lit environments, their performance diminishes significantly in low-light conditions, limiting application in certain healthcare settings.
Despite these technological advances, significant challenges persist. Durability remains a primary concern, as most antimicrobial coatings demonstrate reduced efficacy after repeated cleaning cycles or extended use. Healthcare facilities typically require surfaces that maintain antimicrobial properties for 3-5 years, yet many current solutions show significant degradation within 6-12 months.
Regulatory hurdles present another substantial challenge. Different regions maintain varying standards for antimicrobial claims, with the FDA and EPA in the United States requiring rigorous efficacy and safety testing. European regulations under the Biocidal Products Regulation impose similarly stringent requirements, creating complex compliance landscapes for manufacturers.
Cost-effectiveness continues to impede widespread adoption, with antimicrobial surfaces typically commanding a 15-40% premium over standard materials. Healthcare administrators often struggle to justify this additional expenditure despite potential long-term benefits in infection reduction.
The balance between antimicrobial efficacy and toxicity concerns represents another significant challenge. Some highly effective antimicrobial agents raise concerns regarding potential environmental impacts or human exposure risks, particularly in sensitive healthcare environments like neonatal units or operating rooms.
Standardization of testing protocols remains inconsistent across the industry, making direct comparisons between different antimicrobial technologies difficult. This lack of standardization complicates decision-making processes for healthcare facilities evaluating potential solutions.
Copper and copper alloy surfaces represent another established technology, leveraging copper's inherent antimicrobial properties through contact killing mechanisms. Studies have shown that copper surfaces can reduce bacterial loads by up to 83% in hospital settings compared to standard surfaces. However, implementation costs and aesthetic considerations have limited widespread adoption despite their proven efficacy.
Quaternary ammonium compound (QAC) coatings function by disrupting microbial cell membranes through their positively charged molecules. These coatings offer the advantage of relatively low production costs and ease of application to various surfaces, but concerns regarding potential antimicrobial resistance development have emerged with their prolonged use.
Titanium dioxide (TiO₂) photocatalytic coatings represent an advancing technology that generates reactive oxygen species when exposed to light, effectively destroying microorganisms. While highly effective in well-lit environments, their performance diminishes significantly in low-light conditions, limiting application in certain healthcare settings.
Despite these technological advances, significant challenges persist. Durability remains a primary concern, as most antimicrobial coatings demonstrate reduced efficacy after repeated cleaning cycles or extended use. Healthcare facilities typically require surfaces that maintain antimicrobial properties for 3-5 years, yet many current solutions show significant degradation within 6-12 months.
Regulatory hurdles present another substantial challenge. Different regions maintain varying standards for antimicrobial claims, with the FDA and EPA in the United States requiring rigorous efficacy and safety testing. European regulations under the Biocidal Products Regulation impose similarly stringent requirements, creating complex compliance landscapes for manufacturers.
Cost-effectiveness continues to impede widespread adoption, with antimicrobial surfaces typically commanding a 15-40% premium over standard materials. Healthcare administrators often struggle to justify this additional expenditure despite potential long-term benefits in infection reduction.
The balance between antimicrobial efficacy and toxicity concerns represents another significant challenge. Some highly effective antimicrobial agents raise concerns regarding potential environmental impacts or human exposure risks, particularly in sensitive healthcare environments like neonatal units or operating rooms.
Standardization of testing protocols remains inconsistent across the industry, making direct comparisons between different antimicrobial technologies difficult. This lack of standardization complicates decision-making processes for healthcare facilities evaluating potential solutions.
Current Antimicrobial Surface Solutions in Healthcare
01 Metal-based antimicrobial coatings
Metal-based antimicrobial coatings utilize silver, copper, zinc, and other metal ions or nanoparticles to provide long-lasting protection against a wide range of microorganisms. These metals disrupt bacterial cell membranes and interfere with cellular processes, preventing microbial colonization on surfaces. Such coatings can be applied to various substrates including medical devices, touch surfaces, and industrial equipment to reduce the risk of infection transmission.- Metal-based antimicrobial coatings: Metal-based antimicrobial coatings utilize silver, copper, zinc, and other metal ions or nanoparticles to provide effective antimicrobial properties on various surfaces. These metals disrupt bacterial cell membranes and interfere with cellular processes, preventing microbial growth and colonization. The coatings can be applied to medical devices, touch surfaces, and consumer products to reduce infection transmission and biofilm formation. Metal-based formulations often provide long-lasting protection due to the controlled release of active ions over time.
- Polymer-based antimicrobial coatings: Polymer-based antimicrobial coatings incorporate active ingredients within polymer matrices to create durable protective surfaces. These formulations often use quaternary ammonium compounds, chitosan, or other antimicrobial agents embedded in polymers like polyurethane, silicone, or acrylics. The polymer matrix provides controlled release of the active ingredients while maintaining surface integrity. These coatings can be engineered for specific applications with varying levels of hydrophobicity, durability, and antimicrobial efficacy, making them suitable for healthcare settings, food processing equipment, and consumer products.
- Natural compound-based antimicrobial coatings: Natural compound-based antimicrobial coatings utilize plant extracts, essential oils, enzymes, and other naturally derived substances to inhibit microbial growth on surfaces. These environmentally friendly alternatives often contain components like thymol, carvacrol, eugenol, or plant-derived polyphenols that disrupt bacterial cell membranes or inhibit cellular processes. The formulations can be incorporated into various coating matrices to provide antimicrobial protection for food packaging, medical devices, and consumer products, addressing growing concerns about chemical resistance and environmental impact.
- Photocatalytic antimicrobial coatings: Photocatalytic antimicrobial coatings utilize materials like titanium dioxide (TiO2) that generate reactive oxygen species when exposed to light, particularly UV radiation. These reactive species effectively kill microorganisms by oxidizing their cell components. The coatings provide continuous antimicrobial action as long as they receive appropriate light exposure, making them suitable for self-cleaning surfaces in well-lit environments. Advanced formulations may incorporate dopants or sensitizers to enhance activity under visible light, expanding their application to indoor settings for hospitals, public spaces, and consumer products.
- Multi-functional antimicrobial surface coatings: Multi-functional antimicrobial surface coatings combine antimicrobial properties with additional beneficial characteristics such as anti-fouling, self-cleaning, durability, or enhanced adhesion. These sophisticated formulations often incorporate multiple active ingredients or utilize specialized surface structures to achieve synergistic effects. The coatings may feature hierarchical surface patterns, hydrophobic/hydrophilic domains, or responsive elements that adapt to environmental conditions. Applications include medical implants that resist both bacterial colonization and host rejection, marine coatings that prevent both microbial growth and organism attachment, and consumer products with enhanced longevity and safety profiles.
02 Quaternary ammonium compound coatings
Quaternary ammonium compounds (QACs) are effective antimicrobial agents that can be incorporated into surface coatings. These compounds disrupt microbial cell membranes, leading to cell death. QAC-based coatings provide broad-spectrum antimicrobial activity against bacteria, fungi, and some viruses. They can be formulated to create durable, long-lasting antimicrobial surfaces for healthcare settings, food processing facilities, and public spaces to reduce pathogen transmission.Expand Specific Solutions03 Polymer-based antimicrobial coatings
Polymer-based antimicrobial coatings incorporate active ingredients within polymer matrices to create surfaces that resist microbial colonization. These coatings can utilize various mechanisms including contact-killing, controlled release of antimicrobial agents, or anti-adhesion properties. The polymer matrix provides durability and controlled release of active ingredients, extending the antimicrobial efficacy over time. Applications include medical implants, food packaging, and high-touch surfaces in public spaces.Expand Specific Solutions04 Natural and bio-based antimicrobial coatings
Natural and bio-based antimicrobial coatings utilize plant extracts, essential oils, enzymes, and other naturally derived compounds to inhibit microbial growth on surfaces. These environmentally friendly alternatives offer reduced toxicity compared to synthetic antimicrobials while providing effective protection against various pathogens. Such coatings are particularly valuable in food contact applications, children's products, and environments where reduced chemical exposure is desired.Expand Specific Solutions05 Photocatalytic antimicrobial coatings
Photocatalytic antimicrobial coatings contain materials like titanium dioxide that generate reactive oxygen species when exposed to light. These reactive species can destroy microorganisms on the coated surface by oxidizing their cell components. Such coatings provide continuous self-cleaning and disinfecting properties as long as they receive adequate light exposure. Applications include hospital surfaces, air purification systems, and exterior building materials where sunlight can activate the antimicrobial properties.Expand Specific Solutions
Key Industry Players in Healthcare Antimicrobial Coatings
The antimicrobial surface coatings market in healthcare is currently in a growth phase, with increasing adoption driven by heightened infection control awareness. The global market is projected to reach significant value as healthcare facilities prioritize pathogen reduction technologies. Technologically, the field shows moderate maturity with established solutions, but continuous innovation is evident. Key players represent diverse approaches: academic institutions (MIT, HKUST) focus on fundamental research; specialized coating companies (Kastus Technologies, Jiangsu Biosurf) develop proprietary antimicrobial technologies; while established healthcare manufacturers (Medtronic, Baxter, B. Braun) integrate these solutions into medical devices. Research organizations (CNRS, Empa) bridge the gap between theoretical advances and practical applications, creating a competitive landscape balanced between innovation-focused entities and market-oriented commercial players.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have developed a revolutionary approach to antimicrobial surface coatings for healthcare applications through their Layer-by-Layer (LbL) assembly technology. This platform enables the precise construction of nanolayered antimicrobial films with controlled release properties. The technology incorporates multiple antimicrobial mechanisms including contact-killing polymers, antimicrobial peptides, and controlled-release antibiotic reservoirs within a single coating architecture. Laboratory studies have demonstrated that these coatings can reduce bacterial adhesion by over 99.9% while maintaining activity for extended periods (>6 months) under simulated clinical conditions. A key innovation in MIT's approach is the incorporation of "smart" responsive elements that can trigger enhanced antimicrobial activity in response to environmental changes such as pH shifts associated with bacterial colonization. This creates an adaptive defense system rather than a static barrier. The coating technology has been successfully applied to complex medical device geometries including catheters, orthopedic implants, and wound dressings. Recent developments include the integration of quorum sensing inhibitors that disrupt bacterial communication, preventing biofilm formation without relying on traditional antibiotics, thus addressing concerns about antimicrobial resistance development.
Strengths: Highly customizable platform allowing application-specific optimization; multiple antimicrobial mechanisms reduce resistance development risk; precise control over release kinetics for extended efficacy. Weaknesses: Complex manufacturing process increases production costs; requires specialized equipment for application; still in translational research phase for many potential applications.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed advanced antimicrobial surface coatings for healthcare environments utilizing their proprietary Microban® technology. Their solution incorporates silver ion technology that disrupts bacterial cell walls and inhibits reproduction. The coating is engineered to provide continuous protection throughout the product lifecycle, with efficacy lasting up to 99.99% against common healthcare-associated pathogens including MRSA, E. coli, and C. difficile. DuPont's antimicrobial coatings are designed to be integrated into various healthcare surfaces including countertops, door handles, and medical equipment casings. Their technology allows for the controlled release of active ingredients, providing sustained antimicrobial activity without compromising the material's physical properties or appearance. Recent clinical implementations have shown a reduction in surface bacterial counts by over 90% in hospital settings where these coatings were applied.
Strengths: Long-lasting efficacy with proven clinical results; compatibility with multiple substrate materials; established global distribution network and regulatory compliance. Weaknesses: Higher initial implementation costs compared to standard coatings; potential for development of microbial resistance with prolonged use; limited effectiveness against certain viral pathogens.
Critical Patents and Innovations in Antimicrobial Coatings
Antimicrobial composition for coating surfaces
PatentInactiveUS20190246644A1
Innovation
- A composition combining micronized, high-purity metallic copper particles of various shapes and sizes with a solvent-free polymer binder, applied cold and hardened by polymerization, ensuring comprehensive coverage and long-lasting antimicrobial action.
Antimicrobial coating for long-term disinfection of surfaces
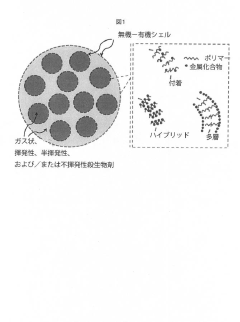
PatentActiveJP2020063260A
Innovation
- Development of colloidal capsules containing biocides within inorganic-organic shells that provide controlled release and contact killing, combining polymers and metal compounds for antimicrobial coatings on surfaces, which can be applied to both porous and non-porous materials.
Regulatory Framework for Healthcare Surface Materials
The regulatory landscape governing antimicrobial surface coatings in healthcare settings is complex and multifaceted, requiring manufacturers to navigate various approval processes before bringing products to market. In the United States, the Environmental Protection Agency (EPA) regulates antimicrobial coatings under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) when they make public health claims. Products must undergo rigorous testing to demonstrate efficacy against specific pathogens and receive EPA registration before commercialization.
Concurrently, the Food and Drug Administration (FDA) oversees antimicrobial coatings used on medical devices through the 510(k) clearance process or Premarket Approval (PMA) pathway, depending on the device classification and intended use. This dual regulatory oversight creates significant compliance challenges for manufacturers seeking to enter the healthcare antimicrobial coatings market.
In the European Union, the Biocidal Products Regulation (BPR) governs antimicrobial coatings, requiring manufacturers to obtain authorization for active substances and demonstrate both safety and efficacy. Additionally, the Medical Device Regulation (MDR) applies when these coatings are incorporated into medical devices, imposing stringent requirements for clinical evaluation and post-market surveillance.
International standards organizations play a crucial role in establishing testing methodologies and performance criteria. ISO 22196 and JIS Z 2801 are widely recognized standards for evaluating antimicrobial activity on surfaces, while ASTM E2180 addresses antimicrobial activity of polymeric materials. These standards provide a framework for consistent evaluation but may not fully reflect real-world conditions in healthcare environments.
Recent regulatory trends indicate increasing scrutiny of antimicrobial claims, particularly regarding the duration of efficacy and potential for contributing to antimicrobial resistance. Regulatory bodies are demanding more comprehensive data on long-term performance and environmental impact, including leaching behavior and potential toxicity concerns of active ingredients.
Healthcare facility accreditation requirements further influence the adoption of antimicrobial surfaces. Organizations such as The Joint Commission and Healthcare Facilities Accreditation Program incorporate infection control standards that indirectly impact surface material selection decisions. These requirements often emphasize evidence-based approaches to infection prevention, creating additional hurdles for novel antimicrobial technologies.
Navigating this complex regulatory framework requires significant investment in testing and documentation, creating barriers to entry for smaller companies. However, regulatory compliance also serves as a competitive advantage for established players who can leverage their regulatory expertise and existing relationships with authorities to expedite approvals for new antimicrobial coating technologies.
Concurrently, the Food and Drug Administration (FDA) oversees antimicrobial coatings used on medical devices through the 510(k) clearance process or Premarket Approval (PMA) pathway, depending on the device classification and intended use. This dual regulatory oversight creates significant compliance challenges for manufacturers seeking to enter the healthcare antimicrobial coatings market.
In the European Union, the Biocidal Products Regulation (BPR) governs antimicrobial coatings, requiring manufacturers to obtain authorization for active substances and demonstrate both safety and efficacy. Additionally, the Medical Device Regulation (MDR) applies when these coatings are incorporated into medical devices, imposing stringent requirements for clinical evaluation and post-market surveillance.
International standards organizations play a crucial role in establishing testing methodologies and performance criteria. ISO 22196 and JIS Z 2801 are widely recognized standards for evaluating antimicrobial activity on surfaces, while ASTM E2180 addresses antimicrobial activity of polymeric materials. These standards provide a framework for consistent evaluation but may not fully reflect real-world conditions in healthcare environments.
Recent regulatory trends indicate increasing scrutiny of antimicrobial claims, particularly regarding the duration of efficacy and potential for contributing to antimicrobial resistance. Regulatory bodies are demanding more comprehensive data on long-term performance and environmental impact, including leaching behavior and potential toxicity concerns of active ingredients.
Healthcare facility accreditation requirements further influence the adoption of antimicrobial surfaces. Organizations such as The Joint Commission and Healthcare Facilities Accreditation Program incorporate infection control standards that indirectly impact surface material selection decisions. These requirements often emphasize evidence-based approaches to infection prevention, creating additional hurdles for novel antimicrobial technologies.
Navigating this complex regulatory framework requires significant investment in testing and documentation, creating barriers to entry for smaller companies. However, regulatory compliance also serves as a competitive advantage for established players who can leverage their regulatory expertise and existing relationships with authorities to expedite approvals for new antimicrobial coating technologies.
Cost-Benefit Analysis of Antimicrobial Implementation
Implementing antimicrobial surface coatings in healthcare settings requires a thorough cost-benefit analysis to justify the initial investment. The upfront costs include material procurement, installation labor, staff training, and potential facility downtime during implementation. For a typical 250-bed hospital, initial implementation costs range from $150,000 to $400,000 depending on coverage area and coating technology selected. Ongoing maintenance adds approximately $30,000-$75,000 annually, including reapplication schedules and monitoring systems.
Against these expenses, healthcare facilities must weigh substantial potential benefits. Hospital-acquired infections (HAIs) cost the U.S. healthcare system approximately $28-45 billion annually. Each prevented infection saves $14,000-$38,000 in direct treatment costs, with additional savings from avoided litigation and reputation damage. Studies from early adopters indicate antimicrobial coatings can reduce surface bacterial counts by 80-99% and decrease HAI rates by 15-35% when implemented as part of comprehensive infection control protocols.
Operational benefits include reduced cleaning time and chemical usage, with some facilities reporting 20-30% efficiency improvements in environmental services departments. Staff absenteeism related to infectious disease exposure typically decreases by 8-12%, representing significant workforce continuity improvements and reduced temporary staffing costs.
Return on investment timelines vary by facility type and implementation scope. Acute care hospitals generally see financial breakeven within 18-36 months, while long-term care facilities may require 24-48 months. The COVID-19 pandemic has accelerated adoption and shortened perceived ROI timelines as infection control priorities have heightened.
Intangible benefits must also factor into decision-making processes. Enhanced patient confidence, improved HCAHPS scores, and competitive differentiation in healthcare markets increasingly influence facility investment decisions. Regulatory compliance advantages are growing as oversight bodies increasingly focus on infection prevention infrastructure.
Risk factors affecting cost-benefit calculations include coating durability variability, potential for microbial adaptation, and implementation quality inconsistencies. Sensitivity analysis suggests that even with conservative efficacy estimates of 10-15% infection reduction, most facilities still achieve positive ROI within 4 years, demonstrating the robust economic case for antimicrobial surface technology adoption in healthcare environments.
Against these expenses, healthcare facilities must weigh substantial potential benefits. Hospital-acquired infections (HAIs) cost the U.S. healthcare system approximately $28-45 billion annually. Each prevented infection saves $14,000-$38,000 in direct treatment costs, with additional savings from avoided litigation and reputation damage. Studies from early adopters indicate antimicrobial coatings can reduce surface bacterial counts by 80-99% and decrease HAI rates by 15-35% when implemented as part of comprehensive infection control protocols.
Operational benefits include reduced cleaning time and chemical usage, with some facilities reporting 20-30% efficiency improvements in environmental services departments. Staff absenteeism related to infectious disease exposure typically decreases by 8-12%, representing significant workforce continuity improvements and reduced temporary staffing costs.
Return on investment timelines vary by facility type and implementation scope. Acute care hospitals generally see financial breakeven within 18-36 months, while long-term care facilities may require 24-48 months. The COVID-19 pandemic has accelerated adoption and shortened perceived ROI timelines as infection control priorities have heightened.
Intangible benefits must also factor into decision-making processes. Enhanced patient confidence, improved HCAHPS scores, and competitive differentiation in healthcare markets increasingly influence facility investment decisions. Regulatory compliance advantages are growing as oversight bodies increasingly focus on infection prevention infrastructure.
Risk factors affecting cost-benefit calculations include coating durability variability, potential for microbial adaptation, and implementation quality inconsistencies. Sensitivity analysis suggests that even with conservative efficacy estimates of 10-15% infection reduction, most facilities still achieve positive ROI within 4 years, demonstrating the robust economic case for antimicrobial surface technology adoption in healthcare environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!