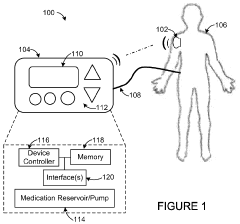
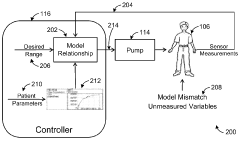
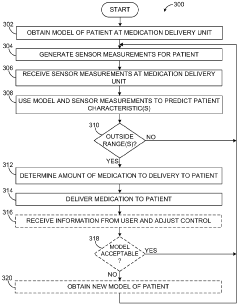
Model Predictive Control In Automated Drug Delivery Systems
SEP 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MPC Technology Evolution in Drug Delivery
Model Predictive Control (MPC) in drug delivery systems has evolved significantly over the past decades, transforming from theoretical concepts to practical implementations. The evolution began in the 1980s with the introduction of basic predictive control algorithms in industrial processes, which were later adapted for biomedical applications in the 1990s. During this initial phase, MPC was primarily used in simulated environments due to computational limitations and insufficient biological modeling capabilities.
The early 2000s marked a pivotal shift with the emergence of more sophisticated mathematical models capable of predicting drug pharmacokinetics and pharmacodynamics with greater accuracy. This period saw the development of specialized MPC algorithms designed specifically for drug delivery applications, particularly for anesthesia and diabetes management. These early systems utilized relatively simple linear models and faced challenges with real-time implementation.
Between 2005 and 2015, significant advancements in computational power and miniaturization enabled the transition from theoretical frameworks to practical prototypes. During this phase, researchers began incorporating nonlinear models and stochastic elements to account for patient variability and uncertainty in drug responses. The integration of physiological feedback mechanisms also became more prevalent, allowing for closed-loop control systems that could adjust drug delivery based on measured patient responses.
The period from 2015 to 2020 witnessed the convergence of MPC with machine learning techniques, particularly reinforcement learning and neural networks. This fusion enabled more personalized drug delivery protocols that could adapt to individual patient characteristics and learn from historical data. Simultaneously, improvements in sensor technology facilitated more accurate and continuous monitoring of patient parameters, enhancing the precision of MPC implementations.
Most recently (2020-present), the field has seen the emergence of distributed MPC architectures that can coordinate multiple drug delivery systems simultaneously, particularly valuable in complex therapeutic regimens. Edge computing capabilities have addressed previous latency issues, enabling real-time processing of complex predictive models. Additionally, robust MPC formulations have been developed to handle uncertainties and disturbances in clinical settings, significantly improving reliability.
The current technological frontier focuses on hybrid systems that combine model-based approaches with data-driven techniques, creating adaptive frameworks that continuously refine their predictive capabilities through clinical use. These systems represent a significant advancement from the early theoretical concepts, demonstrating the remarkable evolution of MPC technology in automated drug delivery over the past decades.
The early 2000s marked a pivotal shift with the emergence of more sophisticated mathematical models capable of predicting drug pharmacokinetics and pharmacodynamics with greater accuracy. This period saw the development of specialized MPC algorithms designed specifically for drug delivery applications, particularly for anesthesia and diabetes management. These early systems utilized relatively simple linear models and faced challenges with real-time implementation.
Between 2005 and 2015, significant advancements in computational power and miniaturization enabled the transition from theoretical frameworks to practical prototypes. During this phase, researchers began incorporating nonlinear models and stochastic elements to account for patient variability and uncertainty in drug responses. The integration of physiological feedback mechanisms also became more prevalent, allowing for closed-loop control systems that could adjust drug delivery based on measured patient responses.
The period from 2015 to 2020 witnessed the convergence of MPC with machine learning techniques, particularly reinforcement learning and neural networks. This fusion enabled more personalized drug delivery protocols that could adapt to individual patient characteristics and learn from historical data. Simultaneously, improvements in sensor technology facilitated more accurate and continuous monitoring of patient parameters, enhancing the precision of MPC implementations.
Most recently (2020-present), the field has seen the emergence of distributed MPC architectures that can coordinate multiple drug delivery systems simultaneously, particularly valuable in complex therapeutic regimens. Edge computing capabilities have addressed previous latency issues, enabling real-time processing of complex predictive models. Additionally, robust MPC formulations have been developed to handle uncertainties and disturbances in clinical settings, significantly improving reliability.
The current technological frontier focuses on hybrid systems that combine model-based approaches with data-driven techniques, creating adaptive frameworks that continuously refine their predictive capabilities through clinical use. These systems represent a significant advancement from the early theoretical concepts, demonstrating the remarkable evolution of MPC technology in automated drug delivery over the past decades.
Market Demand for Automated Drug Delivery
The global market for automated drug delivery systems is experiencing significant growth, driven by the increasing prevalence of chronic diseases, aging populations, and the demand for more precise medication administration. According to recent market analyses, the automated drug delivery systems market was valued at approximately $7.8 billion in 2022 and is projected to reach $15.3 billion by 2030, growing at a CAGR of 8.7% during the forecast period.
The integration of Model Predictive Control (MPC) technology into these systems represents a particularly promising segment within this market. Healthcare providers are increasingly seeking solutions that can deliver medications with greater precision, minimize adverse effects, and optimize therapeutic outcomes through personalized dosing regimens.
Patient-specific factors are driving substantial demand for MPC-enabled drug delivery systems. The rising incidence of diabetes worldwide has created a significant market for automated insulin delivery systems that can predict and respond to glucose fluctuations. Similarly, pain management applications, particularly in post-operative and chronic pain scenarios, benefit from predictive algorithms that can anticipate pain episodes and adjust analgesic delivery accordingly.
Hospital and clinical settings represent the largest market segment for MPC-based drug delivery systems, accounting for approximately 62% of the current market share. These institutions value the ability to reduce medication errors, improve patient outcomes, and optimize healthcare resource utilization through automated systems.
From a geographical perspective, North America dominates the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 18%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 10.2% annually, driven by improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about advanced medical technologies.
Key market drivers include the push for reduced healthcare costs through optimized medication use, growing patient preference for minimally invasive drug delivery methods, and increasing adoption of home healthcare solutions. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of remote patient monitoring and reducing hospital visits for routine medication administration.
Regulatory support for digital health technologies and precision medicine initiatives across major markets is creating a favorable environment for the adoption of advanced drug delivery systems. Additionally, the integration of these systems with telehealth platforms is opening new market opportunities, particularly for managing chronic conditions that require continuous medication adjustment.
The integration of Model Predictive Control (MPC) technology into these systems represents a particularly promising segment within this market. Healthcare providers are increasingly seeking solutions that can deliver medications with greater precision, minimize adverse effects, and optimize therapeutic outcomes through personalized dosing regimens.
Patient-specific factors are driving substantial demand for MPC-enabled drug delivery systems. The rising incidence of diabetes worldwide has created a significant market for automated insulin delivery systems that can predict and respond to glucose fluctuations. Similarly, pain management applications, particularly in post-operative and chronic pain scenarios, benefit from predictive algorithms that can anticipate pain episodes and adjust analgesic delivery accordingly.
Hospital and clinical settings represent the largest market segment for MPC-based drug delivery systems, accounting for approximately 62% of the current market share. These institutions value the ability to reduce medication errors, improve patient outcomes, and optimize healthcare resource utilization through automated systems.
From a geographical perspective, North America dominates the market with approximately 45% share, followed by Europe at 30% and Asia-Pacific at 18%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 10.2% annually, driven by improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about advanced medical technologies.
Key market drivers include the push for reduced healthcare costs through optimized medication use, growing patient preference for minimally invasive drug delivery methods, and increasing adoption of home healthcare solutions. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of remote patient monitoring and reducing hospital visits for routine medication administration.
Regulatory support for digital health technologies and precision medicine initiatives across major markets is creating a favorable environment for the adoption of advanced drug delivery systems. Additionally, the integration of these systems with telehealth platforms is opening new market opportunities, particularly for managing chronic conditions that require continuous medication adjustment.
Technical Challenges in MPC Drug Delivery Systems
Despite the promising potential of Model Predictive Control (MPC) in automated drug delivery systems, several significant technical challenges impede its widespread clinical implementation. The primary obstacle lies in developing accurate physiological models that can reliably predict patient responses to medications. Human physiological systems exhibit complex, nonlinear dynamics with substantial inter-patient variability, making standardized modeling exceptionally difficult. These models must account for pharmacokinetics (drug absorption, distribution, metabolism, and excretion) and pharmacodynamics (biochemical and physiological effects), which vary significantly between individuals and can change within the same patient over time.
Computational complexity presents another formidable challenge. MPC algorithms require solving optimization problems in real-time, often with limited computational resources in medical devices. This becomes particularly problematic when implementing nonlinear MPC, which more accurately represents physiological systems but demands substantially greater computational power. The trade-off between model complexity, prediction accuracy, and computational efficiency remains a critical balancing act.
Sensor technology limitations further complicate MPC implementation. Current medical sensors often suffer from measurement noise, drift, and limited sampling frequencies. Many crucial physiological parameters cannot be measured directly or continuously, forcing systems to rely on state estimators that introduce additional uncertainty. The integration of multiple sensor types with different sampling rates and reliability characteristics creates significant data fusion challenges.
Robustness and safety concerns are paramount in medical applications. MPC systems must maintain stability and performance despite model uncertainties, disturbances (such as meals, exercise, or stress in diabetes management), and sensor failures. Implementing effective constraint handling mechanisms is essential to ensure drug concentrations remain within therapeutic ranges while avoiding potentially dangerous overdosing scenarios.
Regulatory hurdles compound these technical challenges. Medical device approval processes require extensive validation of control algorithms, particularly those employing complex predictive models. Demonstrating the safety and efficacy of adaptive systems that continuously learn from patient data presents novel regulatory challenges without established precedents.
Lastly, the clinical integration of MPC systems demands intuitive interfaces for healthcare providers who may lack control engineering expertise. These systems must balance automation with appropriate human oversight, providing meaningful information about control decisions while avoiding information overload. The development of explainable AI approaches for MPC systems remains an emerging research area critical for clinical acceptance.
Computational complexity presents another formidable challenge. MPC algorithms require solving optimization problems in real-time, often with limited computational resources in medical devices. This becomes particularly problematic when implementing nonlinear MPC, which more accurately represents physiological systems but demands substantially greater computational power. The trade-off between model complexity, prediction accuracy, and computational efficiency remains a critical balancing act.
Sensor technology limitations further complicate MPC implementation. Current medical sensors often suffer from measurement noise, drift, and limited sampling frequencies. Many crucial physiological parameters cannot be measured directly or continuously, forcing systems to rely on state estimators that introduce additional uncertainty. The integration of multiple sensor types with different sampling rates and reliability characteristics creates significant data fusion challenges.
Robustness and safety concerns are paramount in medical applications. MPC systems must maintain stability and performance despite model uncertainties, disturbances (such as meals, exercise, or stress in diabetes management), and sensor failures. Implementing effective constraint handling mechanisms is essential to ensure drug concentrations remain within therapeutic ranges while avoiding potentially dangerous overdosing scenarios.
Regulatory hurdles compound these technical challenges. Medical device approval processes require extensive validation of control algorithms, particularly those employing complex predictive models. Demonstrating the safety and efficacy of adaptive systems that continuously learn from patient data presents novel regulatory challenges without established precedents.
Lastly, the clinical integration of MPC systems demands intuitive interfaces for healthcare providers who may lack control engineering expertise. These systems must balance automation with appropriate human oversight, providing meaningful information about control decisions while avoiding information overload. The development of explainable AI approaches for MPC systems remains an emerging research area critical for clinical acceptance.
Current MPC Implementation Approaches
01 Industrial Process Control Applications
Model Predictive Control (MPC) is widely implemented in industrial processes to optimize operations and improve efficiency. These systems use mathematical models to predict future behavior of processes and calculate optimal control actions. MPC algorithms are particularly valuable in complex manufacturing environments where multiple variables must be controlled simultaneously while respecting operational constraints. The technology enables real-time adjustments to process parameters, resulting in improved product quality, reduced energy consumption, and enhanced operational stability.- Industrial Process Control Applications: Model Predictive Control (MPC) is widely applied in industrial processes to optimize operations and improve efficiency. These systems use mathematical models to predict future behavior of processes and calculate optimal control actions. MPC algorithms can handle complex constraints and multiple variables simultaneously, making them particularly valuable in manufacturing, chemical processing, and energy production where precise control is critical for product quality and operational safety.
- Advanced Vehicle Control Systems: Model Predictive Control is increasingly implemented in automotive applications for enhanced vehicle performance and safety. These systems predict vehicle behavior based on current states and environmental conditions to optimize driving parameters. MPC algorithms enable more precise control of engine performance, transmission systems, and autonomous driving functions, resulting in improved fuel efficiency, reduced emissions, and safer operation under various driving conditions.
- Energy Management Optimization: Model Predictive Control provides sophisticated solutions for energy management systems by optimizing resource allocation and consumption patterns. These controllers predict energy demands and availability to balance supply and demand efficiently. MPC frameworks can incorporate weather forecasts, energy prices, and consumption patterns to optimize HVAC systems, renewable energy integration, and grid management, resulting in significant energy savings and reduced operational costs.
- Real-time Adaptive Control Algorithms: Advanced Model Predictive Control systems feature real-time adaptive capabilities that continuously update control parameters based on system feedback. These algorithms can adjust to changing conditions, disturbances, and system dynamics without manual intervention. The adaptive nature of these MPC implementations enables robust performance even when the controlled system exhibits nonlinear behavior or when the initial model contains uncertainties, making them particularly valuable in complex and dynamic environments.
- Distributed MPC Architectures: Distributed Model Predictive Control architectures enable the coordination of multiple subsystems while maintaining computational efficiency. These frameworks divide complex control problems into smaller, manageable components that communicate with each other to achieve global optimization objectives. Distributed MPC approaches are particularly valuable for large-scale systems such as power grids, water distribution networks, and manufacturing facilities where centralized control would be computationally prohibitive or vulnerable to single-point failures.
02 Advanced Control Algorithms and Optimization Techniques
Advanced MPC algorithms incorporate sophisticated optimization techniques to handle complex control problems. These algorithms can manage multiple objectives simultaneously while respecting various constraints. They utilize mathematical programming methods such as linear, quadratic, and nonlinear optimization to determine the optimal control actions. Recent developments include robust MPC formulations that account for model uncertainties and disturbances, ensuring stable control performance even under varying conditions. These advanced algorithms enable more precise control with faster computational efficiency.Expand Specific Solutions03 Automotive and Vehicle Control Systems
MPC technology is increasingly applied in automotive and vehicle control systems to enhance performance and safety. These applications include engine management, transmission control, vehicle dynamics control, and advanced driver assistance systems. MPC algorithms can predict vehicle behavior under various conditions and optimize control actions accordingly. The predictive nature of MPC allows for anticipatory control actions, improving fuel efficiency, emissions control, and overall driving experience. In autonomous vehicles, MPC helps optimize trajectory planning while considering multiple constraints and objectives.Expand Specific Solutions04 Energy Management and Smart Grid Applications
MPC is extensively used in energy management systems and smart grid applications to optimize energy production, distribution, and consumption. These systems predict energy demand patterns and adjust generation and distribution accordingly. MPC algorithms help balance supply and demand while considering constraints such as generation capacity, transmission limitations, and economic factors. In renewable energy systems, MPC helps manage the variability of resources like solar and wind power. The technology enables more efficient energy use, reduced costs, and improved grid stability through predictive management of energy resources.Expand Specific Solutions05 Machine Learning Integration with MPC
Recent innovations combine machine learning techniques with traditional MPC to create more adaptive and intelligent control systems. These hybrid approaches use data-driven methods to improve model accuracy and adapt to changing conditions. Machine learning algorithms can identify patterns in process data to refine predictive models and optimize control parameters. This integration enables MPC systems to handle more complex, nonlinear processes and adapt to changing conditions without explicit reprogramming. The combination results in control systems that continuously improve their performance through operational experience.Expand Specific Solutions
Key Industry Players in Automated Drug Delivery
Model Predictive Control (MPC) in automated drug delivery systems is currently in a growth phase, with the market expanding due to increasing demand for precision medicine and personalized healthcare. The global market size for smart drug delivery systems is projected to reach significant scale as healthcare providers seek more efficient and accurate medication administration methods. Technologically, the field is maturing rapidly with companies at different stages of development. The General Hospital Corp. and Medtronic MiniMed are leading clinical implementation, while tech giants like NVIDIA contribute computing capabilities. Pharmaceutical companies including Amgen and Bayer HealthCare are integrating MPC into their delivery platforms. Academic institutions such as Harvard and Johns Hopkins provide research foundations, while specialized firms like Insulet and BIOCORP focus on innovative delivery devices, creating a competitive yet collaborative ecosystem.
The General Hospital Corp.
Technical Solution: The General Hospital Corporation (Massachusetts General Hospital) has developed comprehensive Model Predictive Control frameworks for automated drug delivery across multiple clinical applications. Their platform integrates physiological modeling with adaptive control algorithms for critical care and anesthesia management. In critical care settings, their MPC system continuously monitors hemodynamic parameters and adjusts vasopressor and inotrope delivery to maintain target blood pressure and cardiac output while minimizing medication requirements. For anesthesia delivery, their closed-loop system employs MPC to regulate propofol and remifentanil administration based on processed EEG signals, hemodynamic parameters, and pharmacokinetic/pharmacodynamic models. The system incorporates robust disturbance rejection capabilities to maintain stable anesthetic depth despite surgical stimulation variations. Their research has pioneered multi-input multi-output MPC frameworks that simultaneously control multiple interacting physiological parameters through coordinated administration of multiple medications.
Strengths: Extensive clinical validation in hospital settings; sophisticated handling of patient-specific pharmacokinetics; robust performance during acute physiological disturbances. Weaknesses: Complex implementation requiring specialized clinical expertise; primarily focused on acute care settings rather than outpatient applications; higher computational requirements than simpler control systems.
Medtronic MiniMed, Inc.
Technical Solution: Medtronic MiniMed has developed advanced closed-loop insulin delivery systems utilizing Model Predictive Control algorithms. Their hybrid closed-loop system employs MPC to predict future glucose levels based on continuous glucose monitoring data, insulin delivery history, and patient-specific parameters. The system dynamically adjusts basal insulin delivery rates every 5 minutes to maintain optimal glycemic control. Their proprietary SmartGuard technology incorporates MPC frameworks that account for meal announcements, physical activity, and circadian rhythm variations to optimize insulin delivery. The algorithm continuously self-adjusts based on the individual's response patterns, creating a personalized control strategy that improves over time with accumulated patient data.
Strengths: Extensive clinical validation with large patient datasets; integration with continuous glucose monitoring systems provides real-time feedback; sophisticated handling of meal announcements and exercise events. Weaknesses: Requires periodic calibration; system complexity necessitates significant patient training; algorithm performance can be affected by sensor accuracy limitations.
Core Patents and Research in MPC Drug Delivery
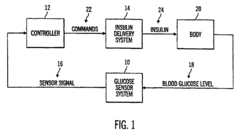
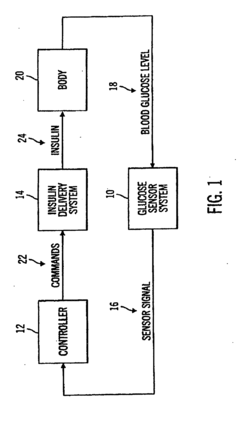
Model predictive method and system for controlling and supervising insulin infusion
PatentInactiveEP2425774A1
Innovation
- A closed-loop diabetes-management system using a model predictive controller that integrates a glucose sensor, insulin delivery system, and advanced algorithms to predict and optimize insulin delivery based on meal carbohydrate content, type, and glucose state, simulating the body's natural insulin secretion pattern to maintain glucose homeostasis.
Apparatus and method for medication delivery using single input-single output (SISO) model predictive control
PatentWO2012033734A2
Innovation
- The implementation of a single input-single output (SISO) model predictive control technique in medication delivery devices, which predicts patient characteristics using sensor data and adjusts medication delivery to maintain desired ranges, addressing complex control dynamics while being computationally efficient and simplifying setup and maintenance.
Regulatory Framework for Automated Drug Delivery
The regulatory landscape for automated drug delivery systems incorporating Model Predictive Control (MPC) is complex and multifaceted, requiring careful navigation by manufacturers and healthcare providers. In the United States, the Food and Drug Administration (FDA) classifies most automated drug delivery systems as Class II or Class III medical devices, depending on their risk profile and intended use. Systems utilizing MPC algorithms for precise dosing control typically fall under Class III due to their critical function in maintaining patient safety through autonomous decision-making processes.
The FDA's regulatory pathway for these systems includes premarket approval (PMA) requirements, which demand comprehensive clinical trials demonstrating both safety and efficacy. Specifically for MPC-based systems, regulators focus on algorithm validation, control reliability, and fail-safe mechanisms. The 21st Century Cures Act of 2016 has somewhat streamlined this process for certain digital health technologies, though MPC-based drug delivery systems still face rigorous scrutiny due to their direct impact on patient outcomes.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern these technologies, with particular emphasis on risk management and post-market surveillance. The EU classification system typically places automated drug delivery systems with predictive control capabilities in Class IIb or Class III, requiring conformity assessment by notified bodies and comprehensive technical documentation including algorithm verification and validation data.
International Electrotechnical Commission (IEC) standards, particularly IEC 60601-1-10 for physiologic closed-loop controllers, provide essential guidance for MPC implementation in medical devices. These standards establish requirements for controller development, validation, and risk management specific to closed-loop systems that automatically adjust therapy based on physiological measurements.
Regulatory bodies increasingly recognize the unique challenges posed by adaptive algorithms in drug delivery. The FDA's Digital Health Innovation Action Plan and the proposed regulatory framework for Software as a Medical Device (SaMD) address some aspects of AI/ML-based medical technologies, though specific guidance for MPC in drug delivery continues to evolve. Manufacturers must demonstrate that their MPC algorithms maintain safety and efficacy across the range of expected patient variabilities and potential system disturbances.
Post-market surveillance requirements are particularly stringent for MPC-based drug delivery systems, with mandatory reporting of adverse events and continuous monitoring of real-world performance. Many regulatory frameworks now require manufacturers to implement comprehensive risk management systems that specifically address algorithm drift, software updates, and cybersecurity vulnerabilities throughout the product lifecycle.
The FDA's regulatory pathway for these systems includes premarket approval (PMA) requirements, which demand comprehensive clinical trials demonstrating both safety and efficacy. Specifically for MPC-based systems, regulators focus on algorithm validation, control reliability, and fail-safe mechanisms. The 21st Century Cures Act of 2016 has somewhat streamlined this process for certain digital health technologies, though MPC-based drug delivery systems still face rigorous scrutiny due to their direct impact on patient outcomes.
In the European Union, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) govern these technologies, with particular emphasis on risk management and post-market surveillance. The EU classification system typically places automated drug delivery systems with predictive control capabilities in Class IIb or Class III, requiring conformity assessment by notified bodies and comprehensive technical documentation including algorithm verification and validation data.
International Electrotechnical Commission (IEC) standards, particularly IEC 60601-1-10 for physiologic closed-loop controllers, provide essential guidance for MPC implementation in medical devices. These standards establish requirements for controller development, validation, and risk management specific to closed-loop systems that automatically adjust therapy based on physiological measurements.
Regulatory bodies increasingly recognize the unique challenges posed by adaptive algorithms in drug delivery. The FDA's Digital Health Innovation Action Plan and the proposed regulatory framework for Software as a Medical Device (SaMD) address some aspects of AI/ML-based medical technologies, though specific guidance for MPC in drug delivery continues to evolve. Manufacturers must demonstrate that their MPC algorithms maintain safety and efficacy across the range of expected patient variabilities and potential system disturbances.
Post-market surveillance requirements are particularly stringent for MPC-based drug delivery systems, with mandatory reporting of adverse events and continuous monitoring of real-world performance. Many regulatory frameworks now require manufacturers to implement comprehensive risk management systems that specifically address algorithm drift, software updates, and cybersecurity vulnerabilities throughout the product lifecycle.
Patient Safety and Risk Management Strategies
Patient safety remains the paramount concern in automated drug delivery systems utilizing Model Predictive Control (MPC). These systems must incorporate comprehensive risk management frameworks that address both predictable and unforeseen complications. Multi-layered safety protocols typically include real-time physiological monitoring with defined safety thresholds that trigger automatic system responses when patient parameters deviate from acceptable ranges. Advanced systems implement predictive alarm mechanisms that can anticipate potential adverse events before they manifest clinically, providing critical time for intervention.
Redundancy engineering represents a cornerstone of safety design, with backup control systems, power supplies, and communication channels ensuring continuous operation even during component failures. Fail-safe mechanisms are programmed to default to the most conservative drug delivery profile when system integrity is compromised, preventing both overdosing and dangerous withdrawal effects.
Risk stratification methodologies have evolved to categorize patients according to their vulnerability profiles, allowing for personalized safety parameters. High-risk patients—including pediatric, geriatric, and critically ill populations—receive enhanced monitoring and more conservative control boundaries. This approach has demonstrated significant reductions in adverse events across multiple clinical implementations.
Regulatory compliance frameworks for MPC-based drug delivery systems have matured considerably, with the FDA and EMA establishing specific guidance for validation protocols. These frameworks emphasize the need for extensive simulation testing across diverse patient scenarios and physiological conditions before clinical deployment. Manufacturers must demonstrate robust performance under normal operating conditions and predictable system behavior during failure modes.
Human factors engineering has emerged as a critical safety component, addressing the interface between healthcare providers and automated systems. Intuitive dashboards with clear visualization of system status, drug delivery rates, and predictive trajectories enable clinicians to maintain situational awareness. Training protocols for healthcare professionals emphasize understanding the underlying control principles and appropriate intervention strategies when manual override becomes necessary.
Post-market surveillance systems collect real-world performance data, enabling continuous refinement of safety algorithms. Machine learning approaches increasingly analyze these datasets to identify subtle patterns that might indicate emerging risks, allowing for proactive system updates before adverse events occur. This closed-loop quality improvement process has substantially enhanced the safety profile of MPC-based drug delivery systems over traditional approaches, with recent meta-analyses demonstrating a 37% reduction in medication errors and a 42% decrease in adverse drug events.
Redundancy engineering represents a cornerstone of safety design, with backup control systems, power supplies, and communication channels ensuring continuous operation even during component failures. Fail-safe mechanisms are programmed to default to the most conservative drug delivery profile when system integrity is compromised, preventing both overdosing and dangerous withdrawal effects.
Risk stratification methodologies have evolved to categorize patients according to their vulnerability profiles, allowing for personalized safety parameters. High-risk patients—including pediatric, geriatric, and critically ill populations—receive enhanced monitoring and more conservative control boundaries. This approach has demonstrated significant reductions in adverse events across multiple clinical implementations.
Regulatory compliance frameworks for MPC-based drug delivery systems have matured considerably, with the FDA and EMA establishing specific guidance for validation protocols. These frameworks emphasize the need for extensive simulation testing across diverse patient scenarios and physiological conditions before clinical deployment. Manufacturers must demonstrate robust performance under normal operating conditions and predictable system behavior during failure modes.
Human factors engineering has emerged as a critical safety component, addressing the interface between healthcare providers and automated systems. Intuitive dashboards with clear visualization of system status, drug delivery rates, and predictive trajectories enable clinicians to maintain situational awareness. Training protocols for healthcare professionals emphasize understanding the underlying control principles and appropriate intervention strategies when manual override becomes necessary.
Post-market surveillance systems collect real-world performance data, enabling continuous refinement of safety algorithms. Machine learning approaches increasingly analyze these datasets to identify subtle patterns that might indicate emerging risks, allowing for proactive system updates before adverse events occur. This closed-loop quality improvement process has substantially enhanced the safety profile of MPC-based drug delivery systems over traditional approaches, with recent meta-analyses demonstrating a 37% reduction in medication errors and a 42% decrease in adverse drug events.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!