Neopentane as a Promising Solvent for Advanced Applications
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Neopentane Overview
Neopentane, also known as 2,2-dimethylpropane, is a branched alkane with the molecular formula C5H12. This colorless, flammable gas has gained significant attention in recent years due to its unique properties and potential applications in various advanced fields. As a member of the pentane family, neopentane stands out for its highly symmetrical structure, which contributes to its distinctive characteristics.
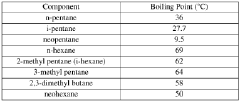
The molecular structure of neopentane consists of a central carbon atom bonded to four methyl groups, resulting in a tetrahedral arrangement. This compact and symmetrical configuration gives neopentane a higher boiling point and lower vapor pressure compared to its isomers, n-pentane and isopentane. These properties make neopentane particularly interesting for use as a solvent in specialized applications.
One of the key advantages of neopentane is its low reactivity and high stability. The absence of linear carbon chains and the presence of four methyl groups surrounding the central carbon atom make it resistant to many chemical reactions. This stability is particularly valuable in applications where chemical inertness is crucial, such as in the production of high-performance lubricants and specialty chemicals.
Neopentane's low freezing point and high vapor pressure at room temperature make it an excellent candidate for use in refrigeration systems and heat transfer applications. Its thermodynamic properties allow for efficient energy transfer, potentially leading to more environmentally friendly cooling solutions. Additionally, the compound's low surface tension and high volatility make it suitable for use in aerosol propellants and as a blowing agent in foam production.
In the field of organic synthesis, neopentane serves as a valuable starting material for the production of various compounds. Its unique structure allows for selective functionalization, opening up possibilities for the creation of novel materials with tailored properties. This aspect of neopentane chemistry has attracted interest from researchers in the pharmaceutical and materials science industries.
The environmental impact of neopentane is an important consideration in its growing application. While it has a lower global warming potential compared to many traditional refrigerants, its production and use still require careful management to minimize environmental risks. Ongoing research focuses on developing sustainable production methods and exploring potential recycling strategies to enhance its eco-friendly profile.
As industries continue to seek innovative solutions for complex challenges, neopentane's unique properties position it as a promising candidate for advanced applications. From enhancing the performance of electronic components to enabling new approaches in drug delivery systems, the potential of neopentane as a versatile solvent and reagent is only beginning to be fully explored.
The molecular structure of neopentane consists of a central carbon atom bonded to four methyl groups, resulting in a tetrahedral arrangement. This compact and symmetrical configuration gives neopentane a higher boiling point and lower vapor pressure compared to its isomers, n-pentane and isopentane. These properties make neopentane particularly interesting for use as a solvent in specialized applications.
One of the key advantages of neopentane is its low reactivity and high stability. The absence of linear carbon chains and the presence of four methyl groups surrounding the central carbon atom make it resistant to many chemical reactions. This stability is particularly valuable in applications where chemical inertness is crucial, such as in the production of high-performance lubricants and specialty chemicals.
Neopentane's low freezing point and high vapor pressure at room temperature make it an excellent candidate for use in refrigeration systems and heat transfer applications. Its thermodynamic properties allow for efficient energy transfer, potentially leading to more environmentally friendly cooling solutions. Additionally, the compound's low surface tension and high volatility make it suitable for use in aerosol propellants and as a blowing agent in foam production.
In the field of organic synthesis, neopentane serves as a valuable starting material for the production of various compounds. Its unique structure allows for selective functionalization, opening up possibilities for the creation of novel materials with tailored properties. This aspect of neopentane chemistry has attracted interest from researchers in the pharmaceutical and materials science industries.
The environmental impact of neopentane is an important consideration in its growing application. While it has a lower global warming potential compared to many traditional refrigerants, its production and use still require careful management to minimize environmental risks. Ongoing research focuses on developing sustainable production methods and exploring potential recycling strategies to enhance its eco-friendly profile.
As industries continue to seek innovative solutions for complex challenges, neopentane's unique properties position it as a promising candidate for advanced applications. From enhancing the performance of electronic components to enabling new approaches in drug delivery systems, the potential of neopentane as a versatile solvent and reagent is only beginning to be fully explored.
Market Potential
The market potential for neopentane as a promising solvent in advanced applications is significant and growing. As industries seek more efficient and environmentally friendly solvents, neopentane's unique properties position it as a valuable alternative in various sectors. The global solvent market, valued at over $20 billion, is expected to expand further, with specialty solvents like neopentane playing a crucial role in this growth.
In the electronics industry, neopentane's low boiling point and high purity make it ideal for precision cleaning of sensitive components. As the demand for smaller, more powerful electronic devices continues to rise, the need for effective cleaning solvents is paramount. This sector alone could drive substantial growth in neopentane usage over the coming years.
The pharmaceutical industry represents another significant market opportunity for neopentane. Its low reactivity and ability to dissolve a wide range of compounds make it suitable for drug formulation and extraction processes. With the global pharmaceutical market projected to reach $1.5 trillion by 2023, the demand for specialized solvents like neopentane is expected to increase correspondingly.
In the field of energy storage, neopentane's potential as an electrolyte solvent in next-generation batteries is particularly promising. As research into more efficient and safer battery technologies intensifies, the demand for high-performance solvents is likely to grow. This application could open up a entirely new market segment for neopentane in the rapidly expanding energy storage sector.
The aerospace and automotive industries also present significant opportunities for neopentane adoption. Its low freezing point and thermal stability make it suitable for use in hydraulic systems and as a blowing agent in foam insulation. As these industries push for lighter, more fuel-efficient vehicles and aircraft, the demand for advanced materials and solvents is expected to rise.
Environmental regulations and sustainability concerns are driving the search for greener solvents across industries. Neopentane's low toxicity and potential for recycling align well with these trends, potentially accelerating its adoption in markets traditionally dominated by more harmful solvents. This shift towards environmentally friendly alternatives could significantly expand neopentane's market share in the coming years.
While the market potential for neopentane is substantial, challenges such as production costs and supply chain considerations will need to be addressed to fully capitalize on these opportunities. However, as research continues and production processes improve, neopentane's unique properties are likely to drive its adoption across a wide range of advanced applications, solidifying its position as a promising solvent in the global market.
In the electronics industry, neopentane's low boiling point and high purity make it ideal for precision cleaning of sensitive components. As the demand for smaller, more powerful electronic devices continues to rise, the need for effective cleaning solvents is paramount. This sector alone could drive substantial growth in neopentane usage over the coming years.
The pharmaceutical industry represents another significant market opportunity for neopentane. Its low reactivity and ability to dissolve a wide range of compounds make it suitable for drug formulation and extraction processes. With the global pharmaceutical market projected to reach $1.5 trillion by 2023, the demand for specialized solvents like neopentane is expected to increase correspondingly.
In the field of energy storage, neopentane's potential as an electrolyte solvent in next-generation batteries is particularly promising. As research into more efficient and safer battery technologies intensifies, the demand for high-performance solvents is likely to grow. This application could open up a entirely new market segment for neopentane in the rapidly expanding energy storage sector.
The aerospace and automotive industries also present significant opportunities for neopentane adoption. Its low freezing point and thermal stability make it suitable for use in hydraulic systems and as a blowing agent in foam insulation. As these industries push for lighter, more fuel-efficient vehicles and aircraft, the demand for advanced materials and solvents is expected to rise.
Environmental regulations and sustainability concerns are driving the search for greener solvents across industries. Neopentane's low toxicity and potential for recycling align well with these trends, potentially accelerating its adoption in markets traditionally dominated by more harmful solvents. This shift towards environmentally friendly alternatives could significantly expand neopentane's market share in the coming years.
While the market potential for neopentane is substantial, challenges such as production costs and supply chain considerations will need to be addressed to fully capitalize on these opportunities. However, as research continues and production processes improve, neopentane's unique properties are likely to drive its adoption across a wide range of advanced applications, solidifying its position as a promising solvent in the global market.
Technical Challenges
Despite its promising potential, the use of neopentane as a solvent for advanced applications faces several technical challenges that need to be addressed. One of the primary concerns is its high volatility, which can lead to significant solvent loss during storage and handling. This characteristic not only increases operational costs but also raises environmental and safety concerns due to potential emissions.
The flammability of neopentane presents another major challenge. Its low flash point and high vapor pressure make it highly combustible, necessitating stringent safety measures in storage, transportation, and usage. This flammability risk limits its applicability in certain industrial processes and requires specialized equipment and handling procedures.
Neopentane's limited solubility range poses challenges in some applications. While it excels in dissolving certain non-polar compounds, its effectiveness with polar substances is limited. This selectivity restricts its versatility as a universal solvent, potentially requiring the use of co-solvents or solvent mixtures in some applications, which can complicate process design and increase costs.
The production and purification of high-grade neopentane for advanced applications present technical hurdles. Achieving the required purity levels can be energy-intensive and expensive, impacting the overall economic viability of neopentane-based processes. Additionally, the development of efficient recycling and recovery systems for neopentane is crucial to minimize waste and improve process economics, but this remains a significant technical challenge.
Environmental concerns also pose challenges to the widespread adoption of neopentane. Although it has a lower global warming potential compared to some other solvents, its volatile organic compound (VOC) status means that its use may be restricted in certain regions due to air quality regulations. Developing effective containment and emission control strategies is essential for its sustainable use.
Compatibility issues with certain materials used in processing equipment and storage containers need to be addressed. Neopentane can cause swelling or degradation of some polymers and elastomers, necessitating careful material selection and potentially limiting equipment options.
Lastly, the scalability of neopentane-based processes from laboratory to industrial scale presents significant engineering challenges. Optimizing heat transfer, ensuring uniform mixing, and maintaining process stability at larger scales require extensive research and development efforts. Overcoming these technical hurdles is crucial for realizing the full potential of neopentane in advanced applications across various industries.
The flammability of neopentane presents another major challenge. Its low flash point and high vapor pressure make it highly combustible, necessitating stringent safety measures in storage, transportation, and usage. This flammability risk limits its applicability in certain industrial processes and requires specialized equipment and handling procedures.
Neopentane's limited solubility range poses challenges in some applications. While it excels in dissolving certain non-polar compounds, its effectiveness with polar substances is limited. This selectivity restricts its versatility as a universal solvent, potentially requiring the use of co-solvents or solvent mixtures in some applications, which can complicate process design and increase costs.
The production and purification of high-grade neopentane for advanced applications present technical hurdles. Achieving the required purity levels can be energy-intensive and expensive, impacting the overall economic viability of neopentane-based processes. Additionally, the development of efficient recycling and recovery systems for neopentane is crucial to minimize waste and improve process economics, but this remains a significant technical challenge.
Environmental concerns also pose challenges to the widespread adoption of neopentane. Although it has a lower global warming potential compared to some other solvents, its volatile organic compound (VOC) status means that its use may be restricted in certain regions due to air quality regulations. Developing effective containment and emission control strategies is essential for its sustainable use.
Compatibility issues with certain materials used in processing equipment and storage containers need to be addressed. Neopentane can cause swelling or degradation of some polymers and elastomers, necessitating careful material selection and potentially limiting equipment options.
Lastly, the scalability of neopentane-based processes from laboratory to industrial scale presents significant engineering challenges. Optimizing heat transfer, ensuring uniform mixing, and maintaining process stability at larger scales require extensive research and development efforts. Overcoming these technical hurdles is crucial for realizing the full potential of neopentane in advanced applications across various industries.
Current Applications
01 Production and purification of neopentane
Various methods for producing and purifying neopentane are described. These include processes for separating neopentane from other hydrocarbons, such as using distillation or membrane separation techniques. The purification methods aim to obtain high-purity neopentane for industrial applications.- Synthesis and production of neopentane: Various methods for synthesizing and producing neopentane are described. These processes often involve catalytic reactions, hydrogenation, or other chemical transformations to obtain neopentane from different precursors. The production methods aim to improve yield, efficiency, and purity of the resulting neopentane.
- Neopentane as a refrigerant or heat transfer fluid: Neopentane is utilized as a refrigerant or heat transfer fluid in various applications due to its thermodynamic properties. It can be used in cooling systems, heat pumps, or other thermal management applications. The compound's low boiling point and other physical characteristics make it suitable for these purposes.
- Neopentane in chemical reactions and processes: Neopentane serves as a reactant or intermediate in various chemical reactions and industrial processes. It can be used in the production of other chemicals, polymers, or materials. The compound's unique structure and properties make it valuable in certain synthetic pathways and manufacturing processes.
- Purification and separation of neopentane: Methods for purifying and separating neopentane from mixtures or other compounds are described. These techniques may involve distillation, adsorption, or other separation processes to obtain high-purity neopentane. The purification methods aim to remove impurities and isolate neopentane for various applications.
- Neopentane in fuel compositions: Neopentane is used as a component in fuel compositions for various applications. It can be blended with other hydrocarbons or additives to enhance fuel properties such as octane rating, volatility, or combustion characteristics. The use of neopentane in fuel formulations aims to improve engine performance and efficiency.
02 Use of neopentane in chemical reactions
Neopentane is utilized as a reactant or intermediate in various chemical processes. It can be used in the synthesis of other organic compounds, particularly in the production of specialty chemicals and pharmaceuticals. The unique structure of neopentane makes it valuable for certain chemical transformations.Expand Specific Solutions03 Neopentane as a refrigerant or propellant
Neopentane finds applications as a refrigerant or propellant due to its physical properties. It can be used in cooling systems, aerosol sprays, and other applications where a low-boiling-point hydrocarbon is required. Its use as an alternative to certain ozone-depleting substances has been explored.Expand Specific Solutions04 Neopentane in polymer production
Neopentane is used in the production of certain polymers and plastics. It can serve as a blowing agent in the manufacture of foam materials or as a component in polymer formulations. The incorporation of neopentane can impart specific properties to the resulting materials.Expand Specific Solutions05 Analytical methods for neopentane
Various analytical techniques have been developed for the detection, quantification, and characterization of neopentane. These methods are important for quality control in industrial processes and environmental monitoring. Chromatographic and spectroscopic techniques are commonly employed for neopentane analysis.Expand Specific Solutions
Industry Leaders
The neopentane solvent market is in an early growth stage, with increasing interest in advanced applications driving expansion. The market size is relatively small but growing steadily as research progresses. Technologically, neopentane as a solvent is still emerging, with varying levels of maturity across applications. Key players like ExxonMobil Chemical, China Petroleum & Chemical Corp, and Merck Sharp & Dohme are actively researching and developing neopentane-based solutions. Other companies such as Sinopec and JSR Corp are also exploring potential uses, indicating a competitive landscape with diverse industry participation. As the technology advances, we can expect increased commercialization efforts and market penetration in specialized chemical and pharmaceutical applications.
ExxonMobil Chemical Patents, Inc.
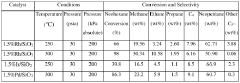
Technical Solution: ExxonMobil has developed advanced applications for neopentane as a solvent, particularly in the petrochemical industry. Their research focuses on utilizing neopentane's unique properties, such as its low boiling point (9.5°C) and high vapor pressure, for enhanced extraction processes and as a blowing agent in polymer foam production[1]. ExxonMobil has also explored neopentane's potential in refrigeration systems, leveraging its environmentally friendly characteristics as a non-ozone-depleting substance[2]. The company has invested in optimizing neopentane production methods, including the development of catalytic processes for more efficient synthesis from isobutylene and methanol[3].
Strengths: Extensive experience in petrochemical applications, strong R&D capabilities, and global market presence. Weaknesses: Potential environmental concerns associated with fossil fuel-based products, and competition from alternative green solvents.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has been exploring neopentane's applications in various fields, with a focus on its use as a high-performance refrigerant and aerosol propellant. The company has developed proprietary technologies for large-scale neopentane production, including advanced distillation and purification processes[4]. Sinopec's research also extends to neopentane's potential in enhanced oil recovery (EOR) techniques, where its low viscosity and high volatility can improve oil displacement efficiency[5]. Additionally, the company is investigating neopentane's role in the production of specialty chemicals and as a reaction medium for certain organic syntheses[6].
Strengths: Large-scale production capabilities, strong presence in the Asian market, and diverse application research. Weaknesses: Potential regulatory challenges in some markets due to VOC emissions, and the need for further safety measures in handling and storage.
Key Innovations
Production of neopentane
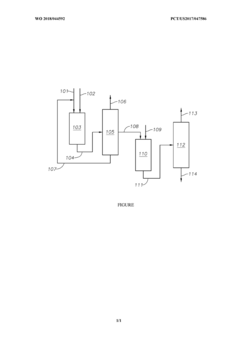
PatentWO2018044592A1
Innovation
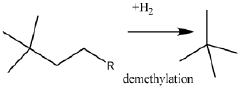
- A process involving the isomerization of C6-C7 paraffins to produce neohexane or neoheptane, followed by demethylation using a catalyst in the presence of hydrogen, which allows for the production of neopentane with yields greater than 40 wt% from readily available C4-C7 paraffinic feed streams, such as light virgin naphtha.
Safety Regulations
The use of neopentane as a solvent in advanced applications necessitates a comprehensive understanding and adherence to safety regulations. Given its flammable nature and potential environmental impact, strict guidelines govern its handling, storage, and disposal.
Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) and the Environmental Protection Agency (EPA) in the United States have established specific standards for neopentane. These regulations typically cover aspects such as permissible exposure limits, personal protective equipment requirements, and proper ventilation systems in workplaces where neopentane is used.
Storage of neopentane must comply with fire safety codes due to its high flammability. This often includes requirements for specialized storage containers, fire-resistant storage areas, and the implementation of robust fire suppression systems. Additionally, transportation of neopentane is subject to hazardous materials regulations, which dictate proper labeling, packaging, and shipping procedures.
Environmental regulations play a crucial role in neopentane usage. Many jurisdictions have implemented strict controls on volatile organic compound (VOC) emissions, which directly impact the use of neopentane in industrial processes. Companies must often invest in emission control technologies or seek alternatives to comply with these environmental standards.
Workplace safety protocols for neopentane handling typically include regular employee training, implementation of engineering controls, and establishment of emergency response procedures. These measures are designed to minimize the risk of accidents and ensure proper response in case of spills or leaks.
In the context of advanced applications, such as in electronics manufacturing or as a blowing agent in foam production, additional industry-specific regulations may apply. These could include purity standards, contamination controls, and specific handling procedures tailored to the particular application.
As global awareness of environmental issues grows, regulations surrounding neopentane and similar solvents are likely to become more stringent. This trend may drive innovation in containment technologies, recycling methods, and the development of safer alternatives. Companies investing in neopentane-based technologies must stay abreast of these evolving regulations to ensure long-term compliance and sustainability.
Internationally, the harmonization of safety standards for neopentane is an ongoing process. While regulations may vary between countries, there is a general trend towards adopting consistent global standards, particularly in industries with international supply chains. This harmonization aims to facilitate trade while maintaining high safety and environmental protection standards across borders.
Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) and the Environmental Protection Agency (EPA) in the United States have established specific standards for neopentane. These regulations typically cover aspects such as permissible exposure limits, personal protective equipment requirements, and proper ventilation systems in workplaces where neopentane is used.
Storage of neopentane must comply with fire safety codes due to its high flammability. This often includes requirements for specialized storage containers, fire-resistant storage areas, and the implementation of robust fire suppression systems. Additionally, transportation of neopentane is subject to hazardous materials regulations, which dictate proper labeling, packaging, and shipping procedures.
Environmental regulations play a crucial role in neopentane usage. Many jurisdictions have implemented strict controls on volatile organic compound (VOC) emissions, which directly impact the use of neopentane in industrial processes. Companies must often invest in emission control technologies or seek alternatives to comply with these environmental standards.
Workplace safety protocols for neopentane handling typically include regular employee training, implementation of engineering controls, and establishment of emergency response procedures. These measures are designed to minimize the risk of accidents and ensure proper response in case of spills or leaks.
In the context of advanced applications, such as in electronics manufacturing or as a blowing agent in foam production, additional industry-specific regulations may apply. These could include purity standards, contamination controls, and specific handling procedures tailored to the particular application.
As global awareness of environmental issues grows, regulations surrounding neopentane and similar solvents are likely to become more stringent. This trend may drive innovation in containment technologies, recycling methods, and the development of safer alternatives. Companies investing in neopentane-based technologies must stay abreast of these evolving regulations to ensure long-term compliance and sustainability.
Internationally, the harmonization of safety standards for neopentane is an ongoing process. While regulations may vary between countries, there is a general trend towards adopting consistent global standards, particularly in industries with international supply chains. This harmonization aims to facilitate trade while maintaining high safety and environmental protection standards across borders.
Environmental Impact
The environmental impact of neopentane as a solvent for advanced applications is a critical consideration in its adoption and widespread use. Neopentane, a branched alkane with the chemical formula C5H12, exhibits several characteristics that influence its environmental footprint.
One of the primary environmental concerns associated with neopentane is its potential as a volatile organic compound (VOC). VOCs can contribute to the formation of ground-level ozone and smog, which can have detrimental effects on air quality and human health. However, neopentane's low reactivity in the atmosphere compared to other hydrocarbons may mitigate some of these concerns.
In terms of global warming potential, neopentane has a relatively low impact compared to many other solvents. Its atmospheric lifetime is shorter than that of many chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs), which were widely used in the past but have since been phased out due to their ozone-depleting properties. This shorter atmospheric lifetime means that neopentane has a lower overall contribution to long-term climate change.
Water pollution is another aspect to consider when evaluating neopentane's environmental impact. Due to its low water solubility, neopentane is less likely to contaminate water sources compared to more water-soluble solvents. However, proper handling and disposal practices are still crucial to prevent any accidental releases into aquatic ecosystems.
Biodegradability is an important factor in assessing the long-term environmental impact of any chemical compound. Neopentane, like other alkanes, can be biodegraded by certain microorganisms under aerobic conditions. This natural breakdown process helps to reduce its persistence in the environment, although the rate of biodegradation may vary depending on specific environmental conditions.
The production process of neopentane also plays a role in its overall environmental impact. Energy consumption and potential emissions during manufacturing should be taken into account when evaluating its sustainability. Advancements in green chemistry and more efficient production methods could potentially reduce the environmental footprint associated with neopentane production.
When considering the use of neopentane in advanced applications, it is essential to compare its environmental impact to that of alternative solvents. In many cases, neopentane may offer environmental advantages over more traditional solvents, particularly those with higher toxicity or greater ozone-depleting potential. However, a comprehensive life cycle assessment would be necessary to fully quantify these comparative benefits.
One of the primary environmental concerns associated with neopentane is its potential as a volatile organic compound (VOC). VOCs can contribute to the formation of ground-level ozone and smog, which can have detrimental effects on air quality and human health. However, neopentane's low reactivity in the atmosphere compared to other hydrocarbons may mitigate some of these concerns.
In terms of global warming potential, neopentane has a relatively low impact compared to many other solvents. Its atmospheric lifetime is shorter than that of many chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs), which were widely used in the past but have since been phased out due to their ozone-depleting properties. This shorter atmospheric lifetime means that neopentane has a lower overall contribution to long-term climate change.
Water pollution is another aspect to consider when evaluating neopentane's environmental impact. Due to its low water solubility, neopentane is less likely to contaminate water sources compared to more water-soluble solvents. However, proper handling and disposal practices are still crucial to prevent any accidental releases into aquatic ecosystems.
Biodegradability is an important factor in assessing the long-term environmental impact of any chemical compound. Neopentane, like other alkanes, can be biodegraded by certain microorganisms under aerobic conditions. This natural breakdown process helps to reduce its persistence in the environment, although the rate of biodegradation may vary depending on specific environmental conditions.
The production process of neopentane also plays a role in its overall environmental impact. Energy consumption and potential emissions during manufacturing should be taken into account when evaluating its sustainability. Advancements in green chemistry and more efficient production methods could potentially reduce the environmental footprint associated with neopentane production.
When considering the use of neopentane in advanced applications, it is essential to compare its environmental impact to that of alternative solvents. In many cases, neopentane may offer environmental advantages over more traditional solvents, particularly those with higher toxicity or greater ozone-depleting potential. However, a comprehensive life cycle assessment would be necessary to fully quantify these comparative benefits.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!