Nylon 66 Vs Polypropylene: UV Resistance Analysis
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Polymer UV Resistance Background and Objectives
Polymers have been extensively utilized across various industries due to their versatility, cost-effectiveness, and adaptable properties. Among the critical challenges faced by polymer materials in outdoor applications is their susceptibility to ultraviolet (UV) radiation, which can significantly compromise their structural integrity and functional performance over time. The comparative analysis of Nylon 66 and Polypropylene (PP) regarding their UV resistance capabilities represents a crucial area of investigation for material scientists and engineers seeking optimal material selections for specific applications.
UV degradation in polymers occurs through photochemical reactions initiated when polymer molecules absorb UV radiation, leading to chain scission, cross-linking, and oxidation processes. These molecular changes manifest as discoloration, embrittlement, surface cracking, and mechanical property deterioration. The economic implications of premature material failure due to UV exposure are substantial, driving continuous research into enhancing polymer UV stability.
Historically, the development of UV-resistant polymers has evolved from simple carbon black additives in the 1950s to sophisticated hindered amine light stabilizers (HALS) and UV absorbers in contemporary formulations. The technological trajectory has consistently aimed at extending service life while maintaining material performance characteristics under prolonged UV exposure conditions.
Nylon 66, a polyamide with excellent mechanical properties and thermal stability, has traditionally demonstrated moderate inherent UV resistance. Its molecular structure, characterized by amide linkages, provides some natural protection against UV degradation. However, its susceptibility to moisture absorption can exacerbate UV-induced degradation through hydrolysis mechanisms, presenting unique challenges in outdoor applications.
Polypropylene, conversely, is a polyolefin with outstanding chemical resistance and processing versatility but inherently poor UV stability due to its tertiary carbon atoms, which are particularly vulnerable to photo-oxidation. The industry has addressed this limitation through extensive stabilization systems, making PP a cost-effective option for UV-exposed applications when properly formulated.
The objective of this technical research report is to conduct a comprehensive comparative analysis of the UV resistance properties of Nylon 66 and Polypropylene. This investigation aims to elucidate the fundamental degradation mechanisms, evaluate the effectiveness of various stabilization strategies, and assess performance metrics under accelerated and natural weathering conditions. The findings will inform material selection decisions for applications requiring prolonged outdoor exposure, such as automotive components, construction materials, and consumer products.
Furthermore, this analysis seeks to identify emerging technologies and innovative approaches for enhancing the UV resistance of both polymers, potentially opening new application domains and extending service life expectations in challenging environmental conditions.
UV degradation in polymers occurs through photochemical reactions initiated when polymer molecules absorb UV radiation, leading to chain scission, cross-linking, and oxidation processes. These molecular changes manifest as discoloration, embrittlement, surface cracking, and mechanical property deterioration. The economic implications of premature material failure due to UV exposure are substantial, driving continuous research into enhancing polymer UV stability.
Historically, the development of UV-resistant polymers has evolved from simple carbon black additives in the 1950s to sophisticated hindered amine light stabilizers (HALS) and UV absorbers in contemporary formulations. The technological trajectory has consistently aimed at extending service life while maintaining material performance characteristics under prolonged UV exposure conditions.
Nylon 66, a polyamide with excellent mechanical properties and thermal stability, has traditionally demonstrated moderate inherent UV resistance. Its molecular structure, characterized by amide linkages, provides some natural protection against UV degradation. However, its susceptibility to moisture absorption can exacerbate UV-induced degradation through hydrolysis mechanisms, presenting unique challenges in outdoor applications.
Polypropylene, conversely, is a polyolefin with outstanding chemical resistance and processing versatility but inherently poor UV stability due to its tertiary carbon atoms, which are particularly vulnerable to photo-oxidation. The industry has addressed this limitation through extensive stabilization systems, making PP a cost-effective option for UV-exposed applications when properly formulated.
The objective of this technical research report is to conduct a comprehensive comparative analysis of the UV resistance properties of Nylon 66 and Polypropylene. This investigation aims to elucidate the fundamental degradation mechanisms, evaluate the effectiveness of various stabilization strategies, and assess performance metrics under accelerated and natural weathering conditions. The findings will inform material selection decisions for applications requiring prolonged outdoor exposure, such as automotive components, construction materials, and consumer products.
Furthermore, this analysis seeks to identify emerging technologies and innovative approaches for enhancing the UV resistance of both polymers, potentially opening new application domains and extending service life expectations in challenging environmental conditions.
Market Demand Analysis for UV-Resistant Polymers
The global market for UV-resistant polymers has experienced significant growth in recent years, driven by increasing applications in outdoor products, automotive components, and construction materials. The demand for materials that can withstand prolonged exposure to ultraviolet radiation without degradation continues to expand across multiple industries, with particular emphasis on polymers like Nylon 66 and Polypropylene.
Current market analysis indicates that the UV-resistant polymer sector is valued at approximately $8.5 billion globally, with projections suggesting a compound annual growth rate of 6.2% through 2028. This growth is primarily fueled by expanding applications in regions with high UV exposure and increasing consumer expectations for product longevity in outdoor environments.
The automotive industry represents one of the largest market segments for UV-resistant polymers, accounting for nearly 28% of total consumption. Vehicle manufacturers increasingly demand materials that maintain structural integrity and aesthetic appearance despite continuous sun exposure. This trend is particularly evident in the growing electric vehicle market, where lightweight yet durable polymers are essential for maximizing range efficiency while ensuring component longevity.
Construction and building materials constitute another significant market segment, representing approximately 23% of UV-resistant polymer demand. The increasing focus on sustainable building practices has led to greater utilization of polymer-based materials in exterior applications, necessitating enhanced UV resistance properties to ensure structural integrity and color stability over extended periods.
Regional market analysis reveals that North America and Europe currently lead in consumption of high-performance UV-resistant polymers, though the Asia-Pacific region demonstrates the fastest growth rate at 7.8% annually. This regional growth is attributed to rapid industrialization, expanding construction sectors, and increasing automotive production in countries like China, India, and Southeast Asian nations.
Consumer goods manufacturers represent an emerging market segment with substantial growth potential, particularly in outdoor furniture, recreational equipment, and packaging applications. This sector increasingly demands materials that combine UV resistance with other performance characteristics such as impact strength, chemical resistance, and recyclability.
Market research indicates a growing preference for polymers that offer comprehensive environmental resistance beyond UV protection, including resistance to moisture, temperature fluctuations, and chemical exposure. This trend favors engineered polymers like Nylon 66, which typically command premium pricing due to their superior performance characteristics across multiple environmental stressors.
The market also shows increasing demand for UV-resistant additives and masterbatches that can enhance the performance of base polymers like Polypropylene, creating opportunities for specialized chemical suppliers to develop innovative solutions that balance performance requirements with cost considerations.
Current market analysis indicates that the UV-resistant polymer sector is valued at approximately $8.5 billion globally, with projections suggesting a compound annual growth rate of 6.2% through 2028. This growth is primarily fueled by expanding applications in regions with high UV exposure and increasing consumer expectations for product longevity in outdoor environments.
The automotive industry represents one of the largest market segments for UV-resistant polymers, accounting for nearly 28% of total consumption. Vehicle manufacturers increasingly demand materials that maintain structural integrity and aesthetic appearance despite continuous sun exposure. This trend is particularly evident in the growing electric vehicle market, where lightweight yet durable polymers are essential for maximizing range efficiency while ensuring component longevity.
Construction and building materials constitute another significant market segment, representing approximately 23% of UV-resistant polymer demand. The increasing focus on sustainable building practices has led to greater utilization of polymer-based materials in exterior applications, necessitating enhanced UV resistance properties to ensure structural integrity and color stability over extended periods.
Regional market analysis reveals that North America and Europe currently lead in consumption of high-performance UV-resistant polymers, though the Asia-Pacific region demonstrates the fastest growth rate at 7.8% annually. This regional growth is attributed to rapid industrialization, expanding construction sectors, and increasing automotive production in countries like China, India, and Southeast Asian nations.
Consumer goods manufacturers represent an emerging market segment with substantial growth potential, particularly in outdoor furniture, recreational equipment, and packaging applications. This sector increasingly demands materials that combine UV resistance with other performance characteristics such as impact strength, chemical resistance, and recyclability.
Market research indicates a growing preference for polymers that offer comprehensive environmental resistance beyond UV protection, including resistance to moisture, temperature fluctuations, and chemical exposure. This trend favors engineered polymers like Nylon 66, which typically command premium pricing due to their superior performance characteristics across multiple environmental stressors.
The market also shows increasing demand for UV-resistant additives and masterbatches that can enhance the performance of base polymers like Polypropylene, creating opportunities for specialized chemical suppliers to develop innovative solutions that balance performance requirements with cost considerations.
Current State and Challenges in Polymer UV Protection
The global polymer industry faces significant challenges in UV protection, with both Nylon 66 and Polypropylene demonstrating distinct vulnerabilities to ultraviolet radiation. Current research indicates that unprotected polymers experience accelerated degradation when exposed to UV radiation, resulting in mechanical property deterioration, color changes, and reduced service life. This phenomenon, known as photodegradation, represents a critical limitation in outdoor applications for these materials.
Nylon 66 exhibits moderate inherent UV resistance compared to other engineering polymers, but still suffers from notable degradation mechanisms including chain scission and crosslinking when exposed to prolonged UV radiation. Industry data suggests that unprotected Nylon 66 can lose up to 50% of its tensile strength after just 1,000 hours of accelerated weathering tests, highlighting the urgency for effective protection strategies.
Polypropylene presents an even greater challenge, with significantly lower inherent UV stability than Nylon 66. Its tertiary carbon atoms are particularly susceptible to UV-induced oxidation, leading to rapid embrittlement and surface crazing. Current market solutions typically involve higher loadings of UV stabilizers for Polypropylene compared to Nylon 66, increasing both cost and potential for additive migration issues.
The primary technical approaches currently employed for UV protection include UV absorbers (UVAs), hindered amine light stabilizers (HALS), and carbon black incorporation. UVAs function by absorbing harmful UV radiation and dissipating it as heat, while HALS act as radical scavengers that interrupt the degradation cycle. Carbon black provides protection through UV absorption and reflection mechanisms. However, each approach presents specific limitations in long-term effectiveness, processing compatibility, and aesthetic constraints.
Recent advancements in nano-scale additives show promise, with titanium dioxide and zinc oxide nanoparticles demonstrating enhanced UV blocking capabilities. However, challenges in dispersion technology and potential environmental concerns regarding nanoparticle release remain significant barriers to widespread adoption.
Regulatory pressures further complicate the landscape, with increasing restrictions on certain traditional UV stabilizers due to environmental persistence concerns. The industry is actively seeking more sustainable alternatives that maintain performance while addressing these regulatory challenges.
Geographical variations in UV intensity create additional complexity, as materials must perform across diverse environmental conditions. This necessitates tailored stabilization packages for different regions, complicating global supply chains and increasing formulation complexity for multinational manufacturers.
Nylon 66 exhibits moderate inherent UV resistance compared to other engineering polymers, but still suffers from notable degradation mechanisms including chain scission and crosslinking when exposed to prolonged UV radiation. Industry data suggests that unprotected Nylon 66 can lose up to 50% of its tensile strength after just 1,000 hours of accelerated weathering tests, highlighting the urgency for effective protection strategies.
Polypropylene presents an even greater challenge, with significantly lower inherent UV stability than Nylon 66. Its tertiary carbon atoms are particularly susceptible to UV-induced oxidation, leading to rapid embrittlement and surface crazing. Current market solutions typically involve higher loadings of UV stabilizers for Polypropylene compared to Nylon 66, increasing both cost and potential for additive migration issues.
The primary technical approaches currently employed for UV protection include UV absorbers (UVAs), hindered amine light stabilizers (HALS), and carbon black incorporation. UVAs function by absorbing harmful UV radiation and dissipating it as heat, while HALS act as radical scavengers that interrupt the degradation cycle. Carbon black provides protection through UV absorption and reflection mechanisms. However, each approach presents specific limitations in long-term effectiveness, processing compatibility, and aesthetic constraints.
Recent advancements in nano-scale additives show promise, with titanium dioxide and zinc oxide nanoparticles demonstrating enhanced UV blocking capabilities. However, challenges in dispersion technology and potential environmental concerns regarding nanoparticle release remain significant barriers to widespread adoption.
Regulatory pressures further complicate the landscape, with increasing restrictions on certain traditional UV stabilizers due to environmental persistence concerns. The industry is actively seeking more sustainable alternatives that maintain performance while addressing these regulatory challenges.
Geographical variations in UV intensity create additional complexity, as materials must perform across diverse environmental conditions. This necessitates tailored stabilization packages for different regions, complicating global supply chains and increasing formulation complexity for multinational manufacturers.
Comparative Analysis of Nylon 66 and PP UV Protection Solutions
01 UV stabilizers and additives for polymer protection
Various UV stabilizers and additives can be incorporated into Nylon 66 and Polypropylene to enhance their resistance to UV degradation. These include hindered amine light stabilizers (HALS), UV absorbers, and antioxidants that work by neutralizing free radicals or absorbing harmful UV radiation. These additives significantly extend the service life of the polymers when exposed to outdoor conditions and prevent yellowing, cracking, and loss of mechanical properties.- UV stabilizers and additives for polymer protection: Various UV stabilizers and additives can be incorporated into Nylon 66 and Polypropylene to enhance their resistance to UV degradation. These include hindered amine light stabilizers (HALS), UV absorbers, and antioxidants that work by neutralizing free radicals or absorbing harmful UV radiation. These additives can significantly extend the service life of these polymers when exposed to outdoor conditions and prevent yellowing, cracking, and loss of mechanical properties.
- Composite materials with enhanced UV resistance: Combining Nylon 66 or Polypropylene with other materials to form composites can improve UV resistance. These composites may include glass fibers, carbon fibers, or mineral fillers that provide physical barriers against UV radiation. Additionally, the incorporation of nanomaterials such as titanium dioxide, zinc oxide, or carbon nanotubes can enhance UV protection while maintaining or improving the mechanical properties of the base polymers.
- Surface treatments and coatings for UV protection: Surface treatments and specialized coatings can be applied to Nylon 66 and Polypropylene products to enhance their UV resistance. These include UV-resistant paints, varnishes, or thin-film coatings that create a protective barrier on the polymer surface. Some treatments involve chemical modification of the polymer surface to incorporate UV-absorbing groups or to create a more stable surface layer that resists photodegradation.
- Processing techniques to improve UV stability: Specific processing techniques can enhance the UV resistance of Nylon 66 and Polypropylene. These include controlled orientation of polymer chains, optimized crystallinity, and specialized extrusion or molding parameters that result in more UV-stable structures. Co-extrusion techniques can create multi-layer structures with UV-resistant outer layers protecting more vulnerable core materials. Heat treatment and annealing processes can also improve long-term UV stability by reducing internal stresses.
- Blends and alloys with UV-resistant polymers: Blending Nylon 66 or Polypropylene with inherently more UV-resistant polymers can improve overall UV stability. These blends may incorporate polymers such as polyesters, polyurethanes, or fluoropolymers that have better natural resistance to UV degradation. Compatibilizers are often used to ensure good miscibility between the different polymer phases. The resulting polymer blends can offer a balance of UV resistance, mechanical properties, and cost-effectiveness for outdoor applications.
02 Composite materials with enhanced UV resistance
Combining Nylon 66 or Polypropylene with other materials to form composites can improve UV resistance. These composites may incorporate carbon fibers, glass fibers, or mineral fillers that shield the polymer matrix from UV radiation. Additionally, layered structures with UV-resistant outer layers protecting the core polymer can be effective. These composite approaches not only enhance UV stability but often improve mechanical properties and thermal resistance as well.Expand Specific Solutions03 Surface treatments and coatings for UV protection
Surface treatments and specialized coatings can be applied to Nylon 66 and Polypropylene products to enhance their UV resistance. These include UV-resistant paints, metallization, plasma treatments, and the application of thin protective films. Such surface modifications create a barrier that prevents UV radiation from reaching and degrading the polymer substrate while maintaining the bulk properties of the material.Expand Specific Solutions04 Chemical modification of polymer structure
Chemical modification of the polymer structure itself can enhance UV resistance in Nylon 66 and Polypropylene. This includes copolymerization with UV-resistant monomers, grafting of protective functional groups, and molecular weight optimization. These modifications alter the chemical structure to reduce susceptibility to photodegradation and increase stability under UV exposure, resulting in polymers with inherently better weathering characteristics.Expand Specific Solutions05 Processing techniques for improved UV stability
Specific processing techniques can enhance the UV resistance of Nylon 66 and Polypropylene products. These include controlled cooling rates during molding, orientation of polymer chains, specialized extrusion methods, and heat treatment processes. Proper processing can create a more ordered molecular structure that is less susceptible to UV degradation, while also ensuring uniform distribution of UV stabilizers throughout the polymer matrix.Expand Specific Solutions
Key Industry Players in UV-Resistant Polymer Development
The UV resistance analysis between Nylon 66 and Polypropylene reveals a competitive landscape in an established but evolving market. The global UV-resistant materials sector is experiencing steady growth, estimated at $25-30 billion annually with 5-7% CAGR. Technologically, polypropylene naturally exhibits poor UV resistance requiring stabilizers, while Nylon 66 offers better inherent stability. Companies like DuPont, Covestro, and Mitsui Chemicals lead with advanced UV stabilization technologies, while Kingfa, Avient, and SK Chemicals focus on specialized formulations. Chinese manufacturers including Shanghai Kingfa and Zhejiang Pret are rapidly advancing with cost-effective solutions, challenging established players through increased R&D investments in UV-resistant polymer technologies.
Mitsui Chemicals, Inc.
Technical Solution: Mitsui Chemicals has developed advanced UV-resistant formulations for both Nylon 66 and Polypropylene through their AdmerTM and TAFMERTM product lines. Their approach for Nylon 66 incorporates novel oligomeric HALS (Hindered Amine Light Stabilizers) that provide superior resistance to extraction and migration compared to traditional low molecular weight stabilizers. For polypropylene, Mitsui has pioneered the use of benzoate-based UV absorbers combined with proprietary antioxidant packages that specifically target the tertiary hydrogen sites vulnerable to photo-oxidation. Their comparative testing has demonstrated that while standard polypropylene typically shows significant degradation after 1000 hours of xenon arc exposure, their enhanced formulations maintain over 70% of mechanical properties after 3000 hours. Mitsui has also developed specialized nucleating agents that improve the crystalline structure of polypropylene, indirectly enhancing UV resistance by reducing light penetration into the polymer matrix.
Strengths: Excellent retention of mechanical properties after extended UV exposure; reduced blooming and plate-out issues during processing; solutions optimized for automotive and outdoor applications. Weaknesses: Higher cost compared to conventional materials; some formulations require specific processing conditions; potential compatibility issues with certain pigment systems.
Kingfa Sci. & Tech. Co., Ltd.
Technical Solution: Kingfa has developed comprehensive UV resistance solutions for both Nylon 66 and Polypropylene materials through their ECOMAX® and COMPLET® product lines. Their approach involves a synergistic combination of primary and secondary antioxidants coupled with specialized UV absorbers tailored to each polymer's degradation mechanism. For Nylon 66, Kingfa employs proprietary oligomeric HALS (Hindered Amine Light Stabilizers) that resist extraction in humid environments, addressing a key weakness of conventional stabilizers. Their polypropylene formulations incorporate benzophenone and benzotriazole UV absorbers alongside novel radical scavengers that specifically target the tertiary carbon sites vulnerable to photo-oxidation. Kingfa's research has demonstrated that their enhanced polypropylene can maintain over 80% of its tensile properties after 4000 hours of accelerated weathering, while their stabilized Nylon 66 shows minimal color shift and mechanical property retention after 2500 hours of exposure.
Strengths: Excellent long-term stabilization performance in harsh outdoor environments; solutions optimized for both mechanical property retention and appearance preservation; cost-effective formulations suitable for mass production. Weaknesses: Some formulations may require adjustment of processing parameters; potential for interaction with certain pigment systems; slightly higher moisture sensitivity in highly loaded systems.
Technical Innovations in Polymer UV Stabilization
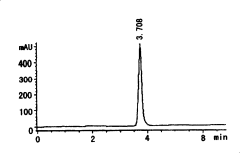
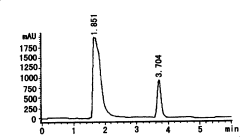
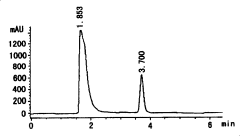
High efficiency liquid phase chromatographic analysis method for 2-benzotrinitrozole-4,6-diiso propyl phenol content in nylon 66
PatentInactiveCN100405059C
Innovation
- Use phenolic dissolving agent to dissolve nylon 66 products and precipitate through alcohol eluent. Use high performance liquid chromatography to analyze the content of 2-benzotriazolyl-4,6-dicumylphenol. Calculate its concentration using the standard curve method, the detection limit is 5.0μg/ml, and the error is less than 5‰.
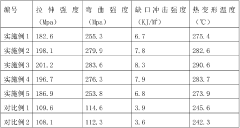
Anti-bending anti-UV hydrolysis-resistant nylon 6/nylon 66 composite material and preparation method thereof
PatentInactiveCN107778857A
Innovation
- Using 12-30% nylon 6, 28-45% nylon 66, 3-10% toughening compatibilizer, 22-40% hydrolysis-resistant glass fiber, 3-8% glass beads, 0.2-1% nylon nucleating agent , 0.2-0.7% anti-UV agent, 0 .A composite material of 1-0.6% antioxidant, 0.5-5% hydrolysis-resistant additive and 0.2-2% lubricating and dispersing agent is prepared through drying, high-speed mixing and twin-screw extrusion granulation process to be resistant to deflection, UV and hydrolysis. Nylon 6/nylon 66 composite material.
Environmental Impact and Sustainability Considerations
The environmental impact and sustainability considerations of polymer materials have become increasingly critical factors in material selection processes across industries. When comparing Nylon 66 and Polypropylene specifically in terms of UV resistance applications, several environmental dimensions must be evaluated.
Nylon 66 production typically requires more energy-intensive processes and generates higher greenhouse gas emissions compared to Polypropylene manufacturing. The production of adipic acid, a key precursor for Nylon 66, is associated with nitrous oxide emissions, a potent greenhouse gas with approximately 300 times the global warming potential of carbon dioxide. This significant environmental footprint must be weighed against the material's extended service life in UV-exposed applications.
Polypropylene, while requiring less energy to produce, presents its own environmental challenges. Its lower inherent UV resistance often necessitates the addition of stabilizers and additives, some of which may pose environmental concerns. These additives can potentially leach into the environment during the product's lifecycle, particularly when exposed to prolonged UV radiation and weathering conditions.
The end-of-life considerations reveal notable differences between these polymers. Polypropylene offers better recyclability prospects, with established recycling streams in many regions. Its lower processing temperature also translates to reduced energy consumption during recycling operations. Conversely, Nylon 66 recycling faces greater technical challenges, particularly when UV stabilizers and other additives have been incorporated.
Water usage patterns differ significantly between these materials. Nylon 66 production typically requires substantially more water than Polypropylene manufacturing. Additionally, the water pollution potential from Nylon production, particularly from chemical intermediates like hexamethylenediamine, presents environmental concerns that must be addressed through proper industrial waste management.
When considering biodegradability, neither polymer performs particularly well in natural environments. However, recent advances in biodegradable additives for Polypropylene show promising developments that may reduce its environmental persistence. Similar technologies for Nylon 66 remain less developed, though research continues in this area.
Carbon footprint assessments across the full lifecycle reveal that the higher initial environmental impact of Nylon 66 may be partially offset by its longer service life in UV-exposed applications, potentially reducing replacement frequency and associated environmental costs. However, this advantage diminishes in applications where Polypropylene with appropriate UV stabilization can achieve comparable performance durability.
Nylon 66 production typically requires more energy-intensive processes and generates higher greenhouse gas emissions compared to Polypropylene manufacturing. The production of adipic acid, a key precursor for Nylon 66, is associated with nitrous oxide emissions, a potent greenhouse gas with approximately 300 times the global warming potential of carbon dioxide. This significant environmental footprint must be weighed against the material's extended service life in UV-exposed applications.
Polypropylene, while requiring less energy to produce, presents its own environmental challenges. Its lower inherent UV resistance often necessitates the addition of stabilizers and additives, some of which may pose environmental concerns. These additives can potentially leach into the environment during the product's lifecycle, particularly when exposed to prolonged UV radiation and weathering conditions.
The end-of-life considerations reveal notable differences between these polymers. Polypropylene offers better recyclability prospects, with established recycling streams in many regions. Its lower processing temperature also translates to reduced energy consumption during recycling operations. Conversely, Nylon 66 recycling faces greater technical challenges, particularly when UV stabilizers and other additives have been incorporated.
Water usage patterns differ significantly between these materials. Nylon 66 production typically requires substantially more water than Polypropylene manufacturing. Additionally, the water pollution potential from Nylon production, particularly from chemical intermediates like hexamethylenediamine, presents environmental concerns that must be addressed through proper industrial waste management.
When considering biodegradability, neither polymer performs particularly well in natural environments. However, recent advances in biodegradable additives for Polypropylene show promising developments that may reduce its environmental persistence. Similar technologies for Nylon 66 remain less developed, though research continues in this area.
Carbon footprint assessments across the full lifecycle reveal that the higher initial environmental impact of Nylon 66 may be partially offset by its longer service life in UV-exposed applications, potentially reducing replacement frequency and associated environmental costs. However, this advantage diminishes in applications where Polypropylene with appropriate UV stabilization can achieve comparable performance durability.
Degradation Mechanisms and Testing Methodologies
Both Nylon 66 and Polypropylene undergo distinct degradation mechanisms when exposed to ultraviolet (UV) radiation, necessitating comprehensive understanding for appropriate material selection in outdoor applications. Nylon 66 primarily degrades through photo-oxidation processes, where UV radiation breaks chemical bonds in the polymer backbone, particularly at the amide linkages. This leads to chain scission, resulting in decreased molecular weight and mechanical properties. The degradation is often characterized by yellowing, surface cracking, and embrittlement over time.
Polypropylene, conversely, experiences degradation through auto-oxidation mechanisms. The tertiary carbon atoms in its structure are particularly vulnerable to UV attack, leading to the formation of hydroperoxides that subsequently decompose into free radicals. These free radicals propagate degradation throughout the polymer matrix, causing chain scission and crosslinking reactions that significantly alter the material's properties. Visual indicators include chalking, surface crazing, and loss of tensile strength.
Environmental factors substantially influence degradation rates for both polymers. Temperature amplifies UV degradation effects, with higher temperatures accelerating the photo-oxidation processes. Humidity plays a more significant role in Nylon 66 degradation due to its hygroscopic nature, potentially catalyzing hydrolysis reactions alongside photo-oxidation. Atmospheric pollutants, particularly ozone and sulfur dioxide, can further accelerate degradation through synergistic effects with UV radiation.
Standardized testing methodologies are essential for quantifying UV resistance. Accelerated weathering tests, including QUV exposure (ASTM G154) and Xenon arc testing (ASTM G155), simulate outdoor conditions with controlled UV intensity, temperature, and humidity cycles. These tests typically measure changes in mechanical properties, color retention, and surface characteristics over time. The Xenon arc method provides a fuller spectrum simulation closer to natural sunlight, while QUV testing emphasizes the UV portion of the spectrum.
Natural weathering tests (ASTM G7) offer real-world exposure data but require longer testing periods, typically 1-3 years. These tests are conducted at standardized exposure sites in different climatic regions to account for geographical variations in UV intensity, temperature fluctuations, and precipitation patterns.
Analytical techniques including FTIR spectroscopy, DSC, and GPC provide detailed insights into chemical changes during degradation. FTIR spectroscopy can track the formation of carbonyl groups, a key indicator of oxidative degradation. Mechanical testing protocols measure changes in tensile strength, impact resistance, and elongation at break, offering practical assessment of material performance degradation over time.
Polypropylene, conversely, experiences degradation through auto-oxidation mechanisms. The tertiary carbon atoms in its structure are particularly vulnerable to UV attack, leading to the formation of hydroperoxides that subsequently decompose into free radicals. These free radicals propagate degradation throughout the polymer matrix, causing chain scission and crosslinking reactions that significantly alter the material's properties. Visual indicators include chalking, surface crazing, and loss of tensile strength.
Environmental factors substantially influence degradation rates for both polymers. Temperature amplifies UV degradation effects, with higher temperatures accelerating the photo-oxidation processes. Humidity plays a more significant role in Nylon 66 degradation due to its hygroscopic nature, potentially catalyzing hydrolysis reactions alongside photo-oxidation. Atmospheric pollutants, particularly ozone and sulfur dioxide, can further accelerate degradation through synergistic effects with UV radiation.
Standardized testing methodologies are essential for quantifying UV resistance. Accelerated weathering tests, including QUV exposure (ASTM G154) and Xenon arc testing (ASTM G155), simulate outdoor conditions with controlled UV intensity, temperature, and humidity cycles. These tests typically measure changes in mechanical properties, color retention, and surface characteristics over time. The Xenon arc method provides a fuller spectrum simulation closer to natural sunlight, while QUV testing emphasizes the UV portion of the spectrum.
Natural weathering tests (ASTM G7) offer real-world exposure data but require longer testing periods, typically 1-3 years. These tests are conducted at standardized exposure sites in different climatic regions to account for geographical variations in UV intensity, temperature fluctuations, and precipitation patterns.
Analytical techniques including FTIR spectroscopy, DSC, and GPC provide detailed insights into chemical changes during degradation. FTIR spectroscopy can track the formation of carbonyl groups, a key indicator of oxidative degradation. Mechanical testing protocols measure changes in tensile strength, impact resistance, and elongation at break, offering practical assessment of material performance degradation over time.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!