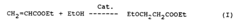Optimizing Ethyl Propanoate Processes in Pharmaceutical Production
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Propanoate Synthesis Background and Objectives
Ethyl propanoate, also known as ethyl propionate, is a crucial ester compound widely used in the pharmaceutical industry as a solvent, flavoring agent, and intermediate in various drug synthesis processes. The optimization of ethyl propanoate production has become increasingly important due to its growing demand in pharmaceutical applications and the need for more efficient and sustainable manufacturing methods.
The synthesis of ethyl propanoate has a rich history dating back to the early 20th century when esterification reactions were first studied in detail. Initially, the production process relied on traditional batch reactions, which were time-consuming and often resulted in low yields. As the pharmaceutical industry expanded, the demand for more efficient production methods grew, leading to significant advancements in synthesis techniques.
Over the years, continuous flow chemistry and catalytic processes have emerged as promising approaches to enhance ethyl propanoate production. These methods offer advantages such as improved reaction control, reduced waste generation, and increased product purity. However, challenges remain in optimizing reaction conditions, catalyst selection, and process scalability.
The evolution of ethyl propanoate synthesis has been closely tied to advancements in green chemistry principles. As environmental concerns have gained prominence, there has been a shift towards more sustainable production methods. This includes the exploration of bio-based feedstocks, the development of recyclable catalysts, and the implementation of energy-efficient processes.
The primary objectives of optimizing ethyl propanoate processes in pharmaceutical production are multifaceted. First and foremost, there is a need to increase production efficiency and yield while maintaining high product quality. This involves fine-tuning reaction parameters, exploring novel catalysts, and implementing advanced process control strategies.
Another key objective is to enhance the sustainability of the production process. This includes reducing energy consumption, minimizing waste generation, and exploring the use of renewable raw materials. Additionally, there is a focus on improving process safety and reducing the environmental impact of ethyl propanoate synthesis.
Researchers and industry professionals are also aiming to develop more versatile and flexible production methods that can easily adapt to changing market demands and regulatory requirements. This includes the design of modular production systems and the integration of real-time monitoring and quality control measures.
As the pharmaceutical industry continues to evolve, there is an increasing emphasis on the development of continuous manufacturing processes for ethyl propanoate. This approach offers potential benefits such as reduced production time, improved consistency, and enhanced process control. However, it also presents challenges in terms of equipment design and process validation.
The synthesis of ethyl propanoate has a rich history dating back to the early 20th century when esterification reactions were first studied in detail. Initially, the production process relied on traditional batch reactions, which were time-consuming and often resulted in low yields. As the pharmaceutical industry expanded, the demand for more efficient production methods grew, leading to significant advancements in synthesis techniques.
Over the years, continuous flow chemistry and catalytic processes have emerged as promising approaches to enhance ethyl propanoate production. These methods offer advantages such as improved reaction control, reduced waste generation, and increased product purity. However, challenges remain in optimizing reaction conditions, catalyst selection, and process scalability.
The evolution of ethyl propanoate synthesis has been closely tied to advancements in green chemistry principles. As environmental concerns have gained prominence, there has been a shift towards more sustainable production methods. This includes the exploration of bio-based feedstocks, the development of recyclable catalysts, and the implementation of energy-efficient processes.
The primary objectives of optimizing ethyl propanoate processes in pharmaceutical production are multifaceted. First and foremost, there is a need to increase production efficiency and yield while maintaining high product quality. This involves fine-tuning reaction parameters, exploring novel catalysts, and implementing advanced process control strategies.
Another key objective is to enhance the sustainability of the production process. This includes reducing energy consumption, minimizing waste generation, and exploring the use of renewable raw materials. Additionally, there is a focus on improving process safety and reducing the environmental impact of ethyl propanoate synthesis.
Researchers and industry professionals are also aiming to develop more versatile and flexible production methods that can easily adapt to changing market demands and regulatory requirements. This includes the design of modular production systems and the integration of real-time monitoring and quality control measures.
As the pharmaceutical industry continues to evolve, there is an increasing emphasis on the development of continuous manufacturing processes for ethyl propanoate. This approach offers potential benefits such as reduced production time, improved consistency, and enhanced process control. However, it also presents challenges in terms of equipment design and process validation.
Pharmaceutical Market Demand Analysis
The pharmaceutical industry's demand for ethyl propanoate has been steadily increasing due to its crucial role in various drug formulations and manufacturing processes. As a key ingredient in the production of certain medications, ethyl propanoate's market demand is closely tied to the growth of the pharmaceutical sector.
The global pharmaceutical market has been experiencing robust growth, with projections indicating continued expansion in the coming years. This growth is driven by factors such as an aging population, increasing prevalence of chronic diseases, and advancements in drug discovery and development. Consequently, the demand for pharmaceutical intermediates and solvents, including ethyl propanoate, is expected to rise in tandem with the industry's overall growth.
Ethyl propanoate finds applications in multiple pharmaceutical processes, including as a solvent for active pharmaceutical ingredients (APIs), a reagent in organic synthesis, and a component in drug delivery systems. Its versatility and relatively low toxicity make it an attractive choice for pharmaceutical manufacturers seeking to optimize their production processes.
The increasing focus on green chemistry and sustainable manufacturing practices in the pharmaceutical industry has also contributed to the growing demand for ethyl propanoate. As a bio-based solvent, it aligns well with the industry's efforts to reduce environmental impact and improve process efficiency.
Regionally, the demand for ethyl propanoate in pharmaceutical production varies. North America and Europe, with their well-established pharmaceutical industries and stringent regulatory environments, represent significant markets for high-quality ethyl propanoate. The Asia-Pacific region, particularly China and India, is experiencing rapid growth in pharmaceutical manufacturing, driving increased demand for ethyl propanoate and other key ingredients.
The COVID-19 pandemic has further highlighted the importance of robust pharmaceutical supply chains and the need for efficient production processes. This has led to increased interest in optimizing manufacturing techniques, including those involving ethyl propanoate, to ensure reliable drug production and supply.
Looking ahead, the pharmaceutical market's demand for ethyl propanoate is expected to continue growing. Factors such as the development of novel drug formulations, the expansion of generic drug markets, and the increasing adoption of continuous manufacturing processes in the pharmaceutical industry are likely to contribute to this trend. As pharmaceutical companies strive to improve their production efficiency and reduce costs, the optimization of ethyl propanoate processes will remain a key area of focus in the industry.
The global pharmaceutical market has been experiencing robust growth, with projections indicating continued expansion in the coming years. This growth is driven by factors such as an aging population, increasing prevalence of chronic diseases, and advancements in drug discovery and development. Consequently, the demand for pharmaceutical intermediates and solvents, including ethyl propanoate, is expected to rise in tandem with the industry's overall growth.
Ethyl propanoate finds applications in multiple pharmaceutical processes, including as a solvent for active pharmaceutical ingredients (APIs), a reagent in organic synthesis, and a component in drug delivery systems. Its versatility and relatively low toxicity make it an attractive choice for pharmaceutical manufacturers seeking to optimize their production processes.
The increasing focus on green chemistry and sustainable manufacturing practices in the pharmaceutical industry has also contributed to the growing demand for ethyl propanoate. As a bio-based solvent, it aligns well with the industry's efforts to reduce environmental impact and improve process efficiency.
Regionally, the demand for ethyl propanoate in pharmaceutical production varies. North America and Europe, with their well-established pharmaceutical industries and stringent regulatory environments, represent significant markets for high-quality ethyl propanoate. The Asia-Pacific region, particularly China and India, is experiencing rapid growth in pharmaceutical manufacturing, driving increased demand for ethyl propanoate and other key ingredients.
The COVID-19 pandemic has further highlighted the importance of robust pharmaceutical supply chains and the need for efficient production processes. This has led to increased interest in optimizing manufacturing techniques, including those involving ethyl propanoate, to ensure reliable drug production and supply.
Looking ahead, the pharmaceutical market's demand for ethyl propanoate is expected to continue growing. Factors such as the development of novel drug formulations, the expansion of generic drug markets, and the increasing adoption of continuous manufacturing processes in the pharmaceutical industry are likely to contribute to this trend. As pharmaceutical companies strive to improve their production efficiency and reduce costs, the optimization of ethyl propanoate processes will remain a key area of focus in the industry.
Current Challenges in Ethyl Propanoate Production
The production of ethyl propanoate in the pharmaceutical industry faces several significant challenges that hinder optimal efficiency and quality. One of the primary issues is the control of reaction kinetics during the esterification process. The reaction between propionic acid and ethanol is reversible, making it difficult to achieve high yields without careful management of reaction conditions.
Temperature control presents another major challenge. The esterification reaction is sensitive to temperature fluctuations, which can affect both the rate of reaction and the equilibrium position. Maintaining a consistent and optimal temperature throughout the production process is crucial but often problematic in large-scale operations.
Catalyst selection and management also pose significant hurdles. While catalysts are essential for accelerating the reaction and improving yields, finding the right catalyst that balances activity, selectivity, and stability can be complex. Additionally, catalyst deactivation and recovery present ongoing operational challenges.
Water removal is a critical aspect of ethyl propanoate production that often proves troublesome. As water is a byproduct of the esterification reaction, its continuous removal is necessary to drive the equilibrium towards the product. However, efficient water removal without disrupting the reaction or compromising product purity is technically demanding.
Purification of the final product is another area of concern. Achieving the high levels of purity required for pharmaceutical applications often involves energy-intensive distillation processes. These processes can be costly and may lead to product loss, impacting overall yield and efficiency.
Raw material quality and consistency also present challenges. Variations in the purity of propionic acid or ethanol can affect reaction kinetics and product quality. Ensuring a stable supply of high-quality raw materials is essential but can be difficult due to market fluctuations and supplier inconsistencies.
Safety considerations add another layer of complexity to ethyl propanoate production. The flammability of ethanol and the corrosive nature of propionic acid necessitate stringent safety measures, which can sometimes conflict with efforts to optimize production efficiency.
Lastly, environmental concerns and regulatory compliance pose ongoing challenges. The need to reduce waste, minimize energy consumption, and adhere to increasingly strict environmental regulations requires continuous innovation in process design and operation.
Addressing these challenges requires a multifaceted approach, combining advances in reaction engineering, process control, catalysis, and separation technologies. Innovations in these areas are crucial for optimizing ethyl propanoate production in the pharmaceutical industry, enhancing both efficiency and sustainability.
Temperature control presents another major challenge. The esterification reaction is sensitive to temperature fluctuations, which can affect both the rate of reaction and the equilibrium position. Maintaining a consistent and optimal temperature throughout the production process is crucial but often problematic in large-scale operations.
Catalyst selection and management also pose significant hurdles. While catalysts are essential for accelerating the reaction and improving yields, finding the right catalyst that balances activity, selectivity, and stability can be complex. Additionally, catalyst deactivation and recovery present ongoing operational challenges.
Water removal is a critical aspect of ethyl propanoate production that often proves troublesome. As water is a byproduct of the esterification reaction, its continuous removal is necessary to drive the equilibrium towards the product. However, efficient water removal without disrupting the reaction or compromising product purity is technically demanding.
Purification of the final product is another area of concern. Achieving the high levels of purity required for pharmaceutical applications often involves energy-intensive distillation processes. These processes can be costly and may lead to product loss, impacting overall yield and efficiency.
Raw material quality and consistency also present challenges. Variations in the purity of propionic acid or ethanol can affect reaction kinetics and product quality. Ensuring a stable supply of high-quality raw materials is essential but can be difficult due to market fluctuations and supplier inconsistencies.
Safety considerations add another layer of complexity to ethyl propanoate production. The flammability of ethanol and the corrosive nature of propionic acid necessitate stringent safety measures, which can sometimes conflict with efforts to optimize production efficiency.
Lastly, environmental concerns and regulatory compliance pose ongoing challenges. The need to reduce waste, minimize energy consumption, and adhere to increasingly strict environmental regulations requires continuous innovation in process design and operation.
Addressing these challenges requires a multifaceted approach, combining advances in reaction engineering, process control, catalysis, and separation technologies. Innovations in these areas are crucial for optimizing ethyl propanoate production in the pharmaceutical industry, enhancing both efficiency and sustainability.
Existing Ethyl Propanoate Synthesis Methods
01 Reaction conditions optimization
Optimizing reaction conditions such as temperature, pressure, and catalyst concentration is crucial for improving the yield and efficiency of ethyl propanoate production. This may involve adjusting parameters to achieve optimal reaction kinetics and minimize side reactions.- Reaction conditions optimization: Optimizing reaction conditions such as temperature, pressure, and catalyst concentration is crucial for improving the yield and efficiency of ethyl propanoate production. This may involve adjusting parameters to find the optimal balance between reaction rate and product selectivity.
- Catalyst development and selection: Developing and selecting appropriate catalysts can significantly enhance the ethyl propanoate production process. This may include exploring novel catalytic materials, improving catalyst stability, and optimizing catalyst regeneration methods to increase overall process efficiency.
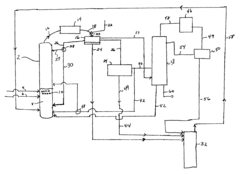
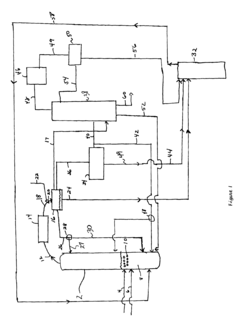
- Continuous flow processes: Implementing continuous flow processes for ethyl propanoate production can lead to improved productivity and reduced operational costs. This approach may involve designing specialized reactors and optimizing flow rates to achieve consistent product quality and higher throughput.
- Purification and separation techniques: Developing efficient purification and separation techniques is essential for obtaining high-quality ethyl propanoate. This may include optimizing distillation processes, implementing membrane separation technologies, or exploring novel extraction methods to improve product purity and reduce energy consumption.
- Process monitoring and control: Implementing advanced process monitoring and control systems can enhance the overall efficiency of ethyl propanoate production. This may involve using real-time analytics, machine learning algorithms, and automated control systems to optimize process parameters and ensure consistent product quality.
02 Catalyst selection and improvement
Selecting appropriate catalysts and improving their performance can significantly enhance the ethyl propanoate production process. This may include developing novel catalysts, modifying existing ones, or optimizing catalyst regeneration methods to increase catalytic activity and selectivity.Expand Specific Solutions03 Continuous flow processes
Implementing continuous flow processes for ethyl propanoate production can lead to improved efficiency, better heat and mass transfer, and enhanced process control. This approach may involve designing specialized reactors or optimizing existing continuous flow systems.Expand Specific Solutions04 Purification and separation techniques
Developing and optimizing purification and separation techniques for ethyl propanoate can improve product quality and overall process efficiency. This may include advancements in distillation, extraction, or membrane separation technologies.Expand Specific Solutions05 Process monitoring and control
Implementing advanced process monitoring and control systems can enhance the overall efficiency and consistency of ethyl propanoate production. This may involve using real-time analytics, machine learning algorithms, or automated control systems to optimize process parameters and respond to variations in operating conditions.Expand Specific Solutions
Key Players in Pharmaceutical Ester Production
The optimization of ethyl propanoate processes in pharmaceutical production is currently in a growth phase, with increasing market demand driven by the pharmaceutical industry's expansion. The global market size for this specific application is estimated to be in the hundreds of millions of dollars, with steady growth projected. Technologically, the process is relatively mature, but there is ongoing research to improve efficiency and sustainability. Companies like Lonza Ltd., MSN Laboratories, and Zydus Lifesciences are at the forefront of innovation in this field, investing in R&D to enhance production methods. Emerging players such as Jiangmen Qianxin Chemical and Taixing Jinjiang Chemical are also contributing to the competitive landscape, particularly in the Asian market.
Lonza Ltd.
Technical Solution: Lonza has developed an innovative continuous flow chemistry process for the production of ethyl propanoate. This method utilizes a microreactor system with precise temperature and pressure control, allowing for enhanced reaction kinetics and improved product quality. The process incorporates in-line purification and real-time monitoring, enabling rapid adjustments to optimize yield and purity. Lonza's approach also integrates green chemistry principles, using bio-based feedstocks and minimizing solvent usage, thus reducing environmental impact[1][3]. The company has implemented advanced process analytical technology (PAT) tools to ensure consistent product quality and facilitate regulatory compliance in pharmaceutical manufacturing[2].
Strengths: Improved efficiency, reduced environmental impact, enhanced product quality, and regulatory compliance. Weaknesses: Potentially higher initial investment costs and the need for specialized equipment and expertise.
Celanese International Corp.
Technical Solution: Celanese has developed a proprietary catalytic process for ethyl propanoate production, focusing on high-volume, cost-effective manufacturing for pharmaceutical applications. Their method employs a novel heterogeneous catalyst system that significantly increases reaction rates and selectivity. The process operates at lower temperatures compared to traditional methods, reducing energy consumption and minimizing side reactions. Celanese has also implemented an advanced distillation system for product purification, achieving high-purity ethyl propanoate suitable for pharmaceutical standards[4]. The company's approach includes a closed-loop solvent recovery system, enhancing sustainability and reducing operational costs[5].
Strengths: Cost-effective large-scale production, energy efficiency, and high product purity. Weaknesses: Potential limitations in flexibility for small-batch production and specialized pharmaceutical grades.
Innovative Catalysts and Reaction Mechanisms
Production of ethyl 3-ethoxy propanoate by acid catalyzed addition of ethanol to ethyl acrylate
PatentInactiveEP0499731A1
Innovation
- EEP is produced through an acid-catalyzed addition of ethanol to ethyl acrylate using strong acid catalysts such as sulfuric acid, hydrochloric acid, or sulfonic acids, with reaction conditions optimized at temperatures between 75°C to 150°C and pressures of 30-50 psig, and the use of inhibitors to manage by-product formation.
Process improvement for continuous ethyl acetate production
PatentInactiveUS6768021B2
Innovation
- The process involves using a membrane separation unit to remove water from the condensed reaction stream, recycling the dried stream back into the production process, and employing an additional distillation zone to produce purified ethyl acetate with minimal acid content, thereby optimizing water management and increasing process capacity.
Green Chemistry Considerations
Green chemistry principles are increasingly important in optimizing ethyl propanoate processes for pharmaceutical production. These principles aim to reduce environmental impact, improve efficiency, and enhance safety throughout the production lifecycle. One key consideration is the use of safer solvents and reaction conditions. Traditional ethyl propanoate synthesis often involves hazardous reagents and generates significant waste. However, newer green approaches utilize biocatalysis or flow chemistry to minimize solvent use and improve atom economy.
Waste reduction is another critical aspect of green chemistry in ethyl propanoate production. Implementing continuous flow processes can significantly decrease solvent consumption and waste generation compared to batch methods. Additionally, recycling and reusing solvents and catalysts can further minimize environmental impact. Some innovative approaches even explore the use of supercritical carbon dioxide as a green solvent alternative.
Energy efficiency is a paramount concern in green chemistry considerations. Optimizing reaction conditions, such as temperature and pressure, can reduce energy consumption while maintaining or improving yields. The use of microwave-assisted synthesis has shown promise in accelerating reactions and reducing energy requirements for ethyl propanoate production.
Raw material selection is another crucial factor in green chemistry. Utilizing bio-based feedstocks, such as ethanol derived from renewable sources, can reduce the carbon footprint of ethyl propanoate production. Some researchers are exploring the use of waste biomass as a sustainable carbon source for ester synthesis.
Catalyst design plays a significant role in green chemistry approaches. Developing highly selective and recyclable catalysts can improve reaction efficiency and reduce waste generation. Heterogeneous catalysts, in particular, offer advantages in terms of ease of separation and reusability.
Process intensification techniques, such as reactive distillation, can combine reaction and separation steps, leading to more compact and energy-efficient production processes. This approach can significantly reduce equipment size and energy consumption in ethyl propanoate manufacturing.
Lastly, life cycle assessment (LCA) is an essential tool in evaluating the overall environmental impact of ethyl propanoate production. By considering factors such as raw material sourcing, energy consumption, and waste generation across the entire production chain, manufacturers can identify areas for improvement and make informed decisions to enhance the sustainability of their processes.
Waste reduction is another critical aspect of green chemistry in ethyl propanoate production. Implementing continuous flow processes can significantly decrease solvent consumption and waste generation compared to batch methods. Additionally, recycling and reusing solvents and catalysts can further minimize environmental impact. Some innovative approaches even explore the use of supercritical carbon dioxide as a green solvent alternative.
Energy efficiency is a paramount concern in green chemistry considerations. Optimizing reaction conditions, such as temperature and pressure, can reduce energy consumption while maintaining or improving yields. The use of microwave-assisted synthesis has shown promise in accelerating reactions and reducing energy requirements for ethyl propanoate production.
Raw material selection is another crucial factor in green chemistry. Utilizing bio-based feedstocks, such as ethanol derived from renewable sources, can reduce the carbon footprint of ethyl propanoate production. Some researchers are exploring the use of waste biomass as a sustainable carbon source for ester synthesis.
Catalyst design plays a significant role in green chemistry approaches. Developing highly selective and recyclable catalysts can improve reaction efficiency and reduce waste generation. Heterogeneous catalysts, in particular, offer advantages in terms of ease of separation and reusability.
Process intensification techniques, such as reactive distillation, can combine reaction and separation steps, leading to more compact and energy-efficient production processes. This approach can significantly reduce equipment size and energy consumption in ethyl propanoate manufacturing.
Lastly, life cycle assessment (LCA) is an essential tool in evaluating the overall environmental impact of ethyl propanoate production. By considering factors such as raw material sourcing, energy consumption, and waste generation across the entire production chain, manufacturers can identify areas for improvement and make informed decisions to enhance the sustainability of their processes.
Regulatory Compliance in Pharmaceutical Synthesis
Regulatory compliance is a critical aspect of pharmaceutical synthesis, particularly in the optimization of ethyl propanoate processes. The production of this ester, commonly used as a solvent and flavoring agent, must adhere to stringent regulations set forth by various governing bodies to ensure product safety and quality.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing pharmaceutical production. The FDA's Current Good Manufacturing Practice (cGMP) regulations provide a framework for ensuring that drugs are consistently produced and controlled according to quality standards. These regulations encompass all aspects of production, including raw materials, facilities, equipment, and training of personnel.
For ethyl propanoate synthesis, compliance with 21 CFR Part 211 (Current Good Manufacturing Practice for Finished Pharmaceuticals) is essential. This regulation covers areas such as organization and personnel, buildings and facilities, equipment, control of components and drug product containers and closures, production and process controls, packaging and labeling control, holding and distribution, laboratory controls, and records and reports.
The European Medicines Agency (EMA) also imposes strict regulations on pharmaceutical production within the European Union. The EMA's guidelines on Good Manufacturing Practice (GMP) are closely aligned with those of the FDA but may have some regional variations. Manufacturers must comply with EudraLex Volume 4, which details GMP requirements for medicinal products in the EU.
Environmental regulations also play a significant role in ethyl propanoate production. The process involves volatile organic compounds (VOCs), which are subject to emission controls under the Clean Air Act in the US and similar legislation in other countries. Manufacturers must implement appropriate control technologies and monitoring systems to ensure compliance with air quality standards.
Water quality regulations are another crucial aspect, as the synthesis process may generate wastewater. The US Environmental Protection Agency (EPA) regulates industrial wastewater discharges under the Clean Water Act, requiring proper treatment and disposal methods.
Occupational safety is a key concern in ethyl propanoate production due to the flammable nature of the compound and its precursors. Compliance with Occupational Safety and Health Administration (OSHA) standards in the US, or equivalent bodies in other countries, is mandatory. This includes proper handling procedures, personal protective equipment, and emergency response protocols.
International harmonization efforts, such as the International Conference on Harmonisation (ICH) guidelines, aim to standardize regulatory requirements across different regions. Adherence to ICH Q7 (Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients) is particularly relevant for ethyl propanoate production when used in pharmaceutical applications.
Continuous monitoring and documentation are essential for maintaining regulatory compliance. This includes implementing robust quality management systems, conducting regular internal audits, and being prepared for inspections by regulatory authorities. Failure to comply with these regulations can result in severe penalties, including fines, production shutdowns, and damage to the company's reputation.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing pharmaceutical production. The FDA's Current Good Manufacturing Practice (cGMP) regulations provide a framework for ensuring that drugs are consistently produced and controlled according to quality standards. These regulations encompass all aspects of production, including raw materials, facilities, equipment, and training of personnel.
For ethyl propanoate synthesis, compliance with 21 CFR Part 211 (Current Good Manufacturing Practice for Finished Pharmaceuticals) is essential. This regulation covers areas such as organization and personnel, buildings and facilities, equipment, control of components and drug product containers and closures, production and process controls, packaging and labeling control, holding and distribution, laboratory controls, and records and reports.
The European Medicines Agency (EMA) also imposes strict regulations on pharmaceutical production within the European Union. The EMA's guidelines on Good Manufacturing Practice (GMP) are closely aligned with those of the FDA but may have some regional variations. Manufacturers must comply with EudraLex Volume 4, which details GMP requirements for medicinal products in the EU.
Environmental regulations also play a significant role in ethyl propanoate production. The process involves volatile organic compounds (VOCs), which are subject to emission controls under the Clean Air Act in the US and similar legislation in other countries. Manufacturers must implement appropriate control technologies and monitoring systems to ensure compliance with air quality standards.
Water quality regulations are another crucial aspect, as the synthesis process may generate wastewater. The US Environmental Protection Agency (EPA) regulates industrial wastewater discharges under the Clean Water Act, requiring proper treatment and disposal methods.
Occupational safety is a key concern in ethyl propanoate production due to the flammable nature of the compound and its precursors. Compliance with Occupational Safety and Health Administration (OSHA) standards in the US, or equivalent bodies in other countries, is mandatory. This includes proper handling procedures, personal protective equipment, and emergency response protocols.
International harmonization efforts, such as the International Conference on Harmonisation (ICH) guidelines, aim to standardize regulatory requirements across different regions. Adherence to ICH Q7 (Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients) is particularly relevant for ethyl propanoate production when used in pharmaceutical applications.
Continuous monitoring and documentation are essential for maintaining regulatory compliance. This includes implementing robust quality management systems, conducting regular internal audits, and being prepared for inspections by regulatory authorities. Failure to comply with these regulations can result in severe penalties, including fines, production shutdowns, and damage to the company's reputation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!